ABSTRACT
Cytokinesis is a complex cellular process that leads to a physical separation of two daughter cells. The key to a successful cytokinesis is a coordinated reorganization of cellular cytoskeleton and membrane trafficking pathways. Consequently, Rab GTPases recently emerged as major regulators of cellular division. Rabs belong to a superfamily of small monomeric GTPases that regulate a diverse array of cellular functions. Rabs in particular are well-established regulators of membrane transport and have been shown to mediate several membrane transport steps including vesicle formation, molecular motor-dependent vesicle transport and targeting of transport vesicles and organelles to their correct destinations. Significantly, several Rab GTPases also have been shown to function in regulating cell division. In this review, we discuss latest findings about the function of Rabs and polarized membrane transport during different steps of cytokinesis as well as during the final stage of cell division known as abscission.
KEYWORDS: abscission, cell polarity, cytokinesis, endosomes, Rab GTPases
Introduction
The last step of cell division is a physical separation of two daughter cells via process known as cytokinesis.1 After replication of genetic material, the mother cell divides by the formation of the cleavage furrow that constricts the cytoplasm leaving two daughter cells connected by a thin intracellular bridge (ICB). The resolution of this bridge, abscission, results in a physical separation of the two daughter cells. Although molecular mechanisms that govern this process are not fully understood, recent evidence supports the roles of recycling endosomes and ESCRT protein complex during abscission.2-4
Originally ESCRT complexes were identified to regulate multi-vesicular body formation and lysosomal sorting.5 However several ESCRT proteins were demonstrated also to be required for cytokinesis.6 Thus it was proposed that the recruitment of various ESCRT complex members, such as CHMP4, to the midbody, lead to a formation of filaments that mediate the final abscission step 7 (Fig. 1). Several excellent reviews have been recently published about the role of ESCRTs in mediating cytokinesis,8,9 thus in this review we will focus instead on describing the role of endosomes during cell division.
Figure 1.
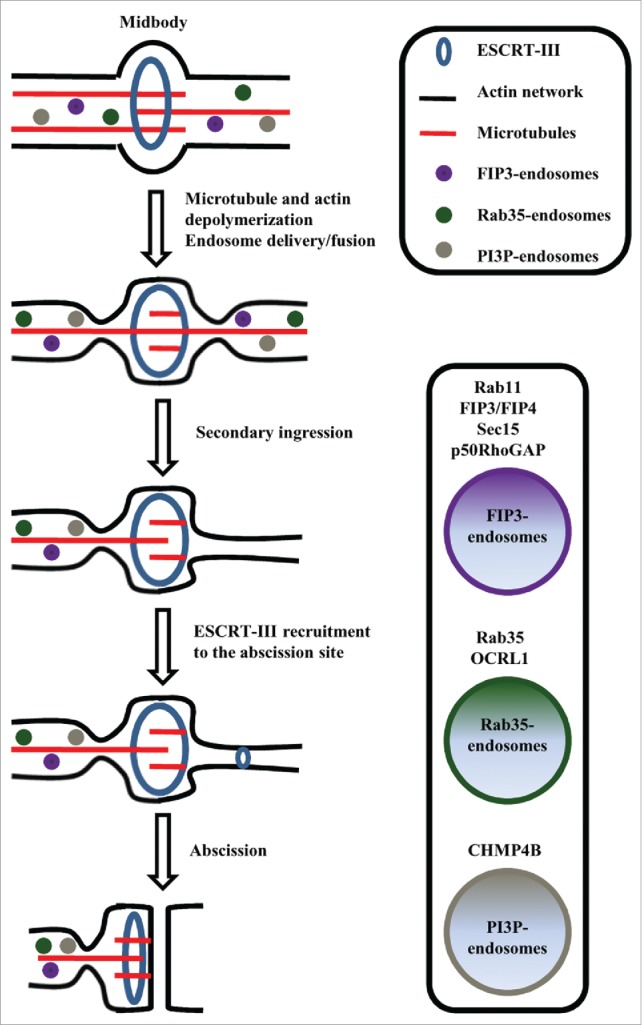
A schematic diagram illustrates the function of endosomes and Rab GTPases during abscission.
Recycling endosomes (REs) also have emerged as important players in mediating abscission.10 Several reports demonstrated that pronounced changes occur in endocytic recycling during mitosis, and that these changes are required for successful completion of cytokinesis.11,12 Originally it was proposed that REs initiate abscission by fusing with each other and the plasma membrane, thus building a separating membrane in a manner similar to the formation of phragmoplast in plant cells. However, recent data from several laboratories have shown that during division animal cells do not form phragmoplast-like structures,13 but fusion of REs instead initiates the formation of a “secondary ingression,” a step that is required for an eventual recruitment of ESCRTs to the abscission site (Fig. 1).
While it is now clearly established that endocytic transport to the midbody and ICB are required for cytokinesis, the function of these endosomes remains to be fully understood. Interestingly, recent work demonstrates that during cell division endosomes mediate localized remodeling of microtubules and actin cytoskeleton, thus directly affecting mitotic spindle formation, orientation and function, as well as the stability and dynamics of the actomyosin contractile ring. Thus, in the rest of this review we will focus on describing these new findings and defining the roles of endosomes and Rab GTPases in regulating cellular cytoskeleton during mitotic division.
The Role of Rabs in Regulation of Cytokinesis and Abscission
Endosome formation, transport, and function are regulated by a complex system of regulatory proteins. However, it is now clear that Rabs are the key regulators of all membrane trafficking steps, including endosomal transport. Rabs are a large sub-family of small monomeric Ras-like GTPases that function by site-specific activation and/or recruitment of various membrane transport regulators, also known and Rab effector proteins.14,15 As a result, in the last decade much effort has been dedicated in identifying and characterizing these Rab-specific effector proteins. In this review we will focus on the Rabs and Rab effector proteins that regulate mitotic cell division.
Rabs and regulation of the mitotic spindle
Correct formation and accurate positioning of the mitotic spindle is required for successful cell division. Mitotic spindle assembly begins in prophase when the centrosomes start nucleating microtubules. The correct positioning of this mitotic spindle often plays a key role during development, especially during asymmetric division of polarized cells. It is now clear that mitotic spindle localization is guided by various intracellular and extracellular cortical polarity cues. Surprisingly, recent work has demonstrated that in mammalian cells a known endocytic Rab11 and its effector protein FIP3 are both localized to the mitotic spindle and control the dynein-dependent endosome allocation at spindle poles.15 It was suggested that Rab11-endosomes might function as carriers to supplement and arrange microtubule nucleating material (GCP4, α-tubulin, γ-tubulin and Ran-GTP) at spindle poles.16 Similarly, Rab11 was also shown to be involved in dynein-dependent mitotic regulation of microtubules in Caenorhabditis elegans.17 It was demonstrated that Rab11-depletion leads to disruption of astral microtubules, delayed mitosis, and also disruption of endosome organization at the mitotic spindle poles and plane of cell division.16 The most common phenotype in Rab11-depleted cells was miss-orientation of the mitotic spindle, with the spindle angle relative to the cell-substrate adhesion plane in most Rab11-depleted cells greater than 20 degrees, compared with spindles parallel to the adhesion plane in control cells.16 Finally, in Rab11-depleted or dominant-negative Rab11 expressing cells, astral microtubule arrays were also disrupted. Taken together, all these recent data demonstrates that the presence of Rab11-dependent molecular mechanisms controlling the organization of astral microtubules and the mitotic spindle play a crucial role in the positioning of spindle poles during the early stages of mitosis.16
Rabs and regulation of abscission
Abscission is a complex cellular event during which the newly formed daughter cells are physically separated from one another. Several studies have revealed multiple proteins that directly regulate abscission. In particular, the actin cytoskeleton has emerged as a main mediator of both cytokinesis and abscission events.18 Cytokinesis starts in anaphase, when the assembly and contraction of the acto-myosin ring drives the formation of the cleavage furrow. Eventually, the remnants of the acto-myosin contractile ring needs to be removed from the intracellular bridge for abscission to take place. Consistent with this idea it was demonstrated that localized disassembly of actin cytoskeleton marks the site of the secondary ingression leading to eventual ESCRT recruitment and abscission.19,20
Phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) is one of the key signaling molecules that promotes actin polymerization and regulates actin cytoskeleton during interphase. It also is known to stabilize the acto-myosin contractile ring and link the actin cytoskeleton to the plasma membrane via binding to septins and ERM (exrin/radixin/moesin) proteins.21 What is not known is how the levels of PtdIns(4,5)P2 are controlled during abscission. Recently Rab35 was proposed as a novel regulator of PtdIns(4,5)P2 levels at the cleavage furrow during telophase. It was shown that overexpression of Rab35 dominant-negative mutant (Rab35-S22N) leads to a decrease in PtdIns(4,5)P2 levels within the ingressing furrow and accumulation of PtdIns(4,5)P2 in the enlarged endocytic organelles. Moreover, Rab35-S22N also reduces recruitment of septin 2 and actin cytoskeleton to the forming furrow, clearly showing its importance in inducing actin disassembly at the cleavage furrow during telophase.22 Significantly, phosphatidylinositol-4,5-bisphosphate 5-phosphatase (OCRL) was identified as a Rab35 effector protein that also accumulates at the cleavage furrow during abscssion.23 The OCRL is a known PtdIns(4,5)P2 phosphatase, thus Rab35-dependent targeting of OCRL leads to the reduction of PtdIns(4,5)P2 at the cleavage furrow. Consequently, by modulating PtdIns(4,5)P2 levels, OCRL also mediates actin cytoskeleton disassembly during late telophase, thus allowing for the formation of the secondary abscission and recruitment of the ESCRT complex to the abscission site to occur.
Rab11 and its effector proteins also recently have emerged as major regulators of cytokinesis and abscission.11,24 Rab11 associates with REs and was originally identified as a regulator of plasma membrane receptor recycling. Rab11 controls the delivery of REs to the cleavage furrow by binding to its FIP3 effector protein,25,26 which can directly bind to a known midbody resident, the Centralspindlin complex.27 Interestingly, the spatiotemporal dynamics of Rab11/FIP3-endosomes change distinctly as cell progresses through cytokinesis. During metaphase Rab11/FIP3-endosomes gather around centrosomes. When the midbody is formed and cells enter telophase, Rab11/FIP3-endosomes travel along the centralspindle microtubules to the cleavage furrow. During late telophase these endosomes accumulate at the intracellular bridge in endocytic pools arranged on either side of the midbody, thus initiating secondary ingression and abscission.19,28 Significantly, Rab11/FIP3-endosomes can be delivered to the cleavage in an asynchronous fashion presumably leading to asymmetric abscission on one side of the midbody and the inheritance of the midbody by one daughter cell.29 Recent studies revealed that Sec 15, a member of the Exocyst complex and another Rab11 effector protein, can interact with the centrosomal appendage protein, centriolin, indicating communication between REs and centrosomes. It was shown that REs also interact with the appendages of the mother (older) centriole, where Rab11 and its GAP, Evi5 accumulates.30 Moreover, it was shown that mother centriole appendage components, centriolin and cenexin/ODF2, control gathering of endosome constituents Rab11, the Rab11GTP-activating protein Evi5 and the Exocyst, at the mother centriole. One of the most important findings was that centriolin depletion disrupts recycling endosome organization and inhibits its function during cytokinesis and abscission. This may mean that mother centriole proteins are direct regulators of Rab11 and RE localization and function during mitotic cell division. Importantly, asymmetric accumulation of Rab11-endosomes at the mother centriole may also play a role in regulating asymmetric RE delivery to the abscission site and consequently leads to asymmetric midbody inheritance.
While Rab11/FIP3 endosomes are now firmly established as important regulators of abscission, the functions of these specialized REs remain to be fully defined. Recently we completed proteome analysis of Rab11/FIP3-endosomes and found that, among other proteins, these endosomes contain p50RhoGAP.19 Since p50RhoGAP is a GAP for RhoA, we proposed that the delivery of p50RhoGAP to the cleavage furrow leads to inactivation of RhoA and localized disassembly of actin cytoskeleton. This then results in the formation of the secondary ingression, leading to the initiation of the abscission site.31 Overall, the localized modulation of actin cytoskeleton by endosomes (Rab35 and Rab11) has emerged as a principal step required for the establishment of the abscission site(s), thus leading to the separation of the daughter cells and either release of the midbody into the media or inheritance of post-mitotic midbody by one of the daughter cells. Significantly, it was recently proposed that post-mitotic midbody (also known as midbody derivative) may function as a signaling platform that directly affects cell function during interphase.32,33 Since the putative midbody functions are not a focus of this review, we direct readers to a couple recent reviews that comprehensively discuss all the recent data and controversies regarding post-mitotic midbody function and regulation.34,35
Rabs, Cytokinesis, and Cell Polarity
Many organs contain series of hollow tubes/cavities that are required for their function. These tubes typically are formed by epithelial cell monolayer surrounding a central apical lumen. Each epithelial cell is polarized by subdividing their plasma membrane into apical and basolateral domains that are separated by tight junctions. This apico-basal polarity is a cornerstone to the function of epithelial cells and consequently is a tightly regulated process. Significantly, recent studies have demonstrated that the formation of apico-basal polarity and initiation as well as formation of the apical lumen is coupled to cell division.36,37 Additionally, it was shown that in 3D tissue culture systems the location of the cytokinetic bridge between the first 2 daughter cells is the first symmetry-breaking event that establishes the location of the apical membrane initiation site (AMIS), and therefore, the positioning of the newly forming apical lumen.36,38 Consequently, Rab11 and Rab35 are now emerging as regulators of both cell division and epithelial cell polarization.
Rab11 during cell division and AMIS formation
Apical lumen formation is a key step during epithelial morphogenesis and is dependent on the formation of a single AMIS.39 The formation of the apical lumen is a complicated process that involves coordinated changes in plasma membrane composition, cytoskeleton organization and endocytic transport. These changes are possible partly due to fusion of REs that contain Rab11 as well as its binding protein FIP5. FIPs (Family of Rab11-Interacting Proteins) are a group of proteins that regulate endocytic membrane trafficking. Particularly, FIP5 interacts with Rab11 in polarized MDCK cells and is responsible for apical protein targeting.40 Several studies have begun to elucidate the molecular machinery regulating apical lumen formation during epithelial morphogenesis. In a recent study, it was shown that the AMIS forms around the midbody during late telophase and that Rab11 effector protein FIP5, and endosomes containing apical proteins associated with it, are all transported to the AMIS along central spindle microtubules.41 Then, these FIP5-endosomes fuse with the plasma membrane at the cleavage furrow neighboring the AMIS, thus supplying apical components (Crumbs-3a and glycoprotein135 (gp135)) needed for the formation and progression of the emerging lumen (Fig. 2). Additionally, it was demonstrated that Kinesin-2 molecular motor directly binds to FIP5, thus mediating the delivery of FIP5-endosomes to the AMIS along central spindle microtubules during telophase.41 FIP5 also binds sorting nexin 18 (SNX18). In particular, this interaction is needed for apical endocytic carrier formation and lumen formation. Surprisingly, it was shown that Kinesin-2 and SNX18 compete for binding to FIP5. In summary, it was proposed that FIP5 may interact with SNX18 and Kinesin-2 in hand-me-down fashion, thus mediating sequential steps of endocytic carrier formation and transport during lumen initiation at late telophase41 (Fig. 2). However, how fusion of the apical transport vesicle with that plasma membrane around the midbody leads to formation of nascent apical lumen bordered by tight junctions remains unclear. Further research combining time-lapse and high-resolution imaging approaches will be needed to determine that.
Figure 2.
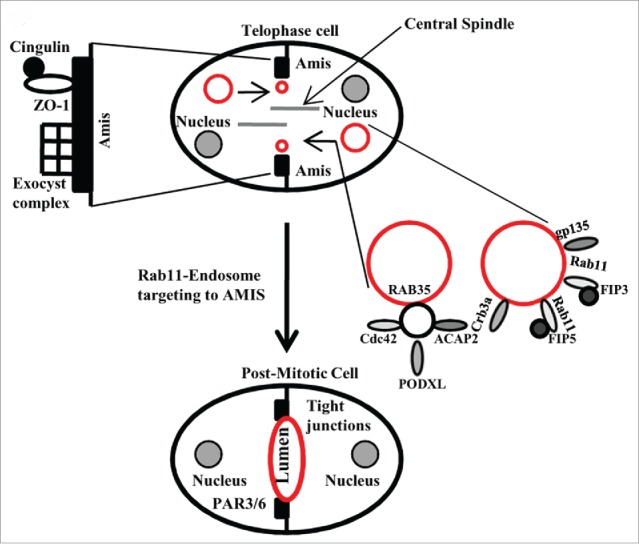
A schematic illustration of the function of midbdoy-associated Rab GTPases during the establishment of apical lumen.
Intriguingly, in addition to FIP5, several other Rab11-binding proteins were also implicated in both cell division and apical lumen formation. Specifically the Exocyst complex was shown to be present at the midbody and was proposed to mediate the targeting of Rab11-containing REs to the abscission site.42 Similarly, the Exocyst was also proposed to target Rab11-endosomes to the AMIS during lumen formation.38 Thus, it is tempting to hypothesize that Rab11 and the Exocyst interaction at the midbody may play a role for targeting Rab11/FIP5-containing apical carriers during lumenogenesis.
The AMIS is a temporary structure that contains various proteins including the Par3/Par6 polarity complex, tight junction proteins such as ZO-1 and Cingulin (CGN), and the Exocyst complex.38 As mentioned above, recent work from several groups has shown that lumen formation is mediated via targeted delivery of apical proteins to the AMIS, which eventually forms around the midbody during cell division. The key to the formation of the single apical lumen is the ability of Rab11-endosomes to be targeted directly and specifically to the AMIS. While the Exocyst complex is clearly required for this targeting, cingulin (CGN) also recently emerged as an important regulator of Rab11/FIP5-endosome transport to AMIS. In a recently published study,43 CGN was shown to bind directly to FIP5, thus functioning as a targeting/tethering factor for Rab11/FIP5-endosomes during the early stages of midbody-dependent lumen formation. Moreover, it was shown that both the branched actin cytoskeleton and midbody microtubules are required for the association of CGN with the AMIS. Since C-terminal tails of glutamylated microtubules bind directly to the basic patch located in the head region of CGN, it is likely that this binding mediates initial CGN recruitment to the midbody.43 Another interesting finding was that Rac1 activation at the midbody induces the recruitment of WAVE/Scar complex, which then promotes the polymerization of branched actin filaments through the activation of Arp2/3. The polymerization of these branched actin filaments is also obligatory for the formation of the AMIS at the midbody, as well as for the formation and development of a single apical lumen. Taken together, it is clear that targeting of apical endosomes to the AMIS depends on several midbody associated factors that include but are not limited to CGN and the Exocyst complex. Thus, lumen formation depends on a combination of several concessive overlapping pathways that likely ensure the fidelity and correct timing of apical lumen formation during late telophase.
Rab35 and apical lumen formation during cell division
Since Rab35 is required for cytokinesis and abscission, it is not too surprising that recent studies also implicated that Rab35 regulates apical lumen formation during the first cell division.44 Just like Rab11, Rab35 regulates the initiation of apico-basal polarity by targeting endocytic carriers containing some of the most important apical factors (Crumbs-3, Cdc42 and gp135) to the AMIS.39 The delivery of these apical proteins depends on binding between Rab35 and the cytoplasmic tail of gp135, and therefore, inactivation of Rab35 results in a complete inversion of apico-basal polarity in 3D cysts.44 Since Rab35 accumulation at the midbody and intracellular bridge precedes apical targeting of gp135, it was proposed that Rab35 functions as a tethering factor that guides apical endosomes to the AMIS during initial steps of lumen formation. Importantly, Rab35 was also shown to bind ACAP2 and OCRL, the proteins that are also required for the formation of apico-basal polarity and lumenogenesis.45
The functioning pathways of both Rab11 and Rab35 in some aspects are similar, and thus they may function together during midbody-dependent lumen formation. Since each of these RabGTPases has their own effector proteins, they can perfectly perform their function without interfering with each other during the complex process of lumen formation and further progression. Alternatively, Rab11 and Rab35 may mediate midbody and/or AMIS targeting of distinct organelles that can carry some of the same apical cargo (such as gp135). In either case, interacting with distinct effector proteins, both Rab11 and Rab35 control the transport of endosome-associated apical components to the AMIS during the initial steps of apical lumen formation, during epithelial morphogenesis. Since the timing and workflow differs among these Rabs, they likely complement each other's function and gaps, ensuring fidelity and successful completion of lumen formation.
The role of cytokinesis in hepatocyte polarization
Most of the studies investigating lumenogenesis used MDCK cells seeded in 3D cultures to study cross-talk between apical lumen formation and cell division. However, a new model that would allow for explanation of the physiologically relevant tissue architecture and function during cell division is in great need. Hepatocytes were shown to serve as a perfect model to study delicate intracellular changes and processes during cell division. Hepatocytes are the major parenchymal cells of the liver and unlike MDCK cells, they do not form large multi-cellular cysts containing a single apical lumen. Instead, hepatocytes form a network of narrow apical tubes known as canaliculi. Canaliculi form between 2 cells and are usually situated at the lateral cell-cell borders and surrounded by a ring of tight junctions. Significantly, it was shown that in hepatocytes, cytokinesis also defines a spatial landmark for polarization and apical lumen/bile canaliculus formation. Based on recent studies, it is proposed that during the final phase of cytokinesis, the epithelial cell polarity regulator Par3 and the tight-junction-associated protein ZO-1 are both delivered to the division site in a step-wise manner before the completion of cytokinesis, perhaps due to the following formation of bile canaliculus. Then, centrosomes migrate close to the disk-shaped tight junctions between the daughter cells to nucleate microtubules that target the Exocyst complex-dependent transport and targeting, leading to canaliculus formation.37 Which Rab GTPase(s) are involved in this process remains to be determined, however considering that the Exocyst complex binds Rab11, it is likely that Rab11GTPase is also involved in canaliculus genesis.
Conclusions and perspectives
Althought the importance of Rab GTPases and endosome-associated protein transport during cytokinesis and abscission is clearly established, the function and regulation of this intracellular protein trafficking pathway is only beginning to emerge. Lack of knowledge comes from technical limitations in studying abscission, since it is a transient cellular event that is difficult to image and quantify. Despite the newest live imaging technigues, the use of multiple approaches rather than a dependence on a single technique will allow us to understand the function of Rab11- and Rab35-associated endosomes and cytoskeleton dynamics during cytokinesis. It is also likely that other Rab GTPases and membrane transport pathways are also invoved in cell division. Indeed, midbody proteomic analysis46 identified several Rab GTPases that are present at the midbody during cytokinesis. Additionally, recently published work also suggested that Rab5, Rab6, Rab8a, Rab21 and Rab24 may also play a role in cytokineis, although their exact function remains to be fully understood.47-51 What, if any, function do these Rab GTPases play during cytokinesis remains to be determined and will require further studies. Additionally, very little is known about the involvement of Rabs and endocytic membrane transport in regulating specialized and assymetric types of cytokinesis and abscission. Thus, while in this review we have presented recent evidence linking cellular endocytic transport proteins with cell division, there are still many questions left to be answered, and they will be a focus for future research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. James Goldenring and Mantas Prekeris for critical reading of the manuscript. We apologize to all colleagues whose work could not be cited due to space limitations.
Funding
The work in Dr. Rytis Prekeris laboratory is supported by support by NIH grant DK064380 (to R.P.), grant from Cancer League of Colorado (to R.P.) as well as grant from Research Council of Lithuania (APP7/2016 to AS/RP).
References
- [1].Guizetti J, Gerlich DW. Cytokinetic abscission in animal cells. Sem in Cell Dev Biol 2010; 21(9):909-16; http://dx.doi.org/ 10.1016/j.semcdb.2010.08.001 [DOI] [PubMed] [Google Scholar]
- [2].Dionne LK, Wang XJ, Prekeris R. Midbody: from cellular junk to regulator of cell polarity and cell fate. Curr Opin in Cell Biol 2015; 35:51-8; http://dx.doi.org/ 10.1016/j.ceb.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen CT, Ettinger AW, Huttner WB, Doxsey SJ. Resurrecting remnants: the lives of post-mitotic midbodies. Trends Cell Biol 2013; 23(3):118-28; PMID:23245592; http://dx.doi.org/ 10.1016/j.tcb.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schiel JA, Prekeris R. Membrane Dynamics during cytokinesis. Curr Opin in Cell Biol 2013; 25:92-98; http://dx.doi.org/ 10.1016/j.ceb.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li M, Rong Y, Chuang YS, Peng D, Emr SD. Ubiquitin-dependent lysosomal membrane protein sorting and degradation. Mol Cell 2015; 57(3):467-78; PMID:25620559; http://dx.doi.org/ 10.1016/j.molcel.2014.12.012 [DOI] [PubMed] [Google Scholar]
- [6].Schiel JA, Childs C, Prekeris R. Endocytic transport and cytokinesis: from regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol 2013; 23(7):319-27; PMID:23522622;http://dx.doi.org/ 10.1016/j.tcb.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schiel JA, Prekeris R. ESCRT or endosomes?: Tales of the separation of two daughter cells. Communicative Integrative Biol 2011; 5:606-08; http://dx.doi.org/ 10.4161/cib.16789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nähse V, Christ L, Stenmark H, Campsteijn C. The Abscission Checkpoint: Making It to the Final Cut. Trends Cell Biol 2017; 27(1):1-11; PMID:27810282; http://dx.doi.org/ 10.1016/j.tcb.2016.10.001 [DOI] [PubMed] [Google Scholar]
- [9].Nakayama K. Regulation of cytokinesis by membrane trafficking involving small GTPases and the ESCRT machinery. Crit Rev Biochem Mol Biol 2016; 51(1):1-6; PMID:26362026; http://dx.doi.org/ 10.3109/10409238.2015.1085827 [DOI] [PubMed] [Google Scholar]
- [10].Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol 2012; 14(10):1068-78; PMID:23000966; http://dx.doi.org/ 10.1038/ncb2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Collins LL, Simon G, Matheson J, Wu C, Miller MC, Otani T, Yu X, Hayashi S, Prekeris R, Gould GW. Rab11-FIP3 is a cell cycle-regulated phosphoprotein. BMC Cell Biol 2012; 13:4; PMID:22401586; http://dx.doi.org/ 10.1186/1471-2121-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 2005; 16(2):849-60; PMID:15601896; http://dx.doi.org/ 10.1091/mbc.E04-10-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prekeris R, Gould WG. Breaking up is hard to do – membrane traffic in cytokinesis. J Cell Sci 2008; 121:1569-76; PMID:18469013; http://dx.doi.org/ 10.1242/jcs.018770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prekeris R. Rabs, Rips, FIPs, and Endocytic Membrane Traffic. ScientificWorld J 2003; 3:870-80; PMID:14532427; http://dx.doi.org/ 10.1100/tsw.2003.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hutagalung AH, Novick PJ. Role of RabGTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91(1):119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hehnly H, Doxsey S. Rab11-endosomes contribute to mitotic spindle orientation. Dev Cell 2014; 28(5):497-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang B, Une Y, Fu X, Yan J, Ge F, Yao J, Sawashita J, Mori M, Tomozawa H, Kametani F, et al.. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc Natl Acad Sci 2008; 105:7263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol 2010; 22(1):50-6; PMID:20031383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schiel A, Simon C, Zaharris C, Weisz J, Castle D, Wu C, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol 2012; 14(10):1068-78; PMID:23000966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Echard A. Connecting membrane traffic to ESCRT and the final cut. Nat Cell Biol 2012; 14(10):983-5; PMID:23033048 [DOI] [PubMed] [Google Scholar]
- [21].Janetopoulos C, Devreotes P. Phosphoinositide signalling plays a key role in cytokinesis. J Cell Biol 2006; 174(4):485-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol 2006; 16(17):1719-25; PMID:16950109 [DOI] [PubMed] [Google Scholar]
- [23].Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A. Rab35GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol 2011; 13(8):981-8; PMID:21706022 [DOI] [PubMed] [Google Scholar]
- [24].Simon GC, Prekeris R. The role of FIP3-dependent endosome transport during cytokinesis. Commun Integr Biol 2008; 1(2):132-3; PMID:19704869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 2005; 16(2):849-60; PMID:15601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun 2004; 319(1):83-94; PMID:15158446 [DOI] [PubMed] [Google Scholar]
- [27].Simon GC, Prekeris R. The role of FIP3-dependent endosome transport during cytokinesis. Commun Integr Biol 2008; 1(2):132-3; PMID:19704869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans 2008; 36(Pt 3):391-4; PMID:18481966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Prekeris R. Analyzing the functions of Rab11-effector proteins during cell division. Methods Cell Biol 2015; 130:19-34; PMID:26360025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S. The centrosome regulates the Rab11-dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol 2012; 22(20):1944-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol 2012; 14(10):1068-78; PMID:23000966; http://dx.doi.org/ 10.1038/ncb2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dionne LK, Wang XJ, Prekeris R. Midbody: from cellular junk to regulator of cell polarity and cell fate. Curr Opin Cell Biol 2015; 35:51-8; PMID:25950842; http://dx.doi.org/ 10.1016/j.ceb.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen CT, Ettinger AW, Huttner WB, Doxsey SJ. Resurrecting remnants: the lives of post-mitotic midbodies. Trends Cell Biol 2013; 23(3):118-28; PMID:23245592; http://dx.doi.org/ 10.1016/j.tcb.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dionne LK, Wang XJ, Prekeris R. Midbody: from cellular junk to regulator of cell polarity and cell fate. Curr Opin Cell Biol 2015; 35:51-8; PMID:25950842; http://dx.doi.org/ 10.1016/j.ceb.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen CT, Ettinger AW, Huttner WB, Doxsey SJ. Resurrecting remnants: the lives of post-mitotic midbodies. Trends Cell Biol 2013; 23(3):118-28; PMID:23245592; http://dx.doi.org/ 10.1016/j.tcb.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li D, Mangan A, Cicchini L, Margolis B., Prekeris R. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep 2014; 15:428-37; PMID:24591568; http://dx.doi.org/ 10.1002/embr.201338128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ting W, Kilangsungla Y, Ben ZS, Doris C, Erfei B. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci 2014; 127:2483-92; PMID:24706948; http://dx.doi.org/ 10.1242/jcs.139923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035-45; PMID:20890297; http://dx.doi.org/ 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 2012; 14:1235-43; PMID:23196841; http://dx.doi.org/ 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willenborg C, Jing J, Wu C, Matern H, Schaack J, Burden J, Prekeris R. Interaction between FIP5 and SNX18 regulates epithelial lumen formation. J Cell Biol 2011; 195:71-86; PMID:21969467; http://dx.doi.org/ 10.1083/jcb.201011112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li D, Kuehn EW, Prekeris R. Kinesin-2 mediates apical endosome transport during epithelial lumen formation. Cell Logist 2014; 4(1):e28928; PMID:24843830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 2005; 24(19):3389-99; PMID:16148947; http://dx.doi.org/ 10.1038/sj.emboj.7600803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mangan JA, Sietsema DV, Li D, Moore JK, Citi S, Prekeris R. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat Commun 2016; 7:12426; PMID:27484926; http://dx.doi.org/ 10.1038/ncomms12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Klinkert K, Rocancourt M, Houdusse A, Echard A. Rab35GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat commun 2016; 7:11166; PMID:27040773; http://dx.doi.org/ 10.1038/ncomms11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mrozowska PS, Fukuda M. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol 2016; 213(3):355-69; PMID:27138252; http://dx.doi.org/ 10.1083/jcb.201512024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Skop AR, Liu H, Yates J 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 2004; 305(5680):61-6; PMID:15166316; http://dx.doi.org/ 10.1126/science.1097931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill E, Clarke M, Barr FA. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J 2000; 19(21):5711-9; PMID:11060022; http://dx.doi.org/ 10.1093/emboj/19.21.5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fontijn RD, Goud B, Echard A, Jollivet F, van Marle J, Pannekoek H, Horrevoets AJ. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol Cell Biol 2001; 21(8):2944-55; PMID:11283271; http://dx.doi.org/ 10.1128/MCB.21.8.2944-2955.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kaplan A, Reiner O. Linking cytoplasmic dynein and transport of Rab8 vesicles to the midbody during cytokinesis by the double cortin domain-containing 5 protein. J Cell Sci 2011; 124(Pt 23):3989-4000; PMID:22159412; http://dx.doi.org/ 10.1242/jcs.085407 [DOI] [PubMed] [Google Scholar]
- [50].Pellinen T, Tuomi S, Arjonen A, Wolf M, Edgren H, Meyer H, Grosse R, Kitzing T, Rantala JK, Kallioniemi O, et al.. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell 2008; 15(3):371-85; PMID:18804435; http://dx.doi.org/ 10.1016/j.devcel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- [51].Militello RD, Munafó DB, Berón W, López LA, Monier S, Goud B, Colombo MI. Rab24 is required for normal cell division. Traffic 2013; 14(5):502-18; PMID:23387408; http://dx.doi.org/ 10.1111/tra.12057 [DOI] [PubMed] [Google Scholar]


