Abstract
The biological properties of trifluoromethyl compounds have led to their ubiquity in pharmaceuticals, yet their chemical properties have made their preparation a substantial challenge, necessitating innovative chemical solutions. We report the serendipitous discovery of a borane-catalyzed formal C(sp3)-CF3 reductive elimination from Au(III) that accesses these compounds by a distinct mechanism proceeding via fluoride abstraction, migratory insertion, and C-F reductive elimination to achieve a net C-C bond construction. The parent bis(trifluoromethyl)Au(III) complexes tolerate a surprising breadth of synthetic protocols, enabling the synthesis of complex organic derivatives without cleavage of the Au-C bond. This feature, combined with the “fluoride-rebound” mechanism, was translated into a protocol for the synthesis of 18F-radiolabeled aliphatic CF3-containing compounds, enabling the preparation of potential tracers for use in positron emission tomography.
The trifluoromethyl (CF3) functional group is both a crucial pharmaceutical moiety and a synthetic frustration. This dual nature stems from a common cause: Strong, noninteracting C-F bonds lend metabolic stability while simultaneously limiting the ability of chemical transformations to forge the relevant linkages and install the CF3 unit. Generally speaking, nucleophilic (i.e., the Ruppert-Prakash reagent), electrophilic (Umemoto’s or Togni’s reagents), or metal-mediated (e.g., CuCF3) chemistry is used to deliver a preformed CF3 unit to an organic substrate, although the latter class of chemistries is often plagued by the slow direct reductive elimination of fluoroalkyl ligands (1–6). Nonetheless, these modern protocols have aided the continuing proliferation of trifluoromethyl-containing compounds in medicinal chemistry, including several high-profile drugs.
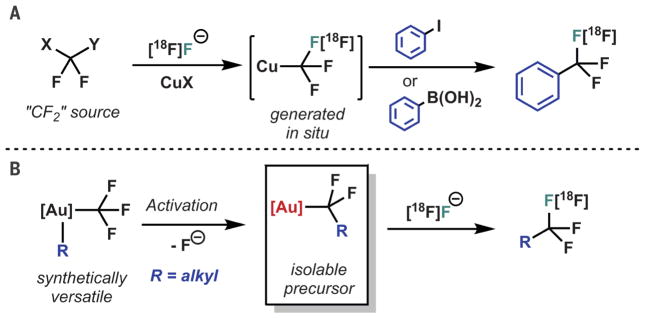
When these same synthetic considerations are extended toward the synthesis of trifluoromethylated positron emission tomography (PET) tracers, the situation becomes more complex (7–9). The biomedical applications of PET rely on the availability of these tracers—radiolabeled compounds in which a positron-emitting isotope, usually 18F (half-life, t1/2 ~ 110 min), is incorporated into a molecule of biological importance. These compounds can be used for high-resolution real-time imaging of tissue-level phenomena, enabling direct interrogation of disease states and mechanisms of bioactivity. However, nucleophilic substitution (i.e., SN2 or SNAr) chemistry, which is used for the majority of organic fluoride radiosynthesis, is generally incompatible with the stereo-electronic demands of the CF3 unit (10). Rather, in the most successful technology, a “CF2” precursor is reacted with radiofluoride to generate the relevant CF3 unit prior to incorporation into the tracer via an organocopper intermediate (Fig. 1A) (11–13).
Fig. 1. Retrosynthetic analysis for CF3 radiosynthesis.
(A) Pregeneration of a metal CF3 unit and transfer to an organic substrate, Y = CO2Me, H; X = Cl, I, S(aryl)2+ (Me, methyl). (B) By virtue of the CF3 activation mechanism reported herein, Au(III) enables synthetic elaboration of the organometallic species prior to activation for implementation for radiochemistry.
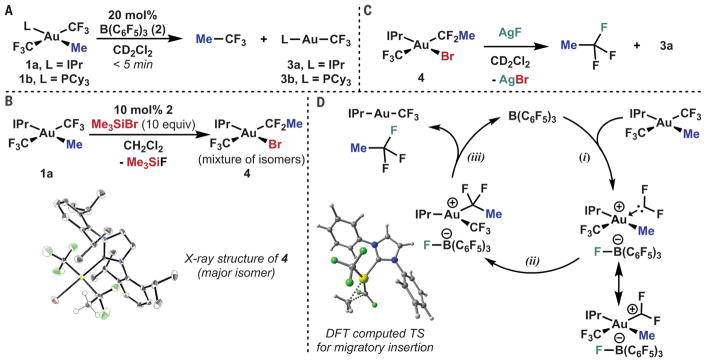
An isolable yet reactive difluoroalkyl metal substituent that affords the corresponding CF3 unit upon treatment with radiofluoride would represent an alternative retrosynthetic approach by which to access these important tracer compounds (14–19). We report the serendipitous discovery of a class of Au(III) complexes that exactly match this profile of reactivity, simultaneously providing synthetic access to the requisite precursors while enabling their implementation for radio-fluorination (Fig. 1B). The successful development of this advance depended on mechanistic investigation of an unexpected trifluoromethylated compound observed upon treatment of complex 1a with B(C6F5)3 (2) (Fig. 2A), namely the formal product of C(sp3)-CF3 reductive elimination from the gold center (20, 21).
Fig. 2. Discovery of formal C(sp3)-CF3 reductive elimination from Au(III).
(A) Borane-catalyzed reductive elimination of trifluoroethane. (B) Trapping of a rearranged intermediate by bromotrimethylsilane. (C) Outer-sphere C-F reductive elimination from a difluoroalkyl Au(III) complex. (D) Proposed mechanism of catalysis by tris(pentafluorophenylborane). IPr, 1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene; Cy, cyclohexyl; TS, transition state.
Our investigation into the mechanism commenced with 19F nuclear magnetic resonance (NMR) monitoring of the formal reductive elimination of Me-CF3 from 1a in the presence of stoichiometric 2 at −15°C. The reaction showed clean first-order kinetics (fig. S11), consistent with catalytic action of 2 (22). At catalytic loadings of 2, complete conversion was observed in less than 5 min at room temperature, affording IPr-Au-CF3 (3a) as the major Au-containing product. The same reactivity was observed with the related phosphine complex 1b (23). Addition of 10 equivalents of Me3SiBr to the 2-catalyzed reductive elimination formed an isolable Au(III) complex (4), bearing bromide and α,α-difluoroethyl substituents, as a mixture of two coordination isomers (Fig. 2B). Treatment of 4 with AgF leads to the gradual formation of MeCF3 and 3 (Fig. 2C). Taken together, these results implicate an overall mechanism (Fig. 2D) in which fluoride abstraction (i) from a CF3 moiety of 1 by the borane 2 results in an intermediate difluorocarbenoid (or alternatively the carbenium resonance form, shown) that undergoes migratory insertion (ii) of the alkyl fragment, followed by formal C-F reductive elimination (iii) to afford trifluoroethane. Additional experiments in support of this proposal include the formation of 1,1-difluoroethyl triflate (MeCF2OTf) when stoichiometric trimethylsilane triflate is used in place of 2 (fig. S5) and the observation of an intermediate aquo complex of the migratory insertion product during low-temperature kinetics in wet CD2Cl2 (figs. S12 to S15) (24).
To various extents, these elementary steps have been observed previously at gold centers; in particular, our earlier work on C-F reductive elimination suggests that an outer-sphere mechanism with considerable carbocationic character is operative (25, 26). Although migratory insertion at Au(III) is comparatively rarer, density functional theory (DFT) calculations suggest that α-insertion to the difluorocarbene occurs readily (enthalpy of activation, ΔH‡ < 4 kcal/mol; see fig. S32 and table S24 for details) (27). Such a low barrier likely explains why competitive hydrolysis of the difluorocarbene, common in metal halocarbene complexes, was not observed here even in wet solvent (28). Regardless, this mechanism is unusual because it represents a formal C(sp3)-CF3 reductive elimination—itself rarely if ever observed—by a catalytic process involving iterative disassembly and reassembly of the CF3 moiety (29–31).
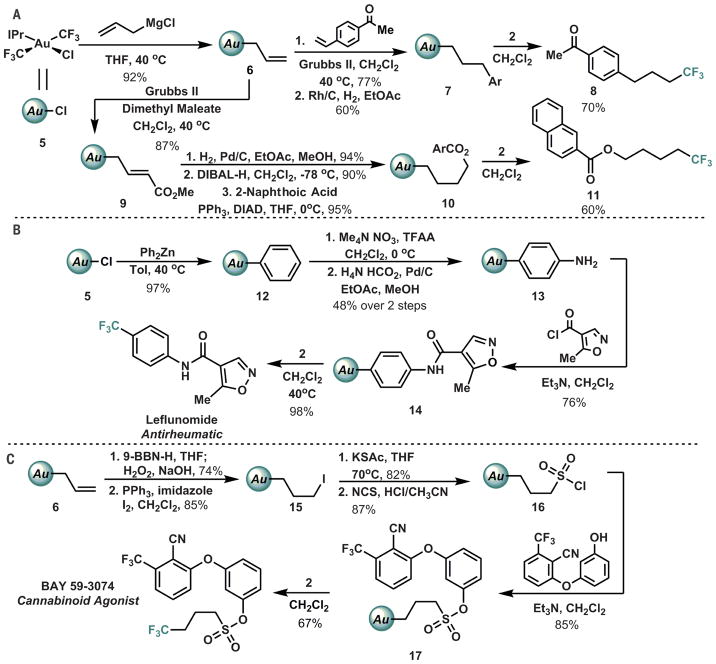
It became clear to us that interception of the rebounding fluoride and introduction of a radio-labeled surrogate could hold promise for the synthesis of PET traces, provided that more complex organic substituents could be used than the parent methyl in 1. Diversification of the organic fragment beyond the simple methyl analog was accomplished by means of a variety of synthetic manipulations (including hydroboration, cross-metathesis, hydrogenation, and aluminum hydride reduction) of η1-allyl 6, allowing the preparation of more functionally diverse complexes (32). Reductive elimination from these elaborated derivatives was likewise triggered with 2, ultimately affording reductive elimination products such as 8 and 11 (Fig. 3A). Similar diversity was seen in the chemistry of arylgold complex 12 (remarkably including aromatic nitration without rupture of the gold-carbon bond), enabling the synthesis of Leflunomide via C-CF3 reductive elimination from an advanced intermediate (Fig. 3B) (33).
Fig. 3. Synthetic versatility of bis(trifluoromethyl) gold complexes.
(A) Elaboration of allyl gold complex via multistep synthesis. (B) Synthesis of Leflunomide. (C) Synthesis of BAY 59–3074. Isolated yields are listed for all reactions (see supplementary materials for details). Grubbs II, Grubbs’ second-generation metathesis catalyst; EtOAc, ethyl acetate; MeOH, methanol; DIBAL-H, diisobutylaluminum hydride; DIAD, diisopropyl azodicarboxylate; Tol, toluene; TFAA, trifluoroacetic anhydride; Et3N, trimethylamine; 9-BBN-H, 9-borabicyclononane; NCS, N-chlorosuccinimide; Ph, phenyl; KSAc, potassium thioacetate; Ar, aryl (aromatic substituent).
To further demonstrate the utility and functional group tolerance of this method, we prepared the Bayer lead compound BAY 59–3074 via straightforward elaboration of 6 (Fig. 3C) (34). In addition to the depicted transformations, we also found the complexes to be tolerant of Simmons-Smith cyclopropanation, osmium-catalyzed dihydroxylation, periodate-mediated diol cleavage, and Levin et al., Science 356, 1272–1276 (2017) 23 June 2017 palladium-catalyzed cross-coupling. Initial Au-C bond construction was also achieved with dialkyl zinc or copper acetylide reagents, further expanding the diversity of accessible structures. Cleavage of the Au-C bond was observed only in the presence of exceptionally strong acids (CF3SO3H) and elemental halogens (e.g., Br2). All of the intermediate gold complexes exhibited complete air and water tolerance and were universally amenable to purification by silica gel column chromatography. As such, we have begun to regard the Au moiety as a triggerable but otherwise inert functional group, enabling routine chemical synthesis akin to typical organic substrates. Although synthetically tolerant metal complexes have previously been reported, such broad compatibility is rare, especially for Au(III), which tends to be highly sensitive toward reductive decomposition (35–39).
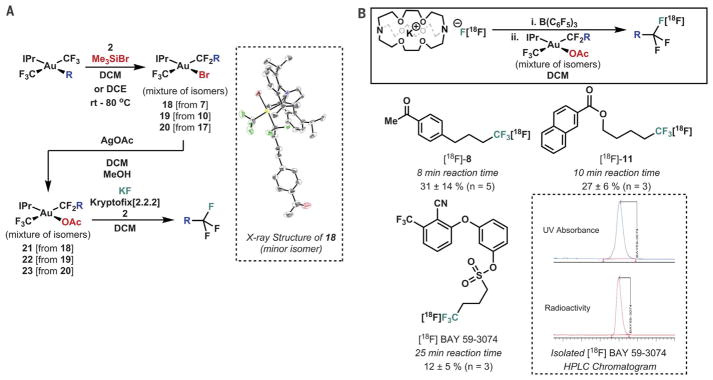
For the radiochemical protocol, we initially envisioned introduction of fluoride to complexes analogous to 4. However, the silver-mediated reductive elimination (Fig. 2C) was deemed too sluggish for these purposes, requiring the development of a modified strategy. Although complexes with several other counterions (such as triflate and tosylate) rapidly eliminated alkyl-CF2X, the corresponding acetate complex exhibited an appropriate blend of stability and reactivity to enable nucleophilic reductive elimination when treated with 2 and a suitable fluoride source (Fig. 4A) (40). Borane 2 was required for rapid reductive elimination to occur; in its absence, the intermediate [Au(III)]-F persisted for long enough to be observed by 19F NMR spectroscopy, and side reactions arising from base-mediated decomposition or glass-etching occurred alongside slow reductive elimination, consistent with an outer-sphere mechanism for C-F bond formation assisted by 2 (41–43). The combined protocol was directly translated to a radiological analog, affording the corresponding radiolabeled reductive elimination products starting from KF and Kryptofix [2.2.2], as shown in Fig. 4B (44).
Fig. 4. Radiosynthesis via C-F reductive elimination from Au(III).
(A) Synthesis of an Au(III) acetate precursor and validation of its reductive elimination chemistry with 19F NMR spectroscopy. (B) Radiosynthesis of aliphatic trifluoromethyl groups via Au(III). Yields given are radiochemical conversions determined by radio–thin-layer chromatography. triggered reductive elimination of functionally tolerant complexes.
Several features of this protocol merit additional comment. First, aromatic complexes remain inaccessible for radiochemical functionalization by this method because of the rapid reductive elimination of the benzylic difluoroalkyl moiety; treatment of phenyl complex 12 with Me3SiBr and 2 results in PhCF2Br rather than an isolable Au(III) complex. As such, the scope of this protocol remains fully complementary to the corresponding Cu-mediated analog via CuCF3 (11–13). Second, isolation of [18F]BAY 59–3074 by preparative high-performance liquid chromatography (HPLC) afforded an isolated radiochemical yield of 6% and a specific activity of 8 mCi/μmol starting from 71 mCi of [18F]fluoride. Such a specific activity is on par with that of many CuCF3-based protocols, although values ranging from 2.7 to 860 mCi/μmol have been obtained depending on the preparative method. [These values are all much lower than those achieved with classical nucleophilic substitution methodologies for alkyl fluoride synthesis (typically in excess of 1000 mCi/μmol), highlighting an important direction for the future development of radio-trifluoromethylation (11–13).] Finally, the cost associated with the use of stoichiometric organo-gold reagents, although substantial by the metrics of process-scale pharmaceutical preparation, are relatively small in this context given the low concentrations required, the cost of 18F production, and the costs of the associated medical imaging technology (45).
The protocol established here represents an important proof of concept in the radiosynthesis of organic trifluoromethyl groups by a retrosynthetic paradigm involving C-F reductive elimination and should prove a fruitful area for further exploration. The broader mechanistic framework consisting of fluoride-rebound reductive elimination upon which this radiochemical reaction is built also has implications for the understanding of transition metal catalysis, as it seems possible that such a mechanism is in fact operative in the related copper-mediated protocols currently in use (in both radiochemical and nonradiochemical contexts) (46–48). Furthermore, this work suggests a promising direction for the development of organometallic reagents via catalytically
Supplementary Material
Acknowledgments
Supported by NSF grant DGE 1106400 and an ARCS Foundation graduate research fellowship (M.D.L.), a Swiss National Science Foundation postdoctoral fellowship (I.J.K.), and National Institute of General Medical Sciences grant R35 GM118190. DFT studies were conducted at the Molecular Graphics and Computation Facility, funded by NSF grant CHE-0840505. We thank H. Celik, W. Wolf, and L. Sofen for technical assistance; R. Bergman, D. Kaphan, R. Thornbury, M. Winston, Y. Wang, and A. Zhukhovitskiy for helpful discussions; and B. Cwick for facilitating the collaboration that prompted the radiochemical applications of this work. Metrical parameters for the structures of compounds 1a, 1b, 4, 5, 6, 7, 18, S2, and S8 are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1537030 to 1537038, respectively, with all other data available in the supplement. M.D.L., J.P.O., and F.D.T. are listed as inventors on a patent application describing Au(III) reagents for radiofluorination, Provisional Application 62/268,842 filed 22 May 2017.
Footnotes
REFERENCES AND NOTES
- 1.Prakash GKS, Yudin AK. Chem Rev. 1997;97:757–786. doi: 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]
- 2.Barata-Vallejo S, Lantaño B, Postigo A. Chem Eur J. 2014;20:16806–16829. doi: 10.1002/chem.201404005. [DOI] [PubMed] [Google Scholar]
- 3.García-Monforte MA, Martínez-Salvador S, Menjón B. Eur J Inorg Chem. 2012;2012:4945–4966. [Google Scholar]
- 4.Zanardi A, Novikov MA, Martin E, Benet-Buchholz J, Grushin VV. J Am Chem Soc. 2011;133:20901–20913. doi: 10.1021/ja2081026. [DOI] [PubMed] [Google Scholar]
- 5.Cho EJ, et al. Science. 2010;328:1679–1681. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball ND, Kampf JW, Sanford MS. J Am Chem Soc. 2010;132:2878–2879. doi: 10.1021/ja100955x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preshlock S, Tredwell M, Gouverneur V. Chem Rev. 2016;116:719–766. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 8.Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJH. Chem Sci. 2014;5:4545–4553. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell MG, et al. Nat Chem. 2016;9:1–3. doi: 10.1038/nchem.2693. [DOI] [PubMed] [Google Scholar]
- 10.Lien VT, Riss PJ. BioMed Res Int. 2014;2014:380124. doi: 10.1155/2014/380124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huiban M, et al. Nat Chem. 2013;5:941–944. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- 12.van der Born D, et al. Angew Chem Int Ed. 2014;53:11046–11050. doi: 10.1002/anie.201406221. [DOI] [PubMed] [Google Scholar]
- 13.Ivashkin P, et al. Chem Eur J. 2014;20:9514–9518. doi: 10.1002/chem.201403630. [DOI] [PubMed] [Google Scholar]
- 14.Liang SH, Vasdev N. Aust J Chem. 2015;68:1319–1328. doi: 10.1071/CH15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, et al. Science. 2011;334:639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teare H, et al. Angew Chem Int Ed. 2010;49:6821–6824. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- 17.Graham TJA, Lambert RF, Ploessl K, Kung HF, Doyle AG. J Am Chem Soc. 2014;136:5291–5294. doi: 10.1021/ja5025645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, et al. J Am Chem Soc. 2014;136:6842–6845. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 19.Lee E, Hooker JM, Ritter T. J Am Chem Soc. 2012;134:17456–17458. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Rubio J, Vicente J. Dalton Trans. 2015;44:19432–19442. doi: 10.1039/c5dt02023a. [DOI] [PubMed] [Google Scholar]
- 21.The mechanism elucidated here indicates that the reaction is not a true C-C reductive elimination, occurring instead by an alternate series of three steps terminating with C-F reductive elimination, and is thus rather a formal C-C reductive elimination. However, for the sake of brevity we have used the phrase “reductive elimination” throughout, with the intention that it be understood as a reference to the overall transformation and not to its microscopic mechanism.
- 22.Chen EYX, Marks TJ. Chem Rev. 2000;100:1391–1434. doi: 10.1021/cr980462j. [DOI] [PubMed] [Google Scholar]
- 23.No thermally induced reductive elimination is observed with 1a or 1b upon heating at 100°C in toluene solution for 24 hours.
- 24.Fluoride abstraction by 2 from 3a and 3b is supported by the observation that addition of superstoichiometric 2 results in the formation of LAuC6F5 as the major Au-containing product. Addition of tetramethylethylene as a scavenger in these reactions results in the formation of the corresponding difluorocyclopropane. See figs. S3, S4, and S8 for details.
- 25.Martínez-Salvador S, Forniés J, Martín A, Menjón B. Angew Chem Int Ed. 2011;50:6571–6574. doi: 10.1002/anie.201101231. [DOI] [PubMed] [Google Scholar]
- 26.Mankad NP, Toste FD. Chem Sci. 2012;3:72–76. doi: 10.1039/C1SC00515D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekhroukh F, Brousses R, Amgoune A, Bourissou D. Angew Chem Int Ed. 2015;54:1266–1269. doi: 10.1002/anie.201409604. [DOI] [PubMed] [Google Scholar]
- 28.Brothers PJ, Roper WR. Chem Rev. 1988;88:1293–1326. [Google Scholar]
- 29.Bour JR, et al. J Am Chem Soc. 2016;138:16105–16111. doi: 10.1021/jacs.6b10350. [DOI] [PubMed] [Google Scholar]
- 30.Leclerc MC, et al. J Am Chem Soc. 2015;137:16064–16073. doi: 10.1021/jacs.5b12003. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, et al. Science. 2011;332:1545–1548. doi: 10.1126/science.1200514. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Salvador S, Falvello LR, Martín A, Menjón B. Chem Eur J. 2013;19:14540–14552. doi: 10.1002/chem.201302142. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett RR, et al. Agents Actions. 1991;32:10–21. doi: 10.1007/BF01983301. [DOI] [PubMed] [Google Scholar]
- 34.De Vry J, et al. J Pharmacol Exp Ther. 2004;310:620–632. doi: 10.1124/jpet.103.062836. [DOI] [PubMed] [Google Scholar]
- 35.Rosillo M, Domínguez G, Pérez-Castells J. Chem Soc Rev. 2007;36:1589–1604. doi: 10.1039/b606665h. [DOI] [PubMed] [Google Scholar]
- 36.Nicholas KM, Pettit R. Tetrahedron Lett. 1971;12:3475–3478. [Google Scholar]
- 37.Harman WD. Chem Rev. 1997;97:1953–1978. doi: 10.1021/cr940316n. [DOI] [PubMed] [Google Scholar]
- 38.Hashmi ASK, Blanco MC, Fischer D, Bats JW. Eur J Org Chem. 2006;2006:1387–1389. [Google Scholar]
- 39.Wolf WJ, Winston MS, Toste FD. Nat Chem. 2014;6:159–164. doi: 10.1038/nchem.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Both isomers of the [Au(III)]-OAc complexes are consumed in these reactions.
- 41.Winston MS, Wolf WJ, Toste FD. J Am Chem Soc. 2015;137:7921–7928. doi: 10.1021/jacs.5b04613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laitar DS, Müller P, Gray TG, Sadighi JP. Organometallics. 2005;24:4503–4505. [Google Scholar]
- 43.Kumar R, Linden A, Nevado C. Angew Chem Int Ed. 2015;54:14287–14290. doi: 10.1002/anie.201505533. [DOI] [PubMed] [Google Scholar]
- 44.This protocol is unoptimized with respect to solvent choice, temperature, or reagent stoichiometries.
- 45.Buck AK, et al. J Nucl Med. 2010;51:401–412. doi: 10.2967/jnumed.108.059584. [DOI] [PubMed] [Google Scholar]
- 46.Wiemers DM, Burton DJ. J Am Chem Soc. 1986;108:832–834. [Google Scholar]
- 47.Tomashenko OA, Grushin VV. Chem Rev. 2011;111:4475–4521. doi: 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]
- 48.Konovalov AI, Lishchynskyi A, Grushin VV. J Am Chem Soc. 2014;136:13410–13425. doi: 10.1021/ja507564p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.