The preoptic area (POA) is a critical brain region for the regulation of body temperature in mammals. Neurons in the POA are believed to integrate information concerning environmental temperature, endocrine and metabolic status, as well as inflammatory and environmental threats, to optimize body temperature through adaptive behaviors, thermogenesis and active and passive heat-loss mechanisms. According to a prevalent model [1], body temperature homeostasis during heat-exposure is proposed to be mediated by warm-activated glutamatergic neurons in the median preoptic nucleus (MnPO), which then act on local GABAergic neurons in the medial preoptic area that inhibit heat production by brown adipose tissue (BAT) and shivering and promote passive heat-loss by increasing cutaneous blood flow.
In a land-mark study, Tan et al. (2016) showed that roughly 60% of preoptic neurons activated by heat exposure are located in a region they call the ventral medial preoptic area (VMPO), and that these neurons express the neuropeptides pituitary adenylate cyclase-activating polypeptide (PACAP) and brain-derived neurotrophic factor (BDNF). The VMPO as used by Tan et al appears to correspond to the anteroventral portion of the MnPO (avMnPO). Selective stimulation of BDNF/PACAP cells in this region was sufficient to replicate several behavioral and autonomic aspects of the response to heat-exposure, including inhibition of BAT thermogenesis and increased passive heat-loss through cutaneous vasodilatation of the tail. Interestingly, the inhibition of BAT thermogenesis during stimulation was at least partly due to the activation of a monosynaptic inhibitory pathway from BDNF VMPO neurons to the dorsal hypothalamic area/dorsomedial hypothalamus, and activation of this pathway had no effect on cutaneous blood flow.
In a paper published in October in the Journal of Physiology, we used optogenetic stimulation to address the role of glutamatergic neurons in the MnPO in thermoregulation [2]. Our experiments showed that activation of glutamatergic MnPO neurons decreases core temperature substantially, an effect we attributed to cutaneous vasodilatation. In addition to hypothermia, in many animals stimulation also caused water consumption [3], which we found was due to activation of a largely separate set of glutamatergic neurons in the more dorsal part of the MnPO. The hypothermic effect, by contrast, was due to activation of glutamatergic neurons in the avMNPO. Intriguingly, this location is identical to the location of BDNF/PACAP neurons activated by heat-exposure in Tan et al. (2016), raising the possibility that at least the vasodilation component of the hypothermic response observed in Tan et al was due to activation of glutamatergic neurons that express BDNF/PACAP. Interestingly, Tan et al. (2016) reported that 69% of BDNF VMPO neurons, and 83% of all heat-activated VMPO neurons, are GABAergic, while Zhao et al. (2016), reported that two-thirds of MnPO neurons that express Fos during heat-exposure also express BDNF, but that only a third of MnPO BDNF neurons express vesicular GABA transporter (VGAT), with the remainder expressing vesicular glutamate transport 2 (VGluT2) [5]. Taken together, these data suggest that there is a population of glutamatergic BDNF/PACAP neurons which our results suggest may constitute a specific vasomotor thermoregulatory pathway. Moreover, the collective evidence suggests that heat-activated GABAergic MnPO neurons inhibit thermogenesis by inhibiting excitatory DHA/DMH neurons that control BAT, whereas glutamatergic MnPO neurons promote cutaneous vasodilatation through a separate network of neurons.
How glutamatergic MnPO neurons regulate cutaneous blood flow is unclear as these excitatory neurons innervate several brain regions that are thought to promote heat conservation by regulating sympathetic outflow to cutaneous vasoconstrictor neurons (CVC) [3]. One possible route would be that direct projections from glutamatergic MnPO neurons to the DHA/DMH, and the medullary raphe and parapyramidal region, may represent inputs to local inhibitory neurons within these targets that modulate the activity of sympathoexcitatory output neurons [3]. Alternatively, inputs from glutamatergic MnPO neurons to inhibitory GABAergic neurons in the lateral preoptic area (LPO) [1–5] and the periaqueductal gray matter may result in inhibition of sympathoexcitatory pathways that conserve heat.
Collectively, our work along with the studies of Tan et al. (2016) and Zhao et al. (2016), demonstrate that the neurochemical phenotype of neurons in the POA that contribute to adaptive regulation of body temperature are not binary, but highly heterogeneous (Figure 1). Of course, none of the studies presented here address whether these neurons are necessary for the autonomic response to heat-exposure, and it is plausible that they are involved in metabolic, immunological and behavioral thermoregulation as opposed to solely responding to changes in environmental and brain temperature. Hence, more work is needed. Fortunately, modern methods that identify specific genetically-defined cell types involved in a response and then use genetically-directed activation and inhibition to study the physiology are providing a new level of precision in our ability to unravel these circuits and the mysteries surrounding thermoregulation and other hypothalamic functions.
Figure 1.
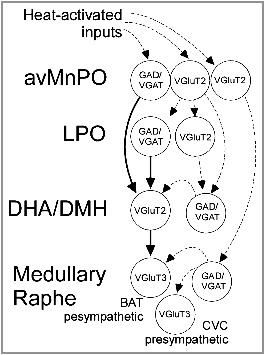
Possible organization of the central pathways mediating the autonomic response to heat-exposure. Excitatory and inhibitory neurons in the anteroventral median preoptic (avMnPO) receive input from thermo-sensory pathways. Inhibitory MnPO neurons monosynaptically inhibit excitatory neurons in the dorsal hypothalamic area and dorsomedial nucleus of the hypothalamus (DHA/DMH) reducing brown adipose tissue (BAT) thermogenesis. Excitatory avMnPO neurons drive inhibitory neurons, possibly in the lateral preoptic (LPO), DHA/DMH and the medullary raphe that inhibit BAT thermogenesis and the activity of cutaneous vasoconstrictor neurons (CVC). Solid lines indicate confirmed pathways, dashed lines indicate putative connections.
Funding Statement
National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke
References
- [1].Morrison SF. Central control of body temperature. F1000Res. 2016;5 DOI: 10.12688/f1000research.7958.1 PubMed PMID: 27239289; PubMed Central PMCID: PMC4870994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abbott SBG, Saper CB. Median preoptic glutamatergic neurons promote thermoregulatory heat loss and water consumption in mice. J Physiol. 2017;595(20):6569–6583. DOI: 10.1113/JP274667 PubMed PMID: 28786483; PubMed Central PMCID: PMC5638873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abbott SB, Machado NL, Geerling JC, et al. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci. 2016;36(31):8228–8237. DOI: 10.1523/JNEUROSCI.1244-16.2016 PubMed PMID: 27488641; PubMed Central PMCID: PMC4971367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tan CL, Cooke EK, Leib DE, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59, e15 DOI: 10.1016/j.cell.2016.08.028 PubMed PMID: 27616062; PubMed Central PMCID: PMC5062957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao ZD, Yang WZ, Gao C, et al. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci U S A. 2017;114(8):2042–2047. DOI: 10.1073/pnas.1616255114 PubMed PMID: 28053227; PubMed Central PMCID: PMC5338448. [DOI] [PMC free article] [PubMed] [Google Scholar]


