ABSTRACT
Hot flushes are due to estrogen withdrawal and characterized by the episodic activation of heat dissipation effectors. Recent studies (in humans and rats) have implicated neurokinin 3 (NK3) receptor signaling in the genesis of hot flushes. Although transgenic mice are increasingly used for biomedical research, there is limited information on how 17β-estradiol and NK3 receptor signaling alters thermoregulation in the mouse. In this study, a method was developed to measure tail skin temperature (TSKIN) using a small data-logger attached to the surface of the tail, which, when combined with a telemetry probe for core temperature (TCORE), allowed us to monitor thermoregulation in freely-moving mice over long durations. We report that estradiol treatment of ovariectomized mice reduced TCORE during the light phase (but not the dark phase) while having no effect on TSKIN or activity. Estradiol also lowered TCORE in mice exposed to ambient temperatures ranging from 20 to 36°C. Unlike previous studies in the rat, estradiol treatment of ovariectomized mice did not reduce TSKIN during the dark phase. Subcutaneous injections of an NK3 receptor agonist (senktide) in ovariectomized mice caused an acute increase in TSKIN and a reduction in TCORE, consistent with the activation of heat dissipation effectors. These changes were reduced by estradiol, suggesting that estradiol lowers the sensitivity of central thermoregulatory pathways to NK3 receptor activation. Overall, we show that estradiol treatment of ovariectomized mice decreases TCORE during the light phase, reduces the thermoregulatory effects of senktide and modulates thermoregulation differently than previously described in the rat.
KEYWORDS: estrogen, hot flush, kisspeptin, menopause, vasodilation, neurokinin B
Introduction
Hot flushes are secondary to estrogen withdrawal and occur in the majority of menopausal women. They are characterized by an episodic activation of heat dissipation effectors including skin vasodilation, sweating and changes in behavior.1 Activation of the physiological mechanisms to dissipate body heat may be so effective that core temperature drops.2 In postmenopausal women, estrogen withdrawal leads to hypertrophy of KNDy (kisspeptin, neurokinin B and dynorphin) neurons in the hypothalamic infundibular (arcuate) nucleus.3-6 We have proposed that KNDy neurons participate in the mechanism of hot flushes via projections to neurokinin 3 (NK3) receptor-expressing neurons in the median preoptic nucleus.3,7-10 In support of this hypothesis, infusion of neurokinin B causes hot flushes in women11 and genetic variation in the tachykinin receptor 3 gene (the gene for the NK3 receptor) is associated with hot flushes.12 Moreover, treatment with NK3 receptor antagonists reduces the number and severity of hot flushes in women.13-15 Thus, there is now strong clinical evidence that NK3 receptor signaling is a critical factor in the generation of hot flushes.
KNDy neurons are conserved across multiple species, including the mouse,16 rat,17,18 goat,19 ewe,20,21 monkey,4,5,22,23 and human.3,5,24,25 Studies on the effects of E2 and NK3 receptor signaling on thermoregulatory heat dissipation effectors have relied predominantly on a rat model. Vasodilation of the tail skin of the rat is a major heat dissipation effector that has been evaluated by measuring changes in tail skin temperature.26,27 E2 treatment of OVX rats reduces TSKIN during the dark (active) phase, lowers TCORE at high ambient temperatures and shifts the thermoneutral zone.28-30 Our studies show that KNDy neurons may activate heat dissipation effectors in the rat via projections to NK3 receptor-expressing neurons in the median preoptic nucleus.7-10,31
Transgenic mice are critical models for addressing questions on central nervous system pathways and control mechanisms. There is little information, however, on the effect of E2 on the thermal physiology of laboratory mice and virtually no studies on whether, in the mouse, E2 modulates peripheral vasodilation. In part, this may be due to the challenge of measuring TSKIN in freely-moving mice over long periods of time. Thermocouples attached to the tail require restraint to keep the mice from damaging the wires and telemetry sensor leads are too large to surgically implant. Imaging with thermal cameras32 would be challenging over long periods of time, given the range of behaviors in freely moving mice (such as sitting on the tail). Fortunately, the development of a small temperature data logger allowed us to measure TSKIN in the mouse by attaching this device to the ventral surface of the tail. Combined with an implanted radio-telemetry probe to measure TCORE, body temperature regulation could be monitored in freely-moving mice in their home cages. In this study, circadian temperature rhythms of TCORE and TSKIN were measured in intact, OVX and OVX + E2 treated mice. We also determined if E2 altered the response to various environmental temperatures and the thermoregulatory effects of systemic injections of senktide, an NK3 receptor agonist. These studies show the effects of E2 treatment and senktide injections on thermoregulation and provide critical baseline data for future studies using transgenic mice.
Materials and methods
Animals: Female Hsd:ICR (CD-1) mice (2.5-3 months of age, 25–39 g; Envigo, Houston, TX) received ad libitum access to water and a low-phytoestrogen diet (Harlan Teklad 2019 Global 19% Protein Extruded Rodent Diet, Envigo). They were housed in the University of Arizona Animal Care Facility with a 12-h light, 12-h dark cycle (lights on at 0700 h). The TAMBIENT of the animal room ranged from 23–25°C with 50% humidity. The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Arizona and followed NIH guidelines. The mice were weighed weekly.
Monitoring TSKIN and TCORE and activity in the mouse
TSKIN was monitored using a Star-Oddi DST (data storage tag) Nano-T temperature probe (EMKA Technologies, Falls Church, VA). The temperature probes were confirmed to be accurate against a National Institute of Standards and Technology certified thermocouple recorder. After testing various materials, sizes and attachment methods, the final housing was manufactured from 0.5 inch diameter Delrin acetal rods (WB Enterprises, Tucson Arizona). The clear window at the end of the Star-Oddi DST probe allowed the temperature sensor to be oriented adjacent to the ventral surface of the tail with the window oriented towards the mouse. Different groove widths (5.0, 5.5 and 6.0 mm) were made to accommodate various tail sizes so the temperature sensor could be placed a consistent distance from the base of the tail. A 4 cm line was marked from the base of the tail to mark the distal attachment of the housing. Loctite 454 prism instant adhesive gel glue (Fisher Scientific, Pittsburgh, VA) was applied on the lateral grooves of the housing and then the housing was attached to the tail with the mouse under brief isoflurane anesthesia (Fig. 1). Fig. 1E shows a representative TSKIN recording over a 3 day period.
Figure 1.
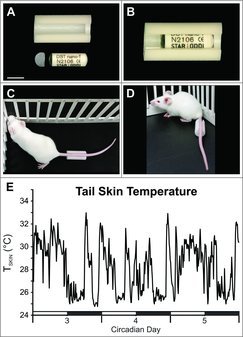
Photographs of the Delrin plastic housing and cap unassembled (A) and assembled (B) with a Star-Oddi DST nano-T probe. A clear window in at the end of the probe allowed the temperature sensor to be oriented toward the ventral surface of the tail. The housing was glued on each side of the tail at a consistent distance from the base (C & D). E, A representative graph from an individual OVX mouse shows wide fluctuations in TSKIN. The dark phase is indicated by black bars. Scale bar in A = 10 mm, applies to A.
Tails were inspected daily to ensure that there was no swelling, erythema or other signs of irritation. If signs of tail irritation were observed, the probe was removed and reattached two to three days later. If the probe fell off it was reattached (under isoflurane anesthesia) and measurements were resumed in 12 hours.
TCORE and activity were measured concurrently with a DSI telemetry probe (TA-F10, Data Sciences International, St. Paul, MN) implanted in the peritoneal cavity under isoflurane anesthesia. Ambient temperature (TAMBIENT) was recorded using a DSI ambient temperature probe.
Experimental design: Experiment 1: To determine the effects of E2 treatment on circadian rhythms of TCORE and TSKIN in OVX mice
Twenty mice were bilaterally ovariectomized while under isoflurane anesthesia and implanted i.p. with a DSI telemetry probe. After a week of recovery, the mice were implanted subcutaneously with a SILASTIC capsule (1.57 mm inner diameter, 3.18 mm outer diameter, effective length 20 mm; Dow Corning Corp., Midland, MI) containing either sesame oil (OVX, n = 10) or 17β-estradiol (180 µg/mL in sesame oil, OVX + E2, n = 10) while under isoflurane anesthesia (Fig. 2A). Pilot studies showed that this treatment produces low physiologic levels of serum E2 (25.3 ± 4.2 pg/mL, n = 7) when measured 7 days after implantation. Fourteen days after implantation, this treatment results in serum E2 (17.8 ± 1.6 pg/ml, n = 10) which is equivalent to levels in intact diestrous mice.33
Figure 2.
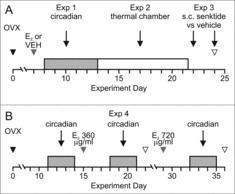
Protocols of Exp. 1–3 (A) and Exp. 4 (B). A, Mice were ovariectomized (OVX) and one week later received s.c. capsules containing 180 µg/mL E2 or vehicle. Circadian rhythms of TCORE, TSKIN and activity were recorded over a 5 day period with mice in their home cages. From days 13–21, the mice were exposed to various TAMBIENT in an environmental chamber. The next day, mice were injected s.c. with either senktide or vehicle. Mice received a second injection two days later in a crossover design. B, Protocol of Exp. 4. Ten mice were OVX and 15 days later implanted with s.c. capsules containing 360 µg/mL E2. The capsules were removed after 7 days and after a wash out period, the mice were implanted with capsules containing 720 µg/mL E2. Circadian rhythms of TCORE, TSKIN and activity were recorded for 3 day intervals in the animals home cages (grey bars). Black arrowheads, OVX; grey arrowheads, capsule implants; open arrowheads, blood sample collection. E2, 17β-estradiol; OVX, ovariectomized; VEH, vehicle.
The DST Nano-T probe was attached to the surface of the tail as described above. The mice were kept in a dedicated room in the animal facility that was relatively free from noise and was only entered during a limited time in the morning by laboratory staff. They were housed individually in plastic shoebox-style cages placed on Physiotel receiver boards (Data Sciences International). The cages allowed free movement and contained nesting material and ad libitum access to water and low phytoestrogen food. Though housed separately, the mice had visual, olfactory and auditory exposure to adjacent animals. TCORE, TSKIN, TAMBIENT and animal activity were recorded every 10 minutes for five consecutive days following capsule implantation.
Experiment 2: To evaluate the effects of E2 treatment on TCORE and TSKIN in OVX mice exposed to a wide range of TAMBIENT
After the circadian recordings were conducted (Exp.1), the mice were brought up to the laboratory to measure TCORE, TSKIN and activity at a wide range of TAMBIENT. They were put into plastic (open to air) grid cages (Nalgene 17.8 × 16.8 × 15.6 cm; Thermo Scientific, Asheville, NC) with ad libitum access to food and water. The grid cages were placed on Physiotel receiver boards in an environmental chamber (Forma Environmental Chamber model 2940; Thermo Scientific, Asheville,NC). Over a period of 9 days, the mice were exposed to two different TAMBIENT each day (order randomly selected) ranging from 20–36°C (in 1°C increments) with 50% humidity. They were left at each TAMBIENT for three hours. TCORE, TSKIN, TAMBIENT and activity were recorded every five minutes (Fig. 2A). To reduce stress, the mice were acclimated to the experimental set-up at a TAMBIENT of 25°C three times prior to temperature recordings.
Experiment 3: To determine the thermoregulatory effects of injecting an NK3 receptor agonist (senktide) in OVX and OVX + E2 mice
We have previously observed that s.c. injections of the NK3 receptor agonist, senktide, results in a marked decrease in TCORE in rats.10 To determine if NK3 receptor signaling also produces hypothermia in mice and if E2 alters this effect, senktide (or vehicle) was injected subcutaneously and TCORE and TSKIN were measured (Fig. 2A). Mice used in Exps. 1 and 2 were placed into plastic grid cages (open to air) with ad libitum access to food and water. The cages were placed on Physiotel receiver boards in a room with a TAMBIENT of 23–24°C. After a 90 min acclimation period, the mice were injected s.c. with either senktide (Tocris Bioscience, s.c., 0.5 mg/kg in saline) or vehicle (between 09:00-11:30). Two days later, each mouse received a second injection of either senktide or vehicle in a crossover design. The injections were performed while the mice briefly were held on a tail snipping platform (Braintree Scientific, Inc.) and then the mice were quickly returned to their cages. TCORE, TAMBIENT and activity were recorded at 1 minute intervals for 90 minutes prior and 120 minutes following injection. In initial experiments, TSKIN was measured every 5 minutes (3 OVX and 2 OVX + E2 mice). When it became evident that the acute rise in TSKIN occurred within 5 minutes, the recordings were increased to 1 minute intervals. The animals were also observed for behaviors such as wet dog shakes, tail rattles, rearing or grooming for 30 minutes after each injection. The mice were sacrificed and trunk blood was collected for measurements of serum estradiol.
Experiment 4: To determine if high concentrations of 17β-estradiol decrease TSKIN in OVX mice during the dark phase
In the first experiment, E2 treatment of OVX mice had no effect on TSKIN during the dark phase. This result was surprising because it is well established that E2 treatment of OVX rats decreases TSKIN during the dark phase.29,34,35 To rule out the possibility that this negative result was due to insufficient serum levels of E2, an additional experiment was performed using higher concentrations of E2 in the SILASTIC capsules. Ten mice were OVX and individually housed in plastic shoebox style cages in the animal facility as described above. Fifteen days later, the mice were implanted s.c. with SILASTIC capsules containing 360 µg/mL E2 in sesame oil. After 7 days, the capsules were removed, and after a wash out period of one week, the mice were implanted with new capsules containing 720 µg/mL E2. TCORE, TSKIN, TAMBIENT and activity were recorded every 10 minutes for three day intervals for each treatment group (Fig 2B). Seven days after implantation of each capsule, blood samples were collected for measurements of serum E2.
Experiment 5: To determine if changes in TCORE and TSKIN are associated with phases of the estrous cycle
This experiment was designed to determine if there were changes in thermoregulation between days of the estrous cycle, when estradiol and other ovarian hormones are fluctuating. Ten mice were implanted (i.p.) with the DSI telemetry probe while under isoflurane anesthesia. The mice were individually housed in plastic shoebox style cages in the animal facility as described above. After a 12-day recovery period, daily vaginal smears (0730 to 0800 h) were performed to monitor estrous cyclicity. TCORE, TSKIN, TAMBIENT and activity were recorded every 5 minutes for 14 days.
Serum assays: Serum samples were sent to the Ligand Assay and Analysis Core Facility at the University of Virginia Center for Research in Reproduction to measure estradiol (Calbiotech Estradiol ELISA). The sensitivity of the assay was 3 pg/mL with an intra-assay coefficient of variation of 6.1% and an inter-assay variation of 8.9%.
Data analysis
Circadian Recordings: Data were calculated for six-hour blocks of time in the light (0900 – 1500 h) and dark (2100 – 0300 h) phases, when entrance into the room where they were housed was restricted. For intact mice, only animals with regular 4 or 5 day estrous cycles (n = 6) were used for analysis. The heat loss index (HLI), an indirect measurement of active tail skin vasomotion,26 was calculated using the formula: HLI = (TSKIN-TAMBIENT)/(TCORE-TAMBIENT). TCORE, TSKIN, HLI and activity were averaged for each mouse and then used to calculate group averages (± SEM). Two way ANOVA (estrogen status vs light/dark phase) was performed for TCORE, TSKIN, HLI, and activity using Tukey's post hoc analysis with α ≤ 0.05.
Ambient Temperature Challenges: To allow for acclimation at each TAMBIENT, data was analyzed using the third hour of recording. Mean TCORE, TSKIN, HLI and activity were calculated for each animal and these values were used to calculate group averages (± SEM). The HLI range was also calculated, which reflects the largest fluctuation of HLI at any given temperature.26 First, TSKIN data was pre-processed to account for any differences in the placement of the tail probe.26 The highest (HLIHIGH) and lowest (HLILOW) HLI was then used to calculate the range for each mouse using the formula: (HLI range = HLIHIGH – HLILOW). HLI range for each mouse was used to calculate group averages (± SEM). Statistical comparisons were completed using two-way ANOVA with repeated measures (E2 status vs TAMBIENT) and Tukey's post hoc analysis with α ≤ 0.05.
Senktide injections: TSKIN data was analyzed only for mice recorded at 1-minute intervals. Baseline values of TCORE and TSKIN were calculated for each animal by averaging values from 60 minutes prior to injection. Values for each animal were subtracted from the baseline of that animal to determine the treatment response from baseline. Mice were excluded from TSKIN analysis if the baseline TSKIN was greater than 30°C (n = 2 OVX and 1 OVX + E2). Data was analyzed using two-way ANOVA with repeated measures (treatment vs time) and Tukey's post hoc analysis (α ≤ 0.05). Behaviors (rearing, grooming and tail rattling) were observed and tallied manually for 30 minutes after injection. A behavior exhibited for 10 seconds or less received 1 count, with a maximum count of 6/minute. The total number of behavioral counts was summed for each mouse over the 30 minute observation period and compared using two-way ANOVA (treatment vs E2 status) with Tukey's post hoc analysis (α < 0.05).
Results
E2 treatment of OVX mice lowers TCORE during the light phase, with no effect on TSKIN
In Exp. 1, circadian rhythms of TCORE were identified in both OVX and OVX + E2 mice. Three to 5 days after capsule implantation, E2 treatment significantly reduced TCORE during the light phase, but not the dark phase (Fig. 3). Similar effects of E2 on TCORE were observed in OVX mice treated with higher concentrations of E2 (Exp. 4, Table 1).
Figure 3.
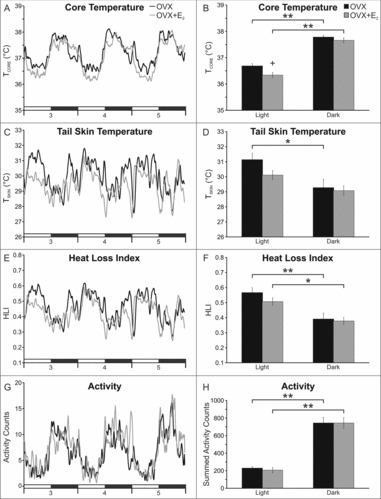
E2 treatment of OVX mice reduces TCORE during the light phase (A, B) with no significant effect on TSKIN (C, D), heat loss index (E, F) or activity (G, H). The line graphs (left) show the mean values for each group 3 to 5 days after E2 treatment. The lines are generated with a moving average of 5 points and the black bars on the X axis denote the dark phase. The bar graphs (right) show data analysis (mean ± SEM) from days 3–5. Light vs dark phase differences were identified (B, D, F, H), except for TSKIN in the OVX + E2 group (D). Unlike previous studies in the rat, E2 did not decrease TSKIN or HLI in the dark phase. n = 9 – 10 mice/group, + Significantly different OVX vs OVX + E2, p = 0.02, * Significantly different light vs dark within treatment group, p < 0.01, ** Significantly different light vs dark within treatment group, p < 0.001.
Table 1.
Treatment of OVX mice with higher concentrations of E2 reduces TCORE during the light phase, with no effect on TSKIN, HLI or activity.
| Phase | OVX | 360 µg/mL E2 | 720 µg/mL E2 | |
|---|---|---|---|---|
| TCORE (°C) | Light | 36.6 ± 0.04* | 36.3 ± 0.07*+ | 36.3 ± 0.05*+ |
| Dark | 37.6 ± 0.07 | 37.7 ± 0.09 | 37.6 ± 0.06 | |
| TSKIN (°C) | Light | 31.4 ± 0.32* | 30.5 ± 0.22 | 31.5 ± 0.38* |
| Dark | 29.8 ± 0.46 | 29.2 ± 0.15 | 30.1 ± 0.48 | |
| HLI | Light | 0.63 ± 0.02* | 0.58 ± 0.01* | 0.65 ± 0.03* |
| Dark | 0.49 ± 0.03 | 0.47 ± 0.01 | 0.50 ± 0.03 | |
| Activity (counts) | Light | 269.8 ± 35.0* | 243.7 ± 41.9* | 166.5 ± 21.9* |
| Dark | 652.0 ± 54.0 | 675.8 ± 92.8 | 705.2 ± 65.0 |
Average (±SEM) core temperature (TCORE), tail skin temperature (TSKIN), heat loss index and summed activity counts of OVX mice receiving different doses of E2.
n = 10 mice/group.
, significantly different, light vs. dark phase, within treatment group, p < 0.01.+, significantly different, compared to OVX, within light phase, p < 0.02.
Circadian rhythms of TSKIN were detected in OVX mice (Fig. 3). There was also a trend for TSKIN to be reduced during the dark phase (compared to light) in OVX + E2 mice (p = 0.08). HLI and activity exhibited a circadian rhythm in both OVX and OVX + E2 treated mice. Notably, E2 had no effect on TSKIN, HLI or activity in either the dark phase or the light phase. This was not due to insufficient amounts of E2, because increasing the concentration of E2 in the capsule by 2- and 4-fold, still had no effect on TSKIN, HLI or activity in the mouse (Exp. 4, Table 1).
E2 treatment of OVX mice lowers TCORE in animals exposed to a wide range of TAMBIENT
E2 reduced the TCORE of OVX mice exposed to TAMBIENT between 20 and 35°C with significant effects at TAMBIENT of 21, 22, 24, 25, 32, 33, 34 and 35°C (Fig. 4A). There was also a non-significant trend (p < 0.07) for TCORE to be reduced by E2 in mice exposed to TAMBIENT of 28, 29, and 30°C. At the high TAMBIENT of 36°C, the TCORE was dramatically elevated, but not significantly different between OVX and OVX + E2 mice (Fig. 4A). There was a tendency for TSKIN and HLI to be lower in OVX + E2 mice, but significant differences were only detected in TSKIN at TAMBIENT of 21, 23, 25 and 26°C and HLI at TAMBIENT of 23, 25 and 26°C (Fig. 4B and C). Activity was comparable between the OVX and OVX + E2 groups at all TAMBIENT (data not shown).
Figure 4.
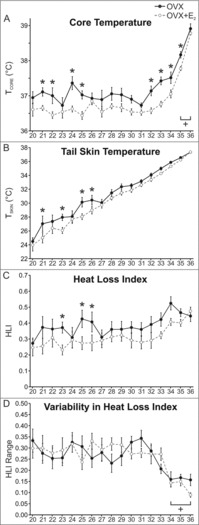
Effects of E2 treatment on TCORE (A), TSKIN (B), HLI (C) and HLI variability (D) in OVX mice exposed to TAMBIENT ranging from 20–36°C. A, TCORE was lower in E2 treated mice at a wide range of TAMBIENT. At 35 and 36°C the TCORE was significantly elevated in both groups. B and C, At select TAMBIENT, both TSKIN and HLI were significantly lower in OVX + E2 mice. D, At the high TAMBIENT of 34–36°C, HLI variability is reduced, reflecting constant vasodilation to dissipate body heat. n = 9 – 10 mice/group. * Significantly different OVX vs OVX+E2, p < 0.05, + Significantly different than the majority of values at the other TAMBIENT, p < 0.05.
In the rat, activation of thermoregulatory effectors has been evaluated by HLI fluctuations.26 In these studies, HLI fluctuations were reduced above and below the thermoneutral zone, reflecting either constant vasodilation or constant vasoconstriction, respectively.26,28 In the present study, there was no difference in the HLI range between OVX and OVX + E2 mice at all TAMBIENT (Fig. 4D). At the high TAMBIENT of 34–36°C, the HLI range was significantly reduced in both groups, indicating constant vasodilation of the tail skin as a mechanism to reduce TCORE. A similar reduction in HLI range (reflecting constant vasoconstriction) was not observed at the lower TAMBIENT.
TCORE, TSKIN and HLI did not vary depending on the phase of the estrous cycle
Circadian rhythms of TCORE, TSKIN, HLI and activity were observed in the intact mice. There was no difference in any of these parameters depending on the phase of the estrous cycle (Fig. 5).
Figure 5.
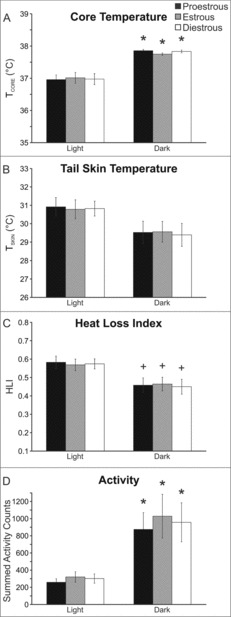
Average TCORE (A), TSKIN (B), heat loss index (C) and activity (D) during the estrous cycle of the mouse. Circadian rhythms were observed but there was no effect of the phase of the estrous cycle. Values represent mean ± SEM, n = 16 cycles averaged from 6 mice. + Significantly different than light phase p < 0.05, * Significantly different than light phase p < 0.01.
Senktide injection in OVX mice caused a transient drop in TCORE and an increase in TSKIN, but these effects were reduced by treatment with E2
In OVX mice, s.c. senktide induced a rapid decline in TCORE accompanied by an acute rise in TSKIN. At 21 minutes post injection, TCORE decreased to a nadir of 1.74± 0.25°C below baseline (Fig. 6A). The rise in TSKIN peaked at 13 minutes post injection to 2.64 ± 0.96°C above the baseline (Fig. 6C). In OVX + E2 mice, senktide also decreased TCORE but this effect was delayed and reduced in magnitude compared to senktide-injected OVX mice (Fig. 6B). Furthermore, the acute rise in TSKIN was not observed in senktide-injected OVX + E2 mice (Fig. 6D). Mice injected with vehicle exhibited a slight but non-significant rise in TCORE and no change in TSKIN.
Figure 6.
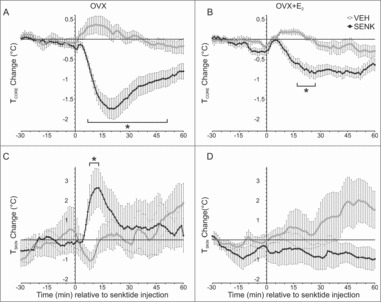
Effects of s.c. injections of the NK3 receptor agonist senktide (or vehicle) on TCORE (top) and TSKIN (bottom) in OVX (left) and OVX + E2 (right) treated mice. Senktide acutely lowered TCORE (A) and increased TSKIN (C) in OVX mice. In OVX + E2 treated mice, the TCORE reduction after senktide was blunted (B) and the acute rise in TSKIN did not occur (D). Values represent mean ± SEM, 0 = time of injection, A and B, n = 9 – 10 mice/group; C, n = 5 mice; D, n = 7 mice, * Significantly different from vehicle, p < 0.01 (TCORE) or 0.05 (TSKIN).
With the exception of one OVX + E2 animal, all mice injected with senktide exhibited tail rattles within thirty minutes post-injection, a finding consistent with a central effect.36 Tail rattling was significantly increased in senktide-injected OVX mice compared to senktide-injected OVX + E2 mice (22 ± 4 vs 11 ± 4 counts respectively, p < 0.05). Tail rattling was not observed in vehicle-injected mice. Grooming and rearing were not different between treatment groups. In addition, the average activity counts recorded by the implanted telemetry probe were not different between groups (data not shown).
Hormone levels and body weights
In mice used in Exp. 1–3, serum E2 was 6.8 ± 0.46 pg/ml (n = 10) 17 days after receiving SILASTIC capsules containing 180 µg/mL E2. As expected,37 these values were decreased compared to blood samples collected at earlier time points (see methods). In OVX mice receiving vehicle, serum E2 was below the level of detection in 5 out of 8 mice, and 3.4 ± 0.2 pg/ml in the remaining 3 mice. In Exp. 4, serum E2 was 36.9 ± 5.0 pg/ml in OVX mice receiving capsules containing 360 µg/mL of E2, and serum E2 was 83.0 ± 4.5 pg/ml in OVX mice implanted with capsules containing 720 µg/mL of E2 (n = 6/group, serum collected 7 days after each implant).
The mice weighed 31.6 ± 1.1 g (OVX) and 32.0 ± 1.0 g (OVX + E2) at the beginning of Exp. 1 (n = 10/group). Body weights were not significantly different between groups 14 days after receiving vehicle or 180 µg/mL E2 capsules (OVX, 33.1 ± 1.0 g vs OVX + E2, 34.5 ± 0.6 g). The mice weighed 36.7 ± 0.9 g at the beginning of experiment 4 (n = 10, Fig 2), 35.3 ± 0.8 g 15 days later, 35.9 ± 0.8 g after one week of 360 µg/mL E2 capsules and 36.4 ± 0.8 g after one week of 720 µg/mL E2 capsules.
Discussion
The present study provides detailed information on the effects of E2 on body temperature and cutaneous vasomotion in the female mouse. We adapted a method previously described in the rat27,29 to record TSKIN in unrestrained, untethered mice. This task became feasible through the availability of the Star Oddi DST nano-T probe, a temperature data logger small enough to be mounted on the ventral surface of the tail. Combined with an intraperitoneal implant of a radio-telemetry probe, circadian rhythms of TCORE, TSKIN and activity were monitored in freely-moving mice over long periods of time with minimal disturbance to the animal.
We observed that E2 treatment of OVX mice significantly decreased TCORE during the light phase of the circadian rhythm recordings, but not the dark phase. The reduction in TCORE was observed in response to the low physiologic doses of E2 used in the first experiment, as well as the higher concentrations used in subsequent experiments. The E2 reduction in TCORE was not secondary to increased cutaneous vasodilation because the TSKIN and HLI was either unchanged (in circadian recordings) or decreased (in environmental chamber recordings) by E2. Of note, E2 also reduced TCORE in OVX mice that were exposed to a wide range of TAMBIENT in an environmental chamber. Because the lower TCORE was defended despite the challenges in TAMBIENT, these data suggest that the regulated balance point for thermal homeostasis was set to a lower TCORE in E2-treated mice.38
It is well established that estrogen regulates energy homeostasis,39 but changes in metabolism do not explain the reduction in TCORE described here. Within the timeframe of our experiment, there was no difference in body weight between OVX and OVX + E2 mice, a finding in agreement with prior studies.40,41 We also observed no difference in activity (measured by telemetry), consistent with previous studies showing no effect of chronic E2 on the locomotor activity42 or sleep43 of mice during the light phase. Although acute E2 increases BAT thermogenesis in several species,44,45 an increase in thermogenesis would be expected to cause an increase, not a decrease in TCORE. Furthermore, a recent study in sheep showed that the thermogenic effect of acute E2 (either as single or repeated injections) is abrogated by chronic treatment.46 Because the reduction of TCORE occurred at TAMBIENT above the threshold for activation of brown adipose tissue (BAT) or shivering thermogenesis, it is also unlikely that turning off these heat-generating thermoeffectors is a mechanism for the lower TCORE. More importantly, Saito et al. showed that chronic E2 treatment of OVX mice does not change energy expenditure during the light phase, which would reflect changes in basal metabolism, adaptive thermogenesis and activity-related metabolism.42 These studies provide evidence that the reduction of TCORE by E2 is secondary to an increase in heat loss, rather than a decrease in heat production.
Similar to the effect of E2 in OVX mice, estrogen replacement reduces TCORE in postmenopausal women.47-49 E2 also reduces TCORE in OVX guinea pigs,50 but in the rat, estrogen treatment has been shown to either increase,51,52 decrease,53 or have no effect on TCORE.29,54,55 These variable effects in the rat could be secondary to the numerous methodological differences, including the type of temperature probes (rectal vs telemetry), the use of restraint, differing TAMBIENT or various hormone replacement regimens. Using virtually identical methods to the current studies, we have previously reported that E2 lowered TCORE in OVX rats, but only when they were subjected to heat stress.8,28
The effects of E2 on cutaneous vasomotion in the mice are also different than previously described in rats. In the OVX rat, E2 treatment dramatically reduces TSKIN in the dark phase, a finding that is well-established in numerous laboratories.29,35,56-58 In contrast, in OVX mice, E2 had no effect on TSKIN during the dark phase. Because these findings could be due to insufficient serum levels of E2, additional experiments were performed in which the concentration of E2 was doubled and then quadrupled. Despite higher serum E2 levels, there was still no reduction in the TSKIN of mice during the dark phase, indicating a species difference between mice and rats. We were surprised that the effects of E2 were so different between rats and mice: E2 reduces TSKIN during the dark phase in OVX rats (but not mice)29, whereas E2 reduces TCORE during the light phase in OVX mice (but only in rats at high TAMBIENT).28 Such studies underscore the importance of considering species differences in designing experiments and interpreting data.
To determine if NK3 receptor signaling modulates TCORE in the OVX mouse (as has been shown in the rat),7,10 senktide was injected in OVX and OVX + E2 mice. In OVX mice, subcutaneous senktide produced an acute rise in TSKIN and acute hypothermia consistent with the activation of heat dissipation effectors. Interestingly, when senktide was injected in OVX + E2 mice, the reduction in core temperature was not as pronounced and the tail skin vasodilation was absent. These data suggest that E2 lowers the sensitivity of thermoregulatory pathways to NK3 receptor activation in mice.
We have previously described a central effect of senktide on body temperature in the rat via NK3 receptor-expressing neurons in the median preoptic nucleus.7,10 The median preoptic nucleus is part of the heat dissipation pathway that receives information from warm-sensitive, cutaneous thermoreceptors.59 In turn, projections from the median preoptic nucleus reduce TCORE via cutaneous vasodilation and activation of other heat dissipation effectors.59 Microinfusion of senktide directly into the median preoptic nucleus of the rat selectively activates fos within the median preoptic nucleus and results in hypothermia.7 These effects are duplicated by subcutaneous injections of senktide.10 If NK3 receptor-expressing neurons in the median preoptic nucleus are ablated, subcutaneous senktide injections do not result in hypothermia or fos activation in the median preoptic nucleus.10 Thus, NK3 receptor-expressing neurons in the median preoptic nucleus are required (and sufficient) for senktide to induce hypothermia in the rat.
While our previous studies strongly implicate the median preoptic nucleus as a site of senktide action, such data do not exclude an effect on NK3 receptor-expressing KNDy neurons that project to the median preoptic nucleus.17,31 KNDy neurons form a bilateral interconnected network within the arcuate nucleus17,31,60 with NKB/NK3R signaling providing a mechanism to synchronize these neurons to influence pulsatile secretion of gonadotropin-releasing hormone.17,61,62 This network may also be important for the generation of hot flushes, because pulses of LH in peripheral plasma (stimulated by pulsatile gonadotropin-releasing hormone) are temporally linked with hot flushes in women.9,63 Importantly, KNDy neurons express estrogen receptor α and ablation of KNDy neurons (but not NK3 receptor-expressing MnPO neurons) interferes with the E2 modulation of thermoregulation in rats.8,10 In mice, E2 treatment decreases NKB (Tac2) and NK3 receptor (Tacr3) gene expression in KNDy neurons,16 reduces KNDy neuron excitability33 and inhibits senktide-induced KNDy neuron firing in hypothalamic tissue slices.64 Thus, the E2 blunting of the thermoregulatory effect of senktide could be mediated by a direct action on KNDy neurons.
The changes in TSKIN and TCORE after senktide injections in OVX mice mimicked the physiological events that accompany hot flushes in women.2,65 Moreover, a recent study has shown that injections of senktide in mice induces hypothermia, a rise in TSKIN and cold seeking behavior.66 Peripheral infusion of neurokinin B in women induces hot flushes11 and two well-controlled clinical trials have shown effective treatment of hot flushes using NK3 receptor antagonists.13,14 Therefore, the thermoregulatory effects of senktide injections in the mice appear to be relevant to the study of human hot flushes. In symptomatic postmenopausal women, a slight elevation in TCORE, stress, spicy foods or a warm environment stimulates the activation of heat dissipation effectors that constitute a flush.2,67-69 Moreover, in postmenopausal women without estrogen replacement therapy, the threshold for activation of sweating is triggered at lower TCORE70 and this effect is reversed by treatment with E2.71 These data suggest that hot flushes may be facilitated by changes in the sensitivity of central thermoregulatory pathways controlling heat dissipation effectors. Based on the blunted response to senktide-injections in E2-treated mice, we speculate that E2 could also reduce the sensitivity of thermoregulatory networks to NK3 receptor activation in women.
In summary, a method was developed which allowed monitoring of TSKIN, TCORE and activity over long time intervals in the freely-moving mouse. Our studies showed significant effects of E2 and NK3 receptor activation on body temperature regulation in the female mouse. These data will provide a foundation for future studies of thermoregulation in transgenic mice to shed light on the mechanism of hot flushes in humans.
Funding Statement
This study was supported by the NIH, National Institute on Aging under grant number R01 AG047887.
Abbreviations
- E2
17β-estradiol
- HLI
heat loss index
- KNDy
arcuate neurons co-expressing kisspeptin, neurokinin B and dynorphin
- MnPO
median preoptic nucleus
- NK3
neurokinin 3
- OVX
ovariectomized
- TAMBIENT
ambient temperature
- TCORE
core temperature
- TSKIN
tail skin temperature
Disclosures
The authors have nothing to disclose.
Acknowledgments
GRANTS: This work was supported by the National Institutes of Health (NIH) National Institute on Aging Grant R01 AG047887. The estradiol assay was performed at the Ligand Assay and Analysis Core, University of Virginia Center for Research in Reproduction supported by the National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934.
Authors acknowledge Kenneth Heeg from WB Enterprises for manufacturing the Delrin tail probe holder. We would also like to thank Ms. Cheryl Johnson and her staff at the University of Arizona Animal Care Facility and Dr. Nathaniel T. McMullen and Filipa Miranda dos Santos for their useful comments on an earlier draft of this manuscript.
References
- 1.Kronenberg F. Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140:1380S-1385S. doi: 10.3945/jn.109.120840 [DOI] [PubMed] [Google Scholar]
- 2.Freedman RR. Physiology of hot flashes. Am J Human Biol. 2001;13:453-464. doi: 10.1002/ajhb.1077 [DOI] [PubMed] [Google Scholar]
- 3.Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239-2247. doi: 10.1210/endo-128-5-2239 [DOI] [PubMed] [Google Scholar]
- 4.Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146-153. doi: 10.1111/j.0953-8194.2004.01143.x [DOI] [PubMed] [Google Scholar]
- 5.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744-2750. doi: 10.1210/jc.2007-0553 [DOI] [PubMed] [Google Scholar]
- 6.Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111-122. doi: 10.1016/j.peptides.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152:4894-4905. doi: 10.1210/en.2011-1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109:19846-19851. doi: 10.1073/pnas.1211517109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211-227. doi: 10.1016/j.yfrne.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology. 2015;156:2552-2562. doi: 10.1210/en.2014-1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C, Abbara A, Ratnasabapathy R, Mogford J, Ng N, et al.. Neurokinin B administration induces hot flushes in women. Scientific Reports. 2015;5:8466. doi: 10.1038/srep08466 PMID:2568306028380486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandall CJ, Manson JE, Hohensee C, Horvath S, Wactawski-Wende J, LeBlanc ES, Vitolins MZ, Nassir R, Sinsheimer JS. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women's Health Initiative Study. Menopause. 2017;24:252-261. doi: 10.1097/GME.0000000000000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, et al.. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:1809-1820. doi: 10.1016/S0140-6736(17)30823-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser GL, Depypere H, Timmerman D, Donders G, Sieprath P, Ramael S, Combalbert J, Hoveyda HR. Clinical Evaluation of the NK3 Receptor Antagonist Fezolinetant (a.k.a. ESN364) for the Treatment of Menopausal Hot Flashes. Endocrine Soc Abstr. 2017;38:OR16-5. [Google Scholar]
- 15.Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 Receptor Antagonism Reveals Roles for Neurokinin B in the Regulation of Gonadotropin Secretion and Hot Flashes in Postmenopausal Women. Neuroendocrinology. 2017;In press: doi: 10.1159/000473893 PMID:28380486 [DOI] [PubMed] [Google Scholar]
- 16.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859-11866. doi: 10.1523/JNEUROSCI.1569-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712-726. doi: 10.1002/cne.21086 [DOI] [PubMed] [Google Scholar]
- 18.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52-64. doi: 10.1111/j.1365-2826.2010.02076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, et al.. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124-3132. doi: 10.1523/JNEUROSCI.5848-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, et al.. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752-5760. doi: 10.1210/en.2007-0961 [DOI] [PubMed] [Google Scholar]
- 21.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90-102. doi: 10.1016/j.brainres.2010.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111-2118. doi: 10.1210/jc.84.6.2111 [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494-4503. doi: 10.1210/en.2010-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20:1376-1381. doi: 10.1111/j.1365-2826.2008.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrabovszky E, Borsay BA, Racz K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Substance p immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PloS one. 2013;8:e72369. doi: 10.1371/journal.pone.0072369 PMID:23977290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667-2679. doi: 10.1152/japplphysiol.01173.2001 [DOI] [PubMed] [Google Scholar]
- 27.Gordon CJ, Puckett E, Padnos B. Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J Pharmacol Toxicol Methods. 2002;47:107-114. doi: 10.1016/S1056-8719(02)00219-8 [DOI] [PubMed] [Google Scholar]
- 28.Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151:1187-1193. doi: 10.1210/en.2009-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151:5389-5394. doi: 10.1210/en.2010-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dacks PA, Krajewski SJ, Rance NE. Ambient temperature and 17β-estradiol modify fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology. 2011;152:2750-2759. doi: 10.1210/en.2010-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680-697. doi: 10.1016/j.neuroscience.2009.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer CW, Ootsuka Y, Romanovsky AA. Body Temperature Measurements for Metabolic Phenotyping in Mice. Front Physiol. 2017;8:520. doi: 10.3389/fphys.2017.00520 PMID:28824441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cholanian M, Krajewsk-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of Arcuate Neurokinin B Neurons in Female Tac2-EGFP Transgenic Mice. Endocrinology. 2014;155:2555-2565. doi: 10.1210/en.2014-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berendsen HHG, Kloosterboer HJ. Oestradiol and mirtazapine restore the disturbed tail-temperature of oestrogen-deficient rats. Eur J Pharmacol. 2003;482:329-333. doi: 10.1016/j.ejphar.2003.09.061 [DOI] [PubMed] [Google Scholar]
- 35.Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O'Byrne K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol. 2006;191:399-405. doi: 10.1677/joe.1.06919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoessl AJ, Dourish CT, Iversen SD. The NK-3 tachykinin receptor agonist senktide elicits 5-HT-mediated behaviour following central or peripheral administration in mice and rats. Br J Pharmacol. 1988;94:285-287. doi: 10.1111/j.1476-5381.1988.tb11527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ström JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest. 2008;68:814-822. doi: 10.1080/00365510802409703 [DOI] [PubMed] [Google Scholar]
- 38.Kanosue K, Crawshaw LI, Nagashima K, Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur J Appl Physiol. 2010;109:5-11. doi: 10.1007/s00421-009-1256-6 [DOI] [PubMed] [Google Scholar]
- 39.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309-338. doi: 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L, Zou F, Yang Y, Xu P, Saito K, Othrell Hinton A Jr., Yan X, Ding H, Wu Q, Fukuda M, et al.. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology. 2015;156:2114-2123. doi: 10.1210/en.2014-1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu P, Zhu L, Saito K, Yang Y, Wang C, He Y, Yan X, Hyseni I, Tong Q, Xu Y. Melanocortin 4 receptor is not required for estrogenic regulations on energy homeostasis and reproduction. Metabolism. 2017;70:152-159. doi: 10.1016/j.metabol.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, Hinton AO, Yan X, Zhao J, Fukuda M, et al.. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Scientific reports. 2016;6:23459. doi: 10.1038/srep23459 PMID:26988598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463:239-243. doi: 10.1016/j.neulet.2009.07.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, et al.. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20:41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López M, Tena-Sempere M. Estradiol and brown fat. Best Pract Res Clin Endocrinol Metab. 2016;30:527-536. doi: 10.1016/j.beem.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 46.Clarke SD, Clarke IJ, Rao A, Evans RG, Henry BA. Differential effects of acute and chronic estrogen treatment on thermogenic and metabolic pathways in ovariectomized sheep. Endocrinology. 2013;154:184-192. doi: 10.1210/en.2012-1758 [DOI] [PubMed] [Google Scholar]
- 47.Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73:1238-1245. [DOI] [PubMed] [Google Scholar]
- 48.Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O'Gorman JT, Derr JA, Kenney WL. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol. 1997;83:477-484. [DOI] [PubMed] [Google Scholar]
- 49.Brooks-Asplund EM, Cannon JG, Kenney WL. Influence of hormone replacement therapy and aspirin on temperature regulation in postmenopausal women. Am J Physiol Regul Integr Comp Physiol. 2000;279:R839-R848. [DOI] [PubMed] [Google Scholar]
- 50.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926-4937. doi: 10.1210/en.2010-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosono T, Chen X-M, Zhang Y-H, Kanosue K. Effects of estrogen on thermoregulatory responses in freely moving female rats. Ann N Y Acad Sci. 1997;813:207-210. doi: 10.1111/j.1749-6632.1997.tb51695.x [DOI] [PubMed] [Google Scholar]
- 52.Marrone BL, Gentry RT, Wade GN. Gonadal hormones and body temperature in rats: effects of estrous cycles, castration and steroid replacement. Physiol Behav. 1976;17:419-425. doi: 10.1016/0031-9384(76)90101-3 [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson CW, Carlisle HJ, Reynolds RW. Estrogenic effects on behavioral thermoregulation and body temperature of rats. Physiol Behav. 1980;24:337-340. doi: 10.1016/0031-9384(80)90096-7 [DOI] [PubMed] [Google Scholar]
- 54.Hosono T, Chen X-M, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1341-R1347. [DOI] [PubMed] [Google Scholar]
- 55.Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol Regul Integr Comp Physiol. 1980;238:R400-R405. [DOI] [PubMed] [Google Scholar]
- 56.Berendsen HHG, Weekers AHJ, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419:47-54. doi: 10.1016/S0014-2999(01)00966-9 [DOI] [PubMed] [Google Scholar]
- 57.Cosmi S, Pawlyk AC, Alfinito PD, Roman J, Zhou T, Deecher DC. Simultaneous telemetric monitoring of tail-skin and core body temperature in a rat model of thermoregulatory dysfunction. J Neurosci Methods. 2009;178:270-275. doi: 10.1016/j.jneumeth.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 58.Girbig D, Keller K, Prelle K, Patchev V, Vonk R, Igl B-W. A dynamic model of circadian rhythms in rodent tail skin temperature for comparison of drug effects. J Circadian Rhythms. 2012;10:1-11. doi: 10.1186/1740-3391-10-1 PMID:22221596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848-8853. doi: 10.1073/pnas.0913358107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cholanian M, Krajewski-Hall SJ, McMullen NT, Rance NE. Chronic oestradiol reduces the dendritic spine density of KNDy (kisspeptin/neurokinin B/dynorphin) neurones in the arcuate nucleus of ovariectomised Tac2-enhanced green fluorescent protein transgenic mice. J Neuroendocrinol. 2015;27:253-263. doi: 10.1111/jne.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112:13109-13114. doi: 10.1073/pnas.1512243112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. doi: 10.7554/eLife.16246 PMID:27549338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casper RF, Yen SSC, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science. 1979;205:823-825. doi: 10.1126/science.462193 [DOI] [PubMed] [Google Scholar]
- 64.Ruka KA, Burger LL, Moenter SM. Regulation of Arcuate Neurons Coexpressing Kisspeptin, Neurokinin B, and Dynorphin by Modulators of Neurokinin 3 and κ-Opioid Receptors in Adult Male Mice. Endocrinology. 2013;154:2761-2771. doi: 10.1210/en.2013-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molnar GW. Body temperatures during menopausal hot flashes. J Appl Physiol. 1975;38:499-503. [DOI] [PubMed] [Google Scholar]
- 66.Krull AA, Larsen SA, Clifton DK, Neal-Perry G, Steiner RA. A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist, senktide. Endocrinology. 2017;158:3259-3268. doi: 10.1210/en.2017-00142 PMID:28531316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molnar GW. Menopausal hot flashes: their cycles and relation to air temperature. Obstet Gynecol. 1981;57:52S-55S. [PubMed] [Google Scholar]
- 68.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29:319-336. doi: 10.1016/0531-5565(94)90012-4 [DOI] [PubMed] [Google Scholar]
- 69.Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, Gompel A, Hickey M, Hunter MS, Lobo RA, et al.. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14:515-528. doi: 10.3109/13697137.2011.608596 [DOI] [PubMed] [Google Scholar]
- 70.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66-70. doi: 10.1016/S0002-9378(99)70437-0 [DOI] [PubMed] [Google Scholar]
- 71.Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77:487-490. doi: 10.1016/S0015-0282(01)03009-6 [DOI] [PubMed] [Google Scholar]


