Abstract
Purpose of review
Taste receptor family 2 (T2R) bitter taste receptors were originally identified and named on the basis of their role in type 2 taste cells of the tongue, in which they serve to detect the presence of potentially harmful ingested chemicals. In 2009, researchers demonstrated that airway epithelial cells also express T2R receptors, but their role in airway physiology and human disease has only recently begun to be identified.
Recent findings
Recent research has demonstrated that at least one airway T2R receptor, taste receptor family 2 isoform 38 protein (T2R38) is activated by secreted bacterial products. Activation of T2R38 in sinonasal epithelial cells stimulates nitric oxide production, increasing ciliary beating and directly killing bacteria. Clinical studies have also found correlations of TAS2R38 genotype with susceptibility to gram-negative upper respiratory infection and established T2R38 as an independent risk factor for chronic rhinosinusitis requiring sinus surgery.
Summary
These recent studies identify a role for T2R38 in sinonasal innate immunity and chronic rhinosinusitis. Clinical implications include the potential development of T2R38-directed topical therapies, as well as using taste testing and/or genotyping to predict susceptibility to infection. Further studies are needed to more clearly determine how TAS2R38 genotype affects patient outcomes in chronic rhinosinusitis and other upper airway diseases.
Keywords: acyl-homoserine lactone, bacterial infection, host–pathogen interactions, interkingdom signaling, nitric oxide
INTRODUCTION
The immune system has been called the human sixth sense [1], because it acts as a sensory system to detect invading pathogens. Supporting this viewpoint, recent evidence suggests that the immune and taste systems utilize some of the same chemo-sensory receptors, namely bitter taste receptors of the taste receptor family 2 (T2R). T2Rs are G-protein-coupled receptors originally identified in type 2 taste receptor cells of the tongue, but expression of T2Rs is now known to extend to multiple organ systems [2,3], including the airway. The need to understand the physiology of extraoral T2Rs is highlighted by the fact that many medicinal compounds are bitter [3]; thus, extraoral T2Rs may mediate important off-target drug effects [4▪].
Recent basic science and clinical studies are establishing T2Rs as part of a novel pathogen detection network in the airway. T2Rs are expressed in a variety of airway cell types and regulate multiple innate immune responses in both mice [5,6,7▪▪,8,9,10▪] and humans [11▪▪,12▪▪]. The focus of this review is on a well studied human T2R isoform, taste receptor family 2 isoform 38 protein (T2R38), which is expressed in motile cilia lining the sinonasal cavity (nose and sinuses) [12▪▪]. T2R38 has recently been linked with sinonasal innate immunity, upper respiratory infection, and chronic rhinosinusitis (CRS), demonstrating that studying extraoral T2Rs has tremendous potential to reveal novel insights into human disease.
CHRONIC RHINOSINUSITIS AND SINONASAL INNATE IMMUNITY
The airway is continuously challenged by inhaled bacteria, fungi, and viruses. The sinonasal cavity is the front line of respiratory defense; host–pathogen interactions occur with every breath [13]. Nevertheless, in most individuals, immune mechanisms ensure that the sinonasal cavity remains free of infection [14–16]. However, upper respiratory defenses do sometimes fail, often resulting in the onset of CRS, a complex syndrome involving ineffective sinonasal mucociliary clearance (MCC), stasis of sinonasal secretions, and subsequent chronic infection and/or persistent inflammation [15,17,18]. Chronic infection and biofilm formation contribute to medically recalcitrant CRS, necessitating surgical intervention [13,15,17–19]. This results in a tremendous impact on quality of life. CRS patients requiring surgery report quality-of-life scores for physical pain and social functioning that are worse than those suffering from chronic obstructive pulmonary disease, congestive heart failure, or angina [15,17–19]. Additionally, many CRS patients are affected with dysosmia [20], which can cause impaired ability to taste food, feelings of isolation, and decreased awareness of everyday danger (e.g. smoke from fires). Treatments that better eradicate early infections may allow patients to retain and/or regain more olfactory function and improve quality of life.
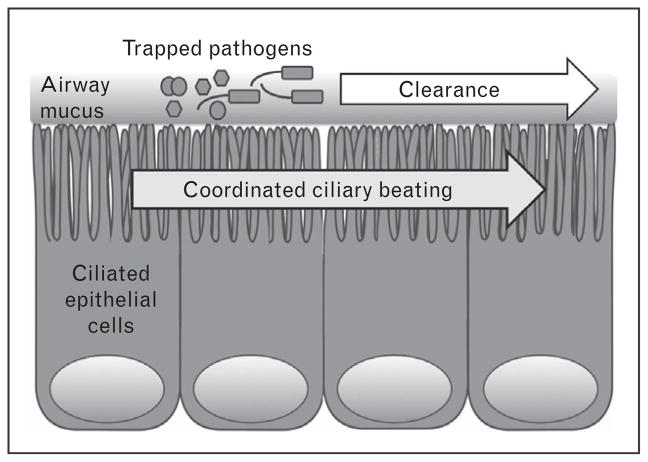
Conventional management of CRS typically involves antibiotics [19]; CRS accounts for one in five antibiotic prescriptions in adults [15,17,18]. However, antibiotics are becoming less effective in the face of the increasing abundance of resistant organisms [21]. The ability to target and stimulate endogenous host defense pathways may be an important alternative therapeutic strategy. However, this requires a greater basic understanding of sinonasal immunity. The primary physical airway defense is MCC [18,22,23] (Fig. 1), which clears the airways of inhaled pathogens, toxins, and irritants. MCC is augmented by more direct antimicrobial mechanisms, including secretion of antimicrobial peptides [14] and production of reactive oxygen and nitrogen species [24,25]. When innate defenses are not enough, epithelial cells can also secrete cytokines and chemokines to recruit dedicated immune cells and activate inflammation [14].
FIGURE 1.
Mucociliary clearance. Inhaled pathogens are trapped by sticky airway mucus secreted by secretory goblet cells and submucosal exocrine glands (not shown).
Coordinated ciliary beating then drives the transport of the debris-laden mucus toward the oropharynx, where it is removed by expectoration or swallowing.
More effective treatment of CRS requires clearer mechanistic insights into the molecular mechanisms of how pathogens activate and/or thwart airway innate defenses. It is also unknown why some individuals are more susceptible to CRS than others. Family and twin studies [26,27▪,28,29] have shown that sinusitis exhibits a degree of heritability, but so far there has been little success in identification of specific genetic modifiers. The idea of genetic susceptibility to CRS is supported by observations of patients with the genetic disease cystic fibrosis, who exhibit increased incidences of CRS and upper airway infections [30–32]. Identification of valid genetic factors controlling CRS susceptibility and/or patient outcomes will likely reveal novel pathophysiological insights and therapeutic modalities. This will allow clinicians to better tailor treatment regimens to individual patients, moving the field further toward the ideal of personalized medicine.
THE ROLE OF T2R38 IN SINONASAL INNATE IMMUNITY
One class of receptors emerging as important components of airway innate immunity is the T2R family of bitter taste receptors. In type II cells of the tongue, T2Rs protect against the ingestion of harmful compounds, including toxic bacterial and plant products [2,3,33,34]. Extraoral expression of T2Rs is found in a variety of organs, including the brain, gut, pancreas, and bladder [2,3]. T2Rs were also recently identified within the motile cilia of cells of the human bronchial [35▪] and sinonasal epithelium [12▪▪]. The physiological roles and ligands for extraoral T2Rs are largely unknown. As many extraoral T2Rs never come into direct contact with ingested food, many known T2R agonists (i.e. bitter products from edible plants) are probably not directly relevant to extraoral function. Instead, it has been hypothesized that extraoral T2Rs detect bitter products from pathogenic bacteria or fungi. A role for T2Rs in immunity might explain their widespread distribution throughout the body. Initial support for this came from studies of solitary chemosensory cells (SCCs) in the mouse nose [36]. SCCs express T2Rs and respond to acyl-homoserine lactones (AHLs) [9], which are quorum-sensing molecules from gram-negative bacteria, including the airway pathogen Pseudomonas aeruginosa [37,38]. Many lactones are bitter [39], suggesting that AHLs are relevant ligands for some extraoral T2Rs.
The hypothesis that T2Rs play a role in immunity may have important clinical consequences. The TAS2R genes encoding T2Rs have many naturally occurring polymorphisms [40] underlying individual taste preferences for bitter foods and beverages, including vegetables [41], coffee [42], scotch [43], and beer [43]. We initially hypothesized that, if T2Rs function in the human airway to detect bacteria and regulate immunity, it is possible that genetic variation in TAS2R genes contributes to susceptibility to respiratory infection and/or CRS.
Sinonasal-ciliated epithelial cells express the bitter taste receptor T2R38, localized within the motile cilia [12▪▪] (Fig. 2a). Sinonasal T2R38 function was studied in human tissue explants, as well as air–liquid interface cultures (ALI [44]) of primary sinonasal cells [12▪▪]. ALI cultures recapitulate a polarized respiratory epithelium with well differentiated ciliated and goblet cells [11▪▪,44]. When human ciliated epithelial cells were stimulated with the T2R38-specific bitter agonist phenylthiocarbamide (PTC; also known as phenylthiourea), they exhibited low-level calcium responses that activated nitric oxide synthase-mediated production of intra-cellular nitric oxide [12▪▪]. Pharmacological inhibition revealed that T2R38 signaling required two important components of the canonical taste pathway characterized in taste cells, namely the transient receptor potential melastatin isoform 5 (TRPM5) ion channel and the phospholipase C isoform beta 2 (PLCβ2) [2,3,12▪▪,33,34], which has now been experimentally confirmed using ALIs derived from nasal septum of wild-type (Wt), TRPM5−/− knockout, and PLCβ2−/− knockout mice [10▪].
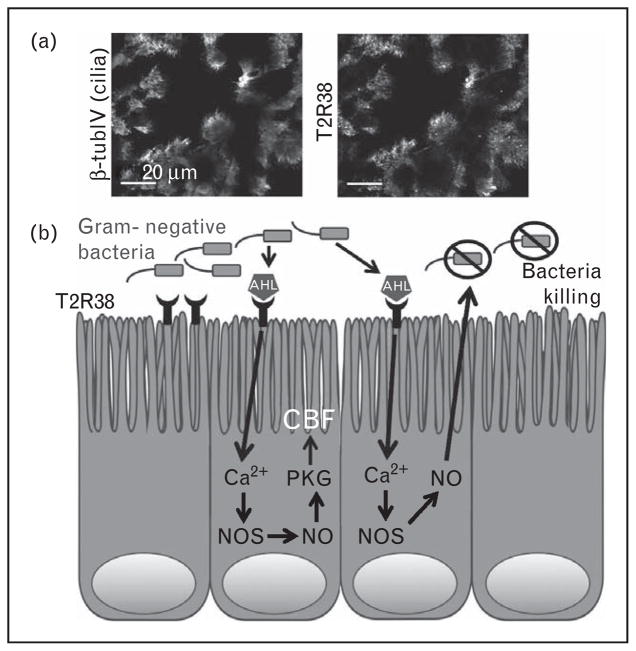
FIGURE 2.
T2R38 in sinonasal innate immunity.
(a) Immunofluorescence confocal micrograph of the apical section of a fixed human sinonasal tissue explant stained using antibodies directed against β-tubulin IV (β-tubIV, with green fluorescent secondary antibody; top panel), a cilia protein, and T2R38 (with red fluorescent secondary antibody; bottom panel), as described in [12▪▪]. Scale bar is 20 μm. (b) T2R38-activation by bacterial quorum-sensing molecules stimulates calcium-mediated nitric oxide production, which increases ciliary beat frequency and directly kills bacteria. AHL, acyl-homoserine lactone; Ca2+, calcium; CBF, ciliary beat frequency; NO, nitric oxide; NOS, nitric oxide synthase; PKG, protein kinase G.
A major result of the nitric oxide produced during T2R38 activation is increased MCC [12▪▪]. Nitric oxide activates guanylyl cyclase, which produces cyclic-guanidine monophosphate and activates protein kinase G, which directly phosphorylates ciliary axoneme proteins to increase beating [45]. However, nitric oxide production is also an important airway defense mechanism independently of MCC [46–48]. Nitric oxide is a highly reactive radical that can diffuse inside bacteria cells. It produces reactive S-nitrosothiols and peroxynitrites that can damage bacterial DNA, membrane lipids, and enzymes [24,25]. High levels of nitric oxide synthase are expressed in the cilia and micro-villi of the sinonasal epithelium [49,50], and thus the sinuses are thought to be a major source of airway nitric oxide. The nitric oxide produced by sinonasal-ciliated epithelial cells in vitro was found to diffuse into the airway surface liquid and have direct bactericidal effects against P. aeruginosa [12▪▪], strongly suggesting that this nitric oxide may have direct antibacterial effects in vivo.
An important piece of evidence supporting the role of T2R38 as a bona-fide contributor to airway immunity was the identification of physiological bacterial ligands that activate T2R38 in vitro. The two major P. aeruginosa AHLs, N-butyryl-L-homo-serine lactone and N-3-oxo-dodecanoyl-L-homoserine lactone [38], activate T2R38 both in sinonasal cells and in a heterologous expression system in vitro [12▪▪]. This was confirmed using a P. aeruginosa strain mutated for the enzymes that synthesize AHLs (strain PAO-JP2; ΔlasI, ΔrhlI; [51]). Sinonasal cells produced robust amounts of nitric oxide in a T2R38-dependent manner when stimulated with physiologically relevant concentrations of AHLs or with dilute conditioned medium from Wt, but not mutant P. aeruginosa. Because many gram-negative bacteria secrete AHLs [52], these data suggest that T2R38 in airway cilia is a sentinel to detect invading gram-negative bacteria and mount nitric oxide-dependent defense responses. Figure 2b depicts a diagram of this pathway.
Human T2R38 functionality is affected dramatically by several well studied polymorphisms in TAS2R38 [53▪,54,55]. Two polymorphisms are common in Caucasians: one encodes a functional T2R38 and the other encodes a nonfunctional T2R38, resulting from differences in amino acids at positions 49, 262, and 296. The functional T2R38 contains Pro (P), Ala (A), and Val (V) (the PAV variant) residues, respectively, at these positions. The nonfunctional T2R38 contains Ala (A), Val (V), and Ile (I) (the AVI variant) at these positions, respectively [56]. Loss of the V at position 296 disrupts AVI receptor activation [57–59]. These polymorphisms have well studied taste phenotypes. Homozygous AVI/AVI individuals (~30% frequency in Caucasians) are nontasters for T2R38-specific agonists, such as PTC [56]; AVI/AVI individuals perceive PTC as either not or weakly bitter. PAV/PAV individuals (~20% frequency in Caucasians [56]) are supertasters for PTC, tasting PTC as intensely bitter. AVI/PAV heterozygotes have varying intermediate levels of taste, correlating with differences of the relative expression levels of AVI and PAV alleles [53▪,56].
The effects of TAS2R38 polymorphisms were studied using primary ALIs derived from genotyped patients. When ALIs were stimulated in vitro with PTC, AHLs, or conditioned medium from P. aerugi-nosa, the antibacterial nitric oxide responses correlated with TAS2R38 polymorphisms. Epithelial cells from PAV/PAV supertasters exhibited markedly enhanced nitric oxide production, MCC, and bacterial killing compared with AVI/AVI or AVI/PAV cells. Cells derived from AVI/AVI nontasters or AVI/PAV heterozygotes had blunted nitric oxide responses that were not effective at killing P. aeru-ginosa in vitro [12▪▪]. This strongly suggested that PAV/PAV individuals might be less susceptible to gram-negative infection, which has now been further studied in a clinical setting.
THE ROLE OF TAS2R38 POLYMORPHISMS IN CHRONIC RHINOSINUSITIS
Preliminary clinical data suggested that PAV/PAV T2R38 supertasters are less susceptible to gram-negative sinonasal infection than PAV/AVI or AVI/AVI patients, who, as described above, have impaired T2R38-dependent responses [12▪▪]. TAS2R38 genotype was compared in genotyped patients (N =56) who had undergone sinonasal surgery for CRS or for non-CRS-related concerns (i.e. pituitary disorder) and who had microbiology results of either no growth (or normal respiratory flora, e.g. Staphylococcus epidermis; n =35) or positive cultures for gram-negative bacteria (n =21) or specifically P. aeruginosa (n =14). None of the patients who had gram-negative or P. aeruginosa growth was PAV/PAV a supertaster. The distribution of PAV/PAV, AVI/PAV, and PAV/PAV genotypes was significantly different between control patients and either gram-negative (P <0.006 by χ2) or P. aeruginosa (P <0.029) patients [12▪▪]. The control and gram-negative/P. aeruginosa patients had no significant differences in the distributions of common polymorphisms in TAS2R19, TAS2R30 (also known as TAS2R47), or TAS2R46 [12▪▪].
Further studies [60▪,61▪▪,62▪▪] have demonstrated that TAS2R38 may be a genetic marker for CRS. A retrospective study [60▪] was carried out using TAS2R38-genotyped patient samples (N =28) from individuals who had undergone primary functional endoscopic sinus surgery (FESS). It was determined that 46% (n =13 patients out of 28) of the FESS patients were AVI/AVI nontasters, 50% (n =14) were AVI/PAV heterozygotes, and 3.6% (n =1) were PAV/PAV supertasters. This was significantly different (P <0.043 by χ2) from the approximate expected distributions of 20% (n =5.6 out of 28) PAV/PAV, 50% (n =14) PAV/AVI, and 30% (n =8.4) AVI/AVI [60▪]. This study suggested that PAV/PAV supertasters are less likely to need surgical intervention for CRS.
This was followed by a subsequent prospective study [61▪▪] of TAS2R38 genotype in patients (N =70) undergoing primary FESS. The distribution of diplotypes in the CRS patients was 37% (n =26) AVI/AVI, 54% (n =38) AVI/PAV, and 8.5% (n =6) PAV/PAV. This was significantly different (P <0.0383 by χ2) from the 29% (n =100) AVI/AVI, 51% (n =177) AVI/PAV, and 20% (n =70) PAV/PAV distributions found in the general Philadelphia population (N =347) [61▪▪]. No significant differences were found in the allele distribution with respect to other risk factors, such as asthma, allergies, aspirin sensitivity, diabetes, smoking exposure, or nasal polyposis, suggesting that TAS2R38 is an independent risk factor for CRS requiring FESS.
To verify whetherT2R polymorphisms correlate with CRS, previously collected pooling-based genome-wide association data were screened for single-nucleotide polymorphisms (SNPs) in taste receptors, using two populations of Canadian CRS patients, as well as a control population [62▪▪]. Included in this study was the TAS2R38 I296 V (rs10246939) SNP, thought to underlie the difference in functionality between PAV and AVI variants. The SNP frequency differences of at least 10% between CRS and control populations were considered significant. I296 V SNP frequency differences were ~15 and 11% between the two CRS populations compared with the control population, confirming that I296 V is associated with CRS [62▪▪]. Interestingly, this study [62▪▪] also found three other missense variants in TAS2R genes that were associated with CRS: two in TAS2R49 (rs12226920 and rs12226919) and one in TAS2R14 (rs1015443). Although highly intriguing, it remains to be determined whether T2R14 or T2R49 plays roles in sinonasal immunity.
CONCLUSION
T2R38 is part of an interkingdom eavesdropping system by which mammalian cells intercept bacterial quorum-sensing communications [12▪▪]. A summary of current evidence linking T2R38s with sinonasal innate immunity is shown in Table 1. Further prospective clinical studies on TAS2R38 genotype and CRS susceptibility, including the influence of TAS2R38 genotype on patient outcomes, are currently ongoing. However, the T2R38 pathway is already a potential therapeutic target to promote endogenous immune responses in patients with upper respiratory infections. One caveat, though, is that there would likely be a large subset of patients who would be suboptimally responsive to the treatment with T2R38 agonists (i.e. PAV/AVI and AVI/AVI individuals). It is, thus, still necessary to further define T2R38 signaling mechanisms in airway cells, as well as to identify other T2Rs that activate similar responses.
Table 1.
Evidence linking T2R38 genotype to sinonasal innate immunity
| Biological and clinical outcome | TAS2R38 genotype | References | ||
|---|---|---|---|---|
| AVI/AVI | PAV/AVI | PAV/PAV | ||
| Intracellular calcium response in vitro | Decreased | Decreased | Increased | [12▪▪] |
| NO production in vitro | Decreased | Decreased | Increased | [12▪▪] |
| Ciliary beat frequency/MCC in vitro | Decreased | Decreased | Increased | [12▪▪] |
| Bactericidal activity in vitro | Decreased | Decreased | Increased | [12▪▪] |
| Sinonasal gram-negative infection in vivo | Increased | Increased | Decreased | [12▪▪] |
| CRS | Increased | – | Decreased | [60▪,61▪▪,62▪▪] |
CRS, chronic rhinosinusitis; MCC, mucociliary clearance; NO, nitric oxide; T2R38, taste receptor family 2 isoform 38 protein; TAS2R38, taste receptor family 2 isoform 38 gene.
It is also likely that other T2R isoforms expressed in other airway cell types may have important clinical relevance. Table 2 [5,6,7▪▪,8,9,10▪,11▪▪, 12▪▪,35▪,63,64▪,65–76] shows a representative list of known T2R expression in the airway. In particular, the genetics of T2Rs in nasal solitary chemo-sensory cells may play an important role in CRS susceptibility. Solitary chemosensory cells control antimicrobial peptide secretion in humans [11▪▪] and both breath-holding [8,9,36] and inflammation [7▪▪] in mice. Clinical consequences remain to be determined.
Table 2.
T2R expression throughout the airway
| Cell type (airway region) | Processes regulated by T2R receptors | Endogenous ligands | References |
|---|---|---|---|
| Ciliated epithelial cells (nose and sinuses) | Nitric oxide production; cilia beating; direct bactericidal effects | Bacterial acyl-homoserine lactone quorum-sensing molecules (T2R38) | [10▪,12▪▪,63] |
| Ciliated epithelial cells (bronchi) | Cilia beating | Unknown | [35▪] |
| Solitary chemosensory cells (nose and sinuses) | Antimicrobial peptide secretion (human); breath-holding and inflammation (mouse) | Bacterial acyl-homoserine lactone quorum-sensing molecules (mouse); unknown (human) | [5,6,7▪▪,8,9,11▪▪,64▪,65–67] |
| Chemosensory brush cells (trachea) | ACh release to stimulate trigeminal neuron activation and breath-holding (mouse); unknown (human) | Bacterial AHL quorum-sensing molecules (mouse); unknown (human) | [68–71] |
| Smooth muscle (bronchi) | Bronchodilation | Unknown | [72–76] |
AHL, acyl-homoserine lactone; T2R, taste receptor family 2; T2R38, taste receptor family 2 isoform 38 protein.
A better understanding of the T2R isoforms expressed in ciliated cells will speed identification of potential compounds that stimulate innate defenses. The role of T2Rs as global mediators of innate immunity was recently supported by a report demonstrating that T2R-expressing chemosensory cells in the rodent urethra respond to the bitter compounds, as well as heat-inactivated uropathogenic Escherichia coli; activation of these cells causes release of ACh to activate the bladder detrusor muscle [77▪]. Further investigation of T2Rs in innate immunity will likely result in a significant clinical impact on the airway and other organ systems.
KEY POINTS.
T2R bitter taster receptors are expressed in several airway cell types.
The T2R38 isoform is expressed in sinonasal-ciliated epithelial cells, in which it detects bacterial quorum-sensing molecules and regulates nitric oxide-dependent innate immune responses.
Polymorphisms in T2R38 that result in decreased receptor functionality have been linked to both impaired innate immune response in vitro and gram-negative infection and CRS in vivo.
Acknowledgments
Some of the research described here was supported by a grant from the Flight Attendants Medical Research Institute (082478) and a philanthropic contribution from the RLG Foundation, Inc., both to N.A.C.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Bedford FL. The missing sense modality: the immune system. Perception. 2011;40:1265–1267. doi: 10.1068/p7119. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K, Ishimaru Y. Oral and extra-oral taste perception. Semin Cell Dev Biol. 2013;24:240–246. doi: 10.1016/j.semcdb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clin Ther. 2013;35:1225–1246. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Clark AA, Liggett SB, Munger SD. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012;26:4827–4831. doi: 10.1096/fj.12-215087. Presents the hypothesis that bitter taste receptors may mediate off-target drug effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finger TE, Bottger B, Hansen A, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99:2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A. 2014;111:6075–6080. doi: 10.1073/pnas.1402251111. Demonstrates that mouse nasal solitary chemosensory cell utilizes bitter taste signaling to mediate cholinergic-induced inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Lee RJ, Chen B, Redding KM, et al. Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun. 2014;20:606–617. doi: 10.1177/1753425913503386. Examines T2R-mediated signaling in mouse motile cilia to examine pathway components involved in nitric oxide-driven innate immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪▪.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. Demonstrates that human sinonasal solitary chemosensory cells express bitter and sweet taste receptors that regulate antimicrobial peptide secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. Demonstrates that T2R38 is expressed in sinonasal cilia and regulates nitric oxide production and innate immune responses. Also contains preliminary data showing that PAV/PAV homozygotes are protected against gram-negative infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:640.e4–653.e4. doi: 10.1016/j.jaci.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen NA. Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol Suppl. 2006;196:20–26. doi: 10.1177/00034894061150s904. [DOI] [PubMed] [Google Scholar]
- 16.Waterer GW. Airway defense mechanisms. Clin Chest Med. 2012;33:199–209. doi: 10.1016/j.ccm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:631–643. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhino-sinusitis. Am J Rhinol Allergy. 2012;26:1–6. doi: 10.2500/ajra.2012.26.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Settipane RA, Peters AT, Chandra R. Chapter 4: chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27:S11–S15. doi: 10.2500/ajra.2013.27.3925. [DOI] [PubMed] [Google Scholar]
- 20.Gaines A. Chapter 13: olfactory disorders. Am J Rhinol Allergy. 2013;27:S45–S47. doi: 10.2500/ajra.2013.27.3898. [DOI] [PubMed] [Google Scholar]
- 21.Manes RP, Batra PS. Bacteriology and antibiotic resistance in chronic rhinosinusitis. Facial Plast Surg Clin North Am. 2012;20:87–91. doi: 10.1016/j.fsc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc. 2011;8:31–39. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 23.Dalgorf DM, Harvey RJ. Chapter 1: sinonasal anatomy and function. Am J Rhinol Allergy. 2013;27:S3–S6. doi: 10.2500/ajra.2013.27.3888. [DOI] [PubMed] [Google Scholar]
- 24.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcinkiewicz J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology. 1997;37:35–41. doi: 10.1016/s0162-3109(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 26.Hamilos DL. Chronic rhinosinusitis patterns of illness. Clin Allergy Immunol. 2007;20:1–13. [PubMed] [Google Scholar]
- 27▪.Hsu J, Avila PC, Kern RC, et al. Genetics of chronic rhinosinusitis: state of the field and directions forward. J Allergy Clin Immunol. 2013;131:977–993. doi: 10.1016/j.jaci.2013.01.028. Discusses the search for genetic modifiers for CRS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greisner WA, 3rd, Settipane GA. Hereditary factor for nasal polyps. Allergy Asthma Proc. 1996;17:283–286. doi: 10.2500/108854196778662192. [DOI] [PubMed] [Google Scholar]
- 29.Cohen NA, Widelitz JS, Chiu AG, et al. Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg. 2006;134:601–604. doi: 10.1016/j.otohns.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Godoy JM, Godoy AN, Ribalta G, Largo I. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg. 2011;145:673–676. doi: 10.1177/0194599811407279. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez C, Ribalta G, Largo I. Retrospective analysis of chronic rhinosinusitis in patients with cystic fibrosis. Acta Otorrinolaringol Esp. 2012;63:286–291. doi: 10.1016/j.otorri.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:70–75. doi: 10.2500/ajra.2012.26.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinnamon SC. Taste receptor signalling: from tongues to lungs. Acta Physiol (Oxf) 2012;204:158–168. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F. Taste perception: from the tongue to the testis. Mol Hum Reprod. 2013;19:349–360. doi: 10.1093/molehr/gat009. [DOI] [PubMed] [Google Scholar]
- 35▪.Shah AS, Ben-Shahar Y, Moninger TO, et al. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. First demonstration that motile cilia of the airway are chemosensory organelles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tizzano M, Finger TE. Chemosensors in the nose: guardians of the airways. Physiology (Bethesda) 2013;28:51–60. doi: 10.1152/physiol.00035.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez PN, Koch G, Thompson JA, et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013;14:12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim U, Wooding S, Ricci D, et al. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 2005;26:199–204. doi: 10.1002/humu.20203. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Zhang J. Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol Biol Evol. 2014;31:303–309. doi: 10.1093/molbev/mst219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes JE, Wallace MR, Knopik VS, et al. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses. 2011;36:311–319. doi: 10.1093/chemse/bjq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;83:821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Lai Y, Chen B, Shi J, et al. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2011;128:1207.e1–1215.e1. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 46.Haight JS, Djupesland PG, Qjan W, et al. Does nasal nitric oxide come from the sinuses? J Otolaryngol. 1999;28:197–204. [PubMed] [Google Scholar]
- 47.Gustafsson LE, Leone AM, Persson MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 48.Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res. 2007;56:58–69. doi: 10.1007/s00011-006-6111-1. [DOI] [PubMed] [Google Scholar]
- 49.Deja M, Busch T, Bachmann S, et al. Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Respir Crit Care Med. 2003;168:281–286. doi: 10.1164/rccm.200207-640OC. [DOI] [PubMed] [Google Scholar]
- 50.Degano B, Valmary S, Serrano E, et al. Expression of nitric oxide synthases in primary ciliary dyskinesia. Hum Pathol. 2011;42:1855–1861. doi: 10.1016/j.humpath.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 51.Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Nair SK. Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012;21:1403–1417. doi: 10.1002/pro.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Lipchock SV, Mennella JA, Spielman AI, Reed DR. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr. 2013;98:1136–1143. doi: 10.3945/ajcn.113.066688. Demonstrates that differential intermediate PTC taste sensitivity in PAV/AVI heterozygotes may be due to differences in mRNA levels of PAV and AVI alleles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed DR, Knaapila A. Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci. 2010;94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachmanov AA, Bosak NP, Lin C, et al. Genetics of taste receptors. Curr Pharm Des. 2014;20:2669–2683. doi: 10.2174/13816128113199990566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bufe B, Breslin PA, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan J, Abrol R, Trzaskowski B, Goddard WA., 3rd 3D structure prediction of TAS2R38 bitter receptors bound to agonists phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) J Chem Inf Model. 2012;52:1875–1885. doi: 10.1021/ci300133a. [DOI] [PubMed] [Google Scholar]
- 58.Biarnes X, Marchiori A, Giorgetti A, et al. Insights into the binding of phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLoS One. 2010;5:e12394. doi: 10.1371/journal.pone.0012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Floriano WB, Hall S, Vaidehi N, et al. Modeling the human PTC bitter-taste receptor interactions with bitter tastants. J Mol Model. 2006;12:931–941. doi: 10.1007/s00894-006-0102-6. [DOI] [PubMed] [Google Scholar]
- 60▪.Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184–187. doi: 10.1002/alr.21140. First retrospective clinical study linking skewed T2R38 genotype distribution in CRS requiring FESS. [DOI] [PubMed] [Google Scholar]
- 61▪▪.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. Follow-up prospective study examining T2R38 genotype in CRS patients requiring FESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪▪.Mfuna Endam L, Filali-Mouhim A, Boisvert P, et al. Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200–206. doi: 10.1002/alr.21275. Analysis of taste receptor SNPs in Canadian CRS patients, confirming T2R38 genotype association with CRS. [DOI] [PubMed] [Google Scholar]
- 63.Lee RJ, Cohen NA. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27:283–286. doi: 10.2500/ajra.2013.27.3911. [DOI] [PubMed] [Google Scholar]
- 64▪.Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3:450–457. doi: 10.1002/alr.21149. Early demonstration that T2Rs in addition to T2R38 are expressed in the sinonasal mucosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulbransen B, Silver W, Finger TE. Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol. 2008;508:62–71. doi: 10.1002/cne.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin W, Ogura T, Margolskee RF, et al. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- 67.Sbarbati A, Osculati F. Solitary chemosensory cells in mammals? Cells Tissues Organs. 2003;175:51–55. doi: 10.1159/000073437. [DOI] [PubMed] [Google Scholar]
- 68.Krasteva G, Canning BJ, Hartmann P, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saunders CJ, Reynolds SD, Finger TE. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol. 2013;49:190–196. doi: 10.1165/rcmb.2012-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat. 2005;206:349–358. doi: 10.1111/j.1469-7580.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91:992–996. doi: 10.1016/j.lfs.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 72.An SS, Wang WC, Koziol-White CJ, et al. TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L304–L311. doi: 10.1152/ajplung.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol. 2011;45:1069–1074. doi: 10.1165/rcmb.2011-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulkkinen V, Manson ML, Safholm J, et al. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol. 2012;303:L956–L966. doi: 10.1152/ajplung.00205.2012. [DOI] [PubMed] [Google Scholar]
- 75.Grassin-Delyle S, Abrial C, Fayad-Kobeissi S, et al. The expression and relaxant effect of bitter taste receptors in human bronchi. Respir Res. 2013;14:134. doi: 10.1186/1465-9921-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinett KS, Koziol-White CJ, Akoluk A, et al. Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50:678–683. doi: 10.1165/rcmb.2013-0439RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪.Deckmann K, Filipski K, Krasteva-Christ G, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci U S A. 2014;111:8287–8292. doi: 10.1073/pnas.1402436111. Study demonstrating that cells in another organ, the bladder, use chemosensory signaling to detect bacteria and regulate defense responses. [DOI] [PMC free article] [PubMed] [Google Scholar]