Abstract
The mechanistic basis for Al toxicity effects on root growth is still a matter of speculation, but it almost certainly involves decreased cell division at the root apex. In this series of experiments, we attempt to determine whether Al enters meristematic cells and binds to nuclei when roots are exposed to a low Al3+ activity in solution. The methodology involved the use of the Al-sensitive stain lumogallion (3-[2,4 dihydroxyphenylazo]-2-hydroxy-5-chlorobenzene sulfonic acid), the DNA stain 4′,6-diamino-phenylindole, and confocal laser scanning microscopy. Soybean (Glycine max L. Merr.) cv Young (Al-sensitive) and PI 416937 (Al-tolerant) genotypes were exposed to 1.45 μm Al3+ for periods ranging from 30 min to 72 h, and then washed with 10 mm citrate to remove apoplastic Al. Fluorescence images show that within 30 min Al entered cells of the sensitive genotype and accumulated at nuclei in the meristematic region of the root tip. Substantial Al also was present at the cell periphery. The images indicated that the Al-tolerant genotype accumulated lower amounts of Al in meristematic and differentiating cells of the root tip and their cell walls. Collectively, the results support an important role for exclusion in Al tolerance.
Under acidic conditions Al can be toxic to plants even at submicromolar levels (Kinraide et al., 1985). The most conspicuous Al toxicity response is a reduction in root growth (Taylor, 1988; Foy, 1992). The mechanistic basis for the growth inhibition remains a matter of speculation. Effects likely are located at the root tip itself (Ryan et al., 1993; Sivaguru and Horst, 1998), and several types of regulatory factors are likely to be involved (Kochian, 1995). Convincing arguments have been advanced for toxicity mechanisms operating in the root apoplast (Horst, 1995; Rengel, 1996) and symplasm (Jones and Kochian, 1995; Kochian, 1995; Jones et al., 1998), at the plasma membrane (Barceló et al., 1996), as well as involving complex interactions at the cell wall/plasma membrane/cytoskeleton continuum (Sivaguru et al., 1999).
It has long been thought that an important part of the growth restriction by Al involves disruption of cell division (Clarkson, 1965). With cell cycles at the root meristem of about 18 to 24 h (Gunning and Steer, 1996), it is unlikely that decreased cell division could be responsible for the rapid decreases in root extension occurring within the 1st h of Al exposure (Jones and Kochian, 1995; Llugany et al., 1995; Sivaguru et al., 1999). Nonetheless, a substantial growth inhibition extending over periods of hours or days would have to be associated with decreased cell production (Lazof and Holland, 1999).
The mechanism(s) responsible for decreased cell division with root exposure to Al is uncertain. Relatively direct disruptions associated with Al binding to DNA or other nuclear material seem possible. It appears that Al can accumulate inside cells at the root tip within 30 min to several hours of exposure (Delhaize et al., 1993a; Lazof et al., 1994a; Vasquez et al., 1999), and it has been shown in many experiments by Matsumoto (1991) and others that intracellular Al binds to cell nuclei and DNA (for a summary of results, see Matsumoto, 1991). Nonetheless, the importance of Al binding at the nucleus in the root growth response remains in question, because experiments typically involved exposure to very high Al concentrations (0.1–1.0 mm AlT). In that concentration range, large amounts of Al penetrate into the root meristem and growth quickly ceases. It is unknown if Al uptake into the root and Al accumulation at nuclei also would occur at Al concentrations in the low micromolar range, which is more realistic agronomically (Gillman and Sumner, 1987) and often used in identification of sensitive and tolerant genotypes. Possible supporting evidence for Al binding to nuclei at lower Al concentrations can be found in an experiment with wheat that used the Al-binding stain morin and AlT concentrations of 18 or 55 μm for 48 h (Tice et al., 1992). Intracellular Al appeared to accumulate in nuclei of differentiated cells 1 to 2 mm behind the root tip, close to the undifferentiated meristematic zone.
In this study, we attempted to determine with greater certainty whether Al accumulates in the symplasm and binds to nuclei in undifferentiated cells at the root meristem when roots are exposed to low Al levels. The experiments involve the use of the fluorescent stain lumogallion (3-[2,4 dihydroxyphenylazo]-2-hydroxy-5-chlorobenzene sulfonic acid), which has been used extensively in analytical chemistry (Hydes and Liss, 1976; Gabriëls et al., 1981; Shuman, 1992; Sutheimer and Cabaniss, 1995). Recently, lumogallion was used in a study with soybean (Glycine max) roots (Kataoka et al., 1997); however, roots were exposed to a high Al concentration (100 μm AlT), and intracellular Al localization was not examined. In our experiments, Al-sensitive and Al-tolerant soybean seedlings were exposed to a solution Al3+ activity of only 1.45 μm. The results indicate that substantial Al accumulates in the nuclei within 30 min and that the accumulation is higher in the Al-sensitive genotype.
RESULTS
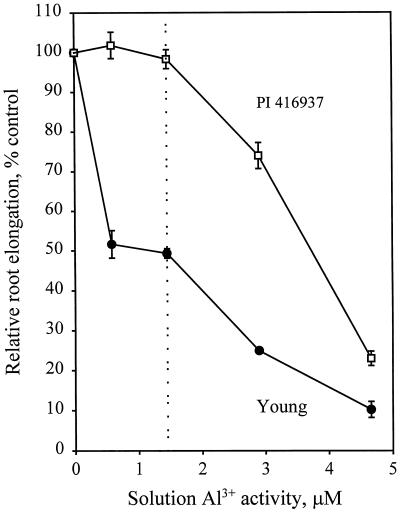
Root Elongation Response to Al
Root elongation responses of the two soybean genotypes, cv Young (Al-sensitive) and PI 416937 (Al-tolerant), across a range of Al3+ activities in solution are shown in Figure 1. As has been shown previously (Carter and Rufty, 1993; Bianchi-Hall et al., 1998), cv Young is more sensitive to Al3+ than PI 416937. The purpose of this experiment was to determine the degree of Al3+ accumulation and binding to nuclei in meristematic cells at a physiologically meaningful Al3+ activity in solution. We define that as being 1.45 μm Al3+, where growth of cv Young was restricted by about 50% and that of PI 416937 by 5%.
Figure 1.
Relative root growth response of soybean genotypes after 72 h of exposure to different activities of Al3+ in solution.
Lumogallion Fluorescence
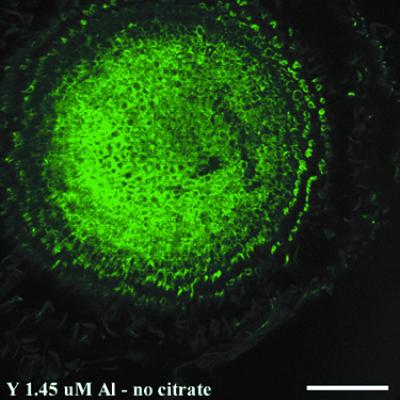
Pseudocolored images from an initial experiment with cv Young show the in situ localization of Al in a cross-section of a soybean root 150 to 250 μm behind the root tip after a 72-h exposure to 1.45 μm Al3+ (Fig. 2). The cross-section was stained with lumogallion. Intense fluorescence was evident across the root section, with the strongest signal being detected toward the root center. Following Al exposure, roots had been washed only with deionized water, so little of the apoplastic Al had been removed and differentiation of apoplastic from symplastic fluorescence was difficult. To improve the definition of cell boundaries, a citrate wash was introduced and used in subsequent experiments. Prior studies have demonstrated that a citrate wash can remove a major portion of the Al adsorbed to cell walls (Zhang and Taylor, 1990; Tice et al., 1992; Archambault et al., 1996; Rengel and Reid, 1997).
Figure 2.
Fluorescence of lumogallion-Al in root cross-sections of cv Young 150 to 250 μm behind the tip without a citrate wash. The root had been exposed to 1.45 μM Al3+ for 72 h and washed with high-purity water for 30 min before embedding and sectioning. Bar = 100 μm.
A number of checks on autofluorescence were conducted in preliminary experiments (data not shown). They included roots not exposed to Al and not stained with lumogallion, those exposed to Al and not stained with lumogallion, and others not exposed to Al but stained with lumogallion. All were consistent with the observed fluorescence being a consequence of lumogallion binding to Al. At high laser intensities, a very low level of Al could be detected in tissues of control plants not exposed to solution Al. We assume that Al originated from impurities in preparatory or analytical reagents (Bloom and Erich, 1996). The low Al background had no impact on the results or interpretations of the study, as high laser intensity was not used.
Radial Penetration of Al in Roots
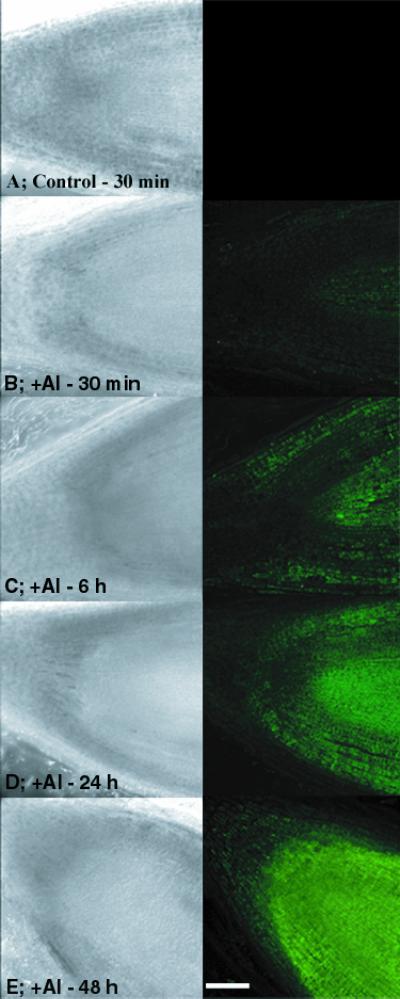
A time course of Al accumulation at the root tip is shown in Figure 3. The images are from longitudinal sections of the Al-sensitive cv Young, following a 10-mm citrate wash. The sections extend about 500 μm back from the root apex, so images are confined to a root region with largely undifferentiated cells. Differential interference contrast (DIC) images are in the left column and corresponding confocal laser images of lumogallion-stained sections are in the right column. The fluorescence level indicates that substantial amounts of Al accumulated in cells at the root meristem after an Al exposure of 30 min, and the amount of Al increased progressively with Al exposure up to 48 h. The highest levels of Al accumulated in outer cell layers and in the root interior. Previous work by Kataoka et al. (1997) demonstrated that Al-lumogallion fluorescence is proportional to Al concentration, as might be expected. Similar experiments were conducted with the more Al-tolerant PI 416937. In contrast to the results with cv Young, very little Al could be detected in cells (see below).
Figure 3.
Longitudinal sections of cv Young roots exposed to 1.45 μm Al3+ for different time intervals and washed with citrate. DIC images are in the left column and lumogallion fluorescence in the right column. Bar = 100 μm.
Al Distribution inside Meristematic Cells
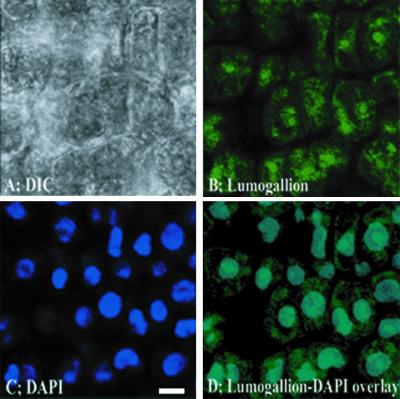
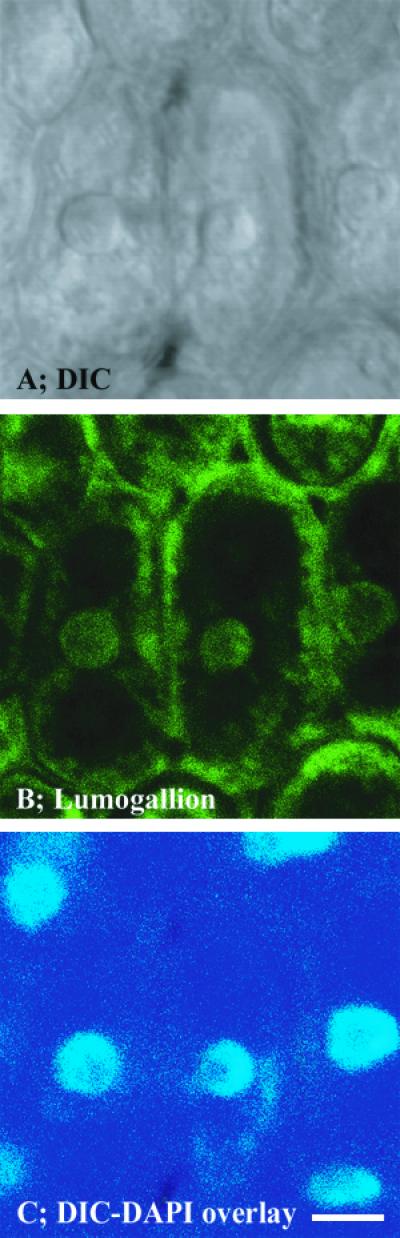
Since detectable levels of Al were clearly present in meristematic cells of the root tip after a short-term Al exposure, we conducted similar experiments with the sensitive genotype cv Young to determine the intracellular distribution of Al in meristematic cells using a higher magnification. Again, roots were exposed to 1.45 μm Al3+ for 30 min, citrate washed, and longitudinal sections were examined. In this case, the images were of cells about 200 μm behind the tip. Concurrently obtained DIC, lumogallion, 4′,6-diamino-phenylindole (DAPI), and lumogallion/DAPI overlay images are shown in Figure 4. The DIC image indicated a focus on thin-walled, individual meristematic cells (Fig. 4A). The lumogallion stain revealed substantial Al inside cells, with Al concentrated in spherical fluorescence zones in the cell interior that were noticeably brighter than the cell cytosol as a whole (Fig. 4B). Images of the nuclear stain DAPI appeared to correspond closely with the fluorescent spheres (Fig. 4C), and overlays of the two fluorescent images indicated identical localization of nuclei and intracellular concentrations of Al (Fig. 4D).
Figure 4.
High-magnification DIC, lumogallion, and DAPI images of meristematic cells from longitudinal sections of roots exposed to 1.45 μm Al3+ for 30 min. Bar = 10 μm.
To further substantiate the Al and nuclei association, experiments were conducted at a somewhat higher Al3+ activity. Seedlings of cv Young were exposed to 2.9 μm Al3+ for 72 h, which corresponds with about 75% inhibition of root extension (compare with Fig. 1). In this case, cross-sections about 500 μm back from the tip were prepared. The images with lumogallion (Fig. 5B) and with DAPI (Fig. 5C) again indicated high Al concentrations at cell nuclei. The image also shows a clear fluorescence signal at the cell periphery, i.e. cell wall/plasma membrane interface.
Figure 5.
Cross-sections from roots of cv Young exposed to 2.9 μm Al3+ for 72 h. Lumogallion-Al and DAPI fluorescence in cells 500 μm back from the root tip. Bar = 10 μm.
Al Accumulation in Cells of Sensitive and Tolerant Genotypes
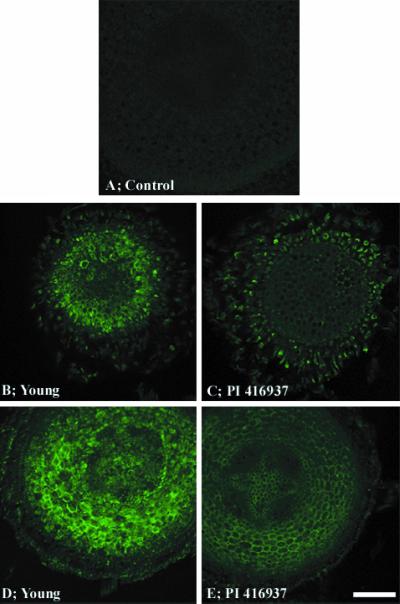
In additional experiments, the extent of Al accumulation in meristematic and more mature tissues in root tips was evaluated with cv Young and the Al-tolerant PI 416937 (Fig. 6). Cross-sections were taken from roots exposed to 1.45 μm Al3+ for 72 h, conditions that correspond to the growth measurements shown in Figure 1, and then washed with 10 mm citrate. The cross-sections were taken from two regions. One was 150 to 250 μm back from the root tip, and the other was from a zone of more differentiated cells 800 to 900 μm from the tip. The lumogallion fluorescence images show differential accumulation of Al between the genotypes (Fig. 6). In both developmental regions, higher Al levels were evident in the sensitive genotype cv Young. Substantial fluorescence was evident at the cell periphery and the cell interior.
Figure 6.
Cross-sections of cv Young and PI 416937 roots exposed to 1.45 μm Al3+ for 72 h. Lumogallion-Al fluorescence in cells 150 to 250 μm (B and C) and 800 to 900 μm (D and E) back from the root tip. Bar = 10 μm.
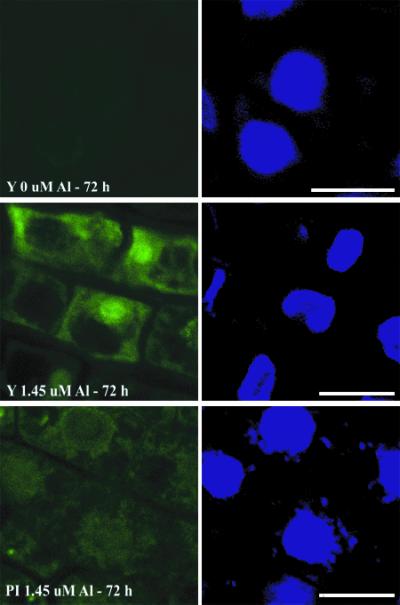
To examine the intracellular distribution of Al in the two genotypes, longitudinal sections of the root tips were prepared from separate experiments, again using 1.45 μm Al3+. Examination of tissues at high magnification shows that less Al was present in the cell wall and cytoplasm of the PI 416937 root tip cells compared with cv Young (Fig. 7). Images with DAPI stain show that the lumogallion-Al fluorescence signal is weaker at cell nuclei of PI 416937 than with cv Young.
Figure 7.
Lumogallion and DAPI images from longitudinal sections of cv Young and PI 416937 roots exposed to 1.45 μm Al3+ for 48 h. Bars = 10 μm.
Al Content in Citrate-Washed Root Tips
Quantitative analysis of Al in 5-mm segments at the root tip is presented in Table I. The chemical analyses confirm the fluorescence images, indicating that the sensitive cv Young accumulated more Al compared with the tolerant PI 416937 at an Al3+ activity of 1.45 μm in solution. The citrate wash removed 31% to 32% of the Al present in the tissue in both genotypes, which is in the same range as that observed in experiments with other species (Zhang and Taylor, 1990; Archambault et al., 1996; Samuels et al., 1997). Therefore, root tips of the Al-sensitive cv Young not only accumulated more Al, but also retained more Al in a fraction that is not desorbed and/or not accessible to citrate.
Table I.
Al accumulation in root apices of soybean cv Young (Al-sensitive) and PI 416937 (Al-tolerant) exposed to 1.45 μm Al3+ for 48 h
| Sample | Washed Root Tip | Citrate Wash | Total Al |
|---|---|---|---|
| nmol g−1 fresh wt | |||
| cv Young − 0 μm Al3+ | 56 | 4 | 60 |
| cv Young + 1.45 μm Al3+ | 408 | 191 | 599 |
| PI 416937 + 1.45 μm Al3+ | 321 | 146 | 467 |
| lsd0.05 | 35 | 21 | 41 |
Following exposure to Al, root tips (approximately 5 mm) were excised and washed in ice-cold 10 mm citrate for 30 min. Following the wash, the tips were digested in high-purity HNO3 and the Al content determined by inductively coupled plasma atomic emission spectrometry. The citrate wash was analyzed by the same method without any additional preparation. Total Al concentration is based on the sum of the Al content in the root tips and corresponding citrate wash fraction. Means are from three independent replicates.
DISCUSSION
The main purpose of these experiments was to determine whether Al accumulated at cell nuclei after exposure to a relatively low Al3+ activity in solution. Results with the fluorescent stain lumogallion showed that it does. The results are consistent with others in the literature from experiments with higher Al concentrations in solution. Collectively, they indicate that Al is able to penetrate the cell symplasm relatively fast (Lazof et al., 1994a; Vitorello and Haug, 1996) and bind to nuclear molecules (Matsumoto, 1991), presumably leading to observed decreases in mitotic activity (Clarkson, 1965; Matsumoto, 1991). That substantial Al accumulation at nuclei can occur with soybean at an Al3+ activity of only 1.45 μm, and to a greater extent in an Al-sensitive than in an Al-tolerant genotype, supports the notion that direct Al interference with nuclear activities is one of the primary controlling events in the restriction of root growth.
The novel aspect of our experiments was the use of the lumogallion stain, which provides advantages in sensitivity and specificity. At the present time, lumogallion and morin seem to be the best fluorescent binding agents for Al; both appear effective in the nanomolar range of Al concentrations (Katyal and Prakash, 1977; Shuman, 1992). In the one instance where lumogallion and morin were used in the same experiment with soybean tissues, lumogallion appeared to have a slight edge in sensitivity (Kataoka et al., 1997). Also, lumogallion binding to Al has been shown to be very specific. Potential interference with Al-lumogallion fluorescence by more than 20 ions was evaluated in different studies (Hydes and Liss, 1976; Gabriëls et al., 1981), and the results suggest that interference is unlikely at ionic concentrations normally found in plant tissue. Furthermore, lumogallion has been shown to detect Al in the presence of organic ligands (Shuman, 1992; Sutheimer and Cabaniss, 1995). Thus, it is reasonable to expect that lumogallion is able to detect low levels of Al bound to cell walls. In our experiment, where a pronounced fluorescence signal was evident at the cell periphery even after a citrate wash, that assumption would appear valid. The possibility exists that morin may not bind to Al in the cell wall as effectively (Tice et al., 1992; Vitorello and Haug, 1996).
An important part of the methodology of the “visualization” of Al at the nucleus was the use of a confocal laser scanning microscope. The presence of significant amounts of Al in the apoplast raises the possibility of Al movement into the symplasm during tissue dissection (Rengel, 1996). With confocal microscopy, penetration of the laser beam through three to four cell layers allowed collection of cellular images in the interior of thick sections separated from the cut surface. Thus, artifacts arising from Al redistribution during tissue preparation are unlikely. This is especially true since the inclusion of a concentrated citrate wash in the tissue preparation procedure would have removed easily exchangeable Al from the root apoplast and reduced the amount of Al prone to redistribution. Furthermore, the combination of images from DIC and from DAPI and Al fluorescence at the cell wall and nucleus allowed much better cellular definition and Al localization compared with other methodologies used for Al localization previously, e.g. energy-dispersive X-ray microanalysis (Delhaize et al., 1993a) and secondary ion mass spectrometry (Lazof et al., 1994a). Confocal laser scanning microscopy coupled with double staining of Al with morin and polynucleotides with propidium iodide was successfully employed to assess the magnitude of Al accumulation and its distribution in yeast cells (Ezaki et al., 1999).
One cannot know, of course, what substrate Al binds to at the nucleus. But the presence of Al in high amounts at the nuclear membrane could interfere with numerous activities such as microtubule binding at the membrane surface during the G2 phase of the cell cycle (Franklin and Cande, 1999) and protein recognition, binding, and transport into the nucleus (Smith and Raikhel, 1999). With thick tissue sections such as those used here, it is not possible to determine how much Al accumulated inside the nucleus. If substantial Al is inside, then binding to DNA and to phosphorylated proteins (Haug and Vitorello, 1997; Martin, 1997) and disturbance of their functions would occur. Previous studies indicated that Al can adversely affect DNA composition (Sampson et al., 1965), chromatin structure, and template activity (Matsumoto, 1991).
The results with lumogallion allow insight into the mechanism of Al tolerance for the two soybean genotypes examined. Proposed mechanisms for tolerance have been broadly classified as those that prevent Al uptake by roots and those that detoxify Al once it is inside the cell (Taylor, 1991; Kochian, 1995; Rengel, 1997). Lower Al accumulation in cells at the root tip of the PI 416937 compared with cv Young (Fig. 5; Table I) indicates an important role for exclusion. Previous measurements of total Al contained at the tip (after a citrate wash) had suggested such a result with soybean (Lazof et al., 1994b). Root tip Al concentrations were found to be lower in Al-tolerant genotypes with other plant species as well (Rincón and Gonzalez, 1992; Tice et al., 1992; Delhaize et al., 1993a; Ryan et al., 1997; Samuels et al., 1997; Larsen et al., 1998). A myriad of processes could contribute to Al exclusion from the meristematic cell region, including increased secretion of mucilage (Horst et al., 1982; Crawford and Wilkens, 1997), polypeptides (Basu et al., 1999), inorganic phosphate (Pellet et al., 1996), and organic acids (Delhaize et al., 1993b; Basu et al., 1994; Delhaize and Ryan, 1995; Pellet et al., 1995; Larsen et al., 1998). The involvement of rhizosphere alkalinization (Degenhardt et al., 1998), efflux of Al from the symplasm (Ezaki et al., 1999), and decreased cell-surface negativity (Wagatsuma and Akiba, 1989) are also possible. There is evidence indicating that Al tolerance in PI 416937 involves three to five genes (Bianchi-Hall et al., 1998), which is consistent with concomitant operation of multiple Al-tolerance mechanisms, as evidently occurs in wheat (Pellet et al., 1996).
Most aspects of the cause and effect relationship between Al accumulation at the nucleus and the inhibition of root growth remain obscure. The time course of Al exposure for cv Young shows increasing Al concentrations at nuclei as the Al exposure period progressed (Fig. 2). It is unknown, however, exactlywhen meristematic activity began to decline. The growth measurements in Figure 1 are at the end of 72 h of Al exposure. It is logical to think that once a “critical” Al concentration is reached at the nucleus, cell division would slow in proportion to the accumulation of Al and the inhibitory effects associated with it. In other experiments, we found that root extension of cv Young is reduced by about 25% during the first 24 h of exposure to 1.45 μm Al3+, and little elongation occurs afterward (data not shown). This would imply a fairly rapid effect on cell division, considering the time required for cell cycles. The pattern of Al accumulation with the more tolerant PI 416937 indicates that exclusion mechanisms were already in place or began soon after initial exposure to Al, minimizing Al entry into the intercellular and symplastic areas. Still, some accumulation of Al at nuclei was detectable after 72 h (Fig. 6). Since growth was decreased only by about 5% at that time, it would appear that low amounts of Al can be tolerated at the nucleus with minimal disruptions in function. It is conceivable that internal detoxification processes, perhaps the formation of non-toxic Al complexes (Ma et al., 1997; Watanabe et al., 1998) at the nuclear membrane surface, were helping to depress inhibitory effects.
MATERIALS AND METHODS
Plant Growth and Al Treatments
Seeds of soybean (Glycine max L. Merr.) cv Young (Al-sensitive) and PI 416937 (Al-tolerant) were rolled in germination paper, placed into a dark chamber at 25°C and 98% RH, and kept moist by capillary action from a 0.1 mm CaSO4 solution. After 72 h, seedlings were selected for uniformity and transferred into 12-L hydroponic chambers containing aerated 0.8 mm CaSO4 for a 16- to 18-h acclimation period. Light (550 μmol m−2 s−1) was provided during an 8-h photoperiod by a combination of high-pressure sodium and metal halide lamps. Solution pH was maintained at 4.3 ± 0.2 by continuous monitoring and adjustment with 0.05 n H2SO4.
Following the acclimation period, Al was added to one-half of the chambers to establish targeted Al3+ activities as predicted with GEOCHEM-PC (Parker et al., 1995). The Al was added from a fresh 100 mm AlCl3 stock solution in dilute HCl. Seedlings were exposed to the solutions for times varying from 30 min to 72 h. For the root growth experiments, taproot length was measured before adding Al and 72 h later.
Specimen Preparation
At the end of Al exposure periods, seedlings were removed from the chambers and root tips (approximately 5 mm) were excised, transferred to tissue processing wells (Electron Microscopy Sciences, Fort Washington, PA), and washed with either ice-cold high-purity water or 10 mm citrate for 30 min (modified from Zhang and Taylor [1990]). The root tips were then embedded in 6% (w/v) type I-A low EEO agarose and sectioned in 100-μm slices using a vi-bratome (Technical Products International, St. Louis). Although thinner sections are ideal for fluorescence confocal microscopy imaging (Gilroy, 1997), the thick sections allowed imaging of cells away from the cut surface, reducing the chances of artifacts (see “Discussion”). Following a 15-min wash in acetate buffer (pH 5.2) at 25°C, the root tip sections were stained with the Al-indicator lumogallion in darkness for 60 min at 50°C in an incubator-shaker at 75 rpm. After staining for Al, root sections were rinsed twice for 15 min in acetate buffer and mounted on glass slides containing DAPI. DAPI is a nuclear stain that preferentially binds to dsDNA. Lumogallion and DAPI were purchased from Molecular Probes (Eugene, OR). The lumogallion solution (10 μm) was prepared in pH 5.2 acetate buffer from a 10-mm stock, and DAPI (1 μg mL−1) was prepared in 90% glycerol. Both solutions were stored in dark containers under refrigeration.
The lumogallion method was modified from Kataoka et al. (1997). The main differences in our methodology were omission of the fixation step to reduce background fluorescence (data not shown) and elimination of a pH 7.0-MOPS rinse after the fixation.
Microscopy
Root tip sections were examined within 4 to 6 h after preparation. Images were collected with a TCS-SP confocal system with an inverted microscope DMIRBE (Leica Microsystems. Wetzlar, Germany) and either a 20×/0.60 numerical aperture or a 40×/1.25 numerical aperture oil objective, therefore affording a theoretical lateral resolution of at least 800 nm. Lasers used were the Coherent UV with excitation lines of 351 and 361 nm to visualize DAPI-stained nuclei and a Uniphase argon laser line at 100% in the Acousto-Optical tuneable filter for the visualization of the Al-lumogallion complex. The argon laser was left in the “parked” setting (lowest possible output), and the photomultiplier used to collect the fluorescence was set between 590 and 620 nm. Emitted fluorescence was collected at wavelengths from 500 to 550 nm. DIC images were collected concurrent with the fluorescence images using a transmitted light detector and argon laser illumination.
Sequential scanning was used to reduce noise. Each image had 512 × 512 pixels, and each plane in the specimen was scanned four times. Stacks of images were collected in the Z plane of the specimen. These stacks are comprised of 32 optical sections that are about 0.4 nm apart. Resultant stacks were projected to form a single image, which was exported to Adobe Photoshop 5.0. To improve clarity and reproduction quality, image colors were proportionally enhanced.
Spectrometric Determination of Al in Root Tips
Root tips (approximately 5 mm) were collected from seedlings of cv Young and the PI 416937 that had been exposed to 0 or 1.45 μm Al3+ for 48 h. The excised tips were rinsed with 10 mm citrate, as described for microscopy studies, transferred to teflon tubes, weighed, and dried at 65°C. The dry tips were digested overnight in 1 mL of Optima grade HNO3 (Al <10 ng kg−1; Fisher Scientific, Loughborough, Leicestershire, UK) and microwaved at high power for 30 min under a stream of nitrogen. Sample volume was brought to 5 mL and Al content was determined by inductively coupled plasma atomic emission spectrometry. The citrate solution used for rinsing root tips was saved and analyzed for Al without any additional preparation. All labware was washed with 20% (v/v) trace metal-grade HNO3 prior to use.
ACKNOWLEDGMENT
We wish to thank Dr. Wayne Robarge (Department of Soil Science, North Carolina State University, Raleigh) for helping with sample preparation and spectrometric root tip Al analysis
Footnotes
This work was partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Ministry of Education-Brazil through a scholarship (grant no. 2575/95–7 to I.R.S.) and by the United Soybean Board (grant no. 7208).
LITERATURE CITED
- Archambault DJ, Zhang G, Taylor GJ. A comparison of the kinetics of aluminum (Al) uptake and distribution in roots of wheat (Triticum aestivum) using different aluminum sources: a revision of the operational definition of symplastic Al. Physiol Plant. 1996;98:578–586. [Google Scholar]
- Barceló J, Poshenrieder CH, Vásquez MD, Gunsé B. Aluminum phytotoxicity: a challenge for plant scientists. Fert Res. 1996;43:217–223. [Google Scholar]
- Basu U, Godbold D, Taylor GJ. Aluminum resistance in Triticum aestivum associated with enhanced exudation of malate. J Plant Physiol. 1994;144:747–753. [Google Scholar]
- Basu U, Good AG, Aung T, Slaski JJ, Basu A, Briggs KG, Taylor GJ. A 23-kDa, root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant. 1999;106:53–61. [Google Scholar]
- Bianchi-Hall CM, Carter TE, Rufty TW, Arellano C, Boerma HR, Ashley DA, Burton JW. Heritability and resource allocation of aluminum tolerance derived from soybean PI 416937. Crop Sci. 1998;38:513–522. [Google Scholar]
- Bloom PR, Erich MS. The quantitation of aqueous aluminum. In: Sposito G, editor. The Environmental Chemistry of Aluminum. Ed 2. Boca Raton, FL: CRC Press; 1996. pp. 1–38. [Google Scholar]
- Carter TE, Rufty TW. Soybean plant introductions exhibiting drought and aluminum tolerance. In: Kuo CG, editor. Adaptation of Food Crops to Temperature and Water Stress, Publication No. 93–410. Asian Vegetable Research and Development Center, Taipei, Taiwan. 1993. pp. 335–346. [Google Scholar]
- Clarkson DT. The effect of aluminum and some other trivalent metal cations on cell division in the root apices of Allium cepa. Ann Bot. 1965;29:310–315. [Google Scholar]
- Crawford SA, Wilkens S. Ultrastructural changes in root cap cells of two Australian native grass species following exposure to aluminum. Aust J Plant Physiol. 1997;24:165–174. [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.): I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993a;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993b;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Sivaguru M, Ezaki Y, Matsumoto H, Gardner RC. Acquisition of Al tolerance in Sacharomyces cereviseae by expression of the BCB or NtGDI1 gene derived from plants. FEMS Microbiol Lett. 1999;171:81–87. doi: 10.1111/j.1574-6968.1999.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Foy CD. Soil chemical factors limiting plant root growth. Adv Soil Sci. 1992;19:97–149. [Google Scholar]
- Franklin AE, Cande WZ. Nuclear organization and chromosome segregation. Plant Cell. 1999;11:523–534. doi: 10.1105/tpc.11.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriëls R, Van Keirsbulch W, Engels H. Spectrofluorimetric determination of aluminum in plants, soils, and irrigation waters. Lab Pract. 1981;30:122–123. [Google Scholar]
- Gillman GP, Sumner ME. Surface charge characterization and soil solution composition of soils from the Southern Piedmont in Georgia. Soil Sci Soc Am J. 1987;51:589–594. [Google Scholar]
- Gilroy S. Fluorescence microscopy of living plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:165–190. doi: 10.1146/annurev.arplant.48.1.165. [DOI] [PubMed] [Google Scholar]
- Gunning BES, Steer MW. Plant Cell Biology: Structure and Function. Sudbury, MA: Jones and Bartlett Publishers; 1996. [Google Scholar]
- Haug AR, Vitorello V. Cellular aspects of aluminum toxicity in plants. In: Yasui M, Strong MJ, Ota K, Verity MA, editors. Mineral and Metal Neurotoxicology. Boca Raton, FL: CRC Press; 1997. pp. 35–41. [Google Scholar]
- Horst WJ. The role of apoplast in aluminum toxicity and resistance of higher plants. J Pflanzenernähr Bodenkd. 1995;158:419–428. [Google Scholar]
- Horst WJ, Wagner A, Marschner H. Mucilage protects root meristems from aluminum injury. Z Planzenphysiol. 1982;105:435–444. [Google Scholar]
- Hydes DJ, Liss PS. Fluorimetric method for determination of low concentrations of dissolved aluminum in natural waters. Analyst. 1976;101:922–931. [Google Scholar]
- Jones DL, Kochian LV. Aluminum inhibition of the inositol 1,4,5-triphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Kochian LV, Gilroy S. Aluminum induces a decrease in cytosolic calcium concentration in BY-2 tobacco cell cultures. Plant Physiol. 1998;116:81–89. [Google Scholar]
- Kataoka T, Mori M, Nakanishi TM, Matsumoto S, Uchiumi A. Highly sensitive analytical method for aluminum movement in soybean root through lumogallion staining. J Plant Res. 1997;110:305–309. [Google Scholar]
- Katyal M, Prakash S. Analytical reactions of hydroxy-flavones. Talanta. 1977;24:367–375. doi: 10.1016/0039-9140(77)80022-2. [DOI] [PubMed] [Google Scholar]
- Kinraide TB, Arnold RC, Baligar VC. A rapid assay for aluminum phytotoxicity at submicromolar concentrations. Physiol Plant. 1985;65:245–250. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Larsen PB, Degenhardt J, Tai C, Stenzler LM, Howell SH, Kochian LV. Aluminum-resistant Arabidopsis mutants that exhibit altered pattern of aluminum accumulation and organic acid release from roots. Plant Physiol. 1998;117:9–18. doi: 10.1104/pp.117.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof DB, Goldsmith JB, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips: a micro analytical study using secondary ion mass spectroscopy. Plant Physiol. 1994a;106:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof DB, Holland MJ. Evaluation of the aluminum-induced root growth inhibition in isolation from low pH effects in Glycine max, Pisum sativum and Phaseolus vulgaris. Aust J Plant Physiol. 1999;26:147–157. [Google Scholar]
- Lazof DB, Rincón M, Rufty TW, Mackown CT, Carter TE. Aluminum accumulation and associated effects on 15NO3- influx in roots of two soybean genotypes differing in Al tolerance. Plant Soil. 1994b;164:291–297. [Google Scholar]
- Llugany M, Poschenrieder C, Barceló J. Monitoring of aluminum-induced inhibition of root elongation in four maize cultivars differing in tolerance to Al and proton toxicity. Physiol Plant. 1995;93:265–271. [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in hydrangea: identification of Al forms in the leaves. Plant Physiol. 1997;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC. Chemistry of aluminum in the central nervous system. In: Yasui M, Strong JM, Ota K, Verity MA, editors. Mineral and Metal Neurotoxycology. Boca Raton, FL: CRC Press; 1997. pp. 75–80. [Google Scholar]
- Matsumoto H. Biochemical mechanism of the toxicity of aluminum and the sequestration of aluminum in plant cells. In: Wright RJ, Baligar VC, Murrmann RP, editors. Plant-Soil Interactions at Low pH. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 825–838. [Google Scholar]
- Parker DR, Norwell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In: Loeppert RH, editor. Soil Chemical Equilibrium and Reaction Models, Soil Science Society of America Special Publication, No. 42. Madison, WI: American Society of Agronomy; 1995. pp. 253–270. [Google Scholar]
- Pellet DM, Grunes LD, Kochian LV. Organic acids exudation as an aluminum tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. Uptake of aluminum by plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Rengel Z. Mechanism of plant resistance of aluminum and heavy metals. In: Basra AS, Basra RK, editors. Mechanisms of Environmental Stress Resistance in Plants. Amsterdam: Harwood Academic Publishers; 1997. pp. 241–276. [Google Scholar]
- Rengel Z, Reid RJ. Uptake of Al across the plasma membrane of plant cells. Plant Soil. 1997;192:31–35. [Google Scholar]
- Rincón M, Gonzalez RA. Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol. 1992;99:1021–1028. doi: 10.1104/pp.99.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Ryan PR, Reid RJ, Smith FA. Direct evaluation of the Ca2+-displacement hypothesis for Al toxicity. Plant Physiol. 1997;113:1351–1357. doi: 10.1104/pp.113.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson M, Clarkson DT, Davies DD. DNA synthesis in aluminum-treated roots of barley. Science. 1965;148:1476–1477. doi: 10.1126/science.148.3676.1476. [DOI] [PubMed] [Google Scholar]
- Samuels TD, Küçükakyüz K, Rincon-Zachary M. Al partitioning patterns and root growth as related to Al sensitivity and Al tolerance in wheat. Plant Physiol. 1997;113:527–534. doi: 10.1104/pp.113.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman MS. Dissociation pathways and species distribution of aluminum bound to an aquatic fulvic acid. Environ Sci Technol. 1992;26:593–598. [Google Scholar]
- Sivaguru M, Baluška F, Volkman D, Felle H, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex: short-term effects on the distal part of the transition zone. Plant Physiol. 1999;119:1073–1082. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–163. [Google Scholar]
- Smith HMS, Raikhel NV. Protein targeting to the nuclear pore: what can we learn from plants? Plant Physiol. 1999;119:1157–1163. doi: 10.1104/pp.119.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutheimer SH, Cabaniss SE. Aqueous Al(III) speciation by high performance cation exchange chromatography with fluorescent detection of the aluminum-lumogallion complex. Anal Chem. 1995;67:2342–2349. [Google Scholar]
- Taylor GJ. The physiology of aluminum phytotoxicity. In: Sigel H, editor. Metal Ions in Biological Systems: Aluminum and Its Role in Biology. Vol. 24. New York: Marcel Dekker; 1988. pp. 123–163. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. In: Randall DD, Blevins DG, Miles CD, editors. Current Topics in Plant Biochemistry and Physiology. Vol. 10. Columbia: University of Missouri; 1991. pp. 57–93. [Google Scholar]
- Tice KR, Parker DR, DeMason DA. Operationally defined apoplastic and symplastic aluminum fraction in root tips of aluminum-intoxicated wheat. Plant Physiol. 1992;100:309–318. doi: 10.1104/pp.100.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez MD, Poschenrieder C, Corrales I, Barceló J. Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol. 1999;119:435–444. doi: 10.1104/pp.119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorello VA, Haug A. Short-term aluminum uptake by tobacco cells: growth dependence and evidence for internalization in a discrete peripheral region. Physiol Plant. 1996;97:536–544. [Google Scholar]
- Wagatsuma T, Akiba R. Low surface negativity of root protoplasts from aluminum-tolerant plant species. Soil Sci Plant Nutr. 1989;35:443–452. [Google Scholar]
- Watanabe T, Osaki M, Yoshihara T, Tadano T. Distribution and chemical speciation of aluminum in the Al accumulator plant, Melastoma malabathricum L. Plant Soil. 1998;201:165–173. [Google Scholar]
- Zhang G, Taylor GJ. Kinetics of aluminum uptake in Triticum aestivum L: identity of the linear phase of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars. Plant Physiol. 1990;94:557–584. doi: 10.1104/pp.94.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]