ABSTRACT
We tested the hypotheses that older adults with cardiovascular co-morbidities will demonstrate greater changes in body temperature and exaggerated changes in blood pressure before initiating thermal behavior. We studied twelve healthy younger adults (Younger, 25 ± 4 y) and six older adults (‘At Risk’, 67 ± 4 y) taking prescription medications for at least two of the following conditions: hypertension, type II diabetes, hypercholesterolemia. Subjects underwent a 90-min test in which they voluntarily moved between cool (18.1 ± 1.8°C, RH: 29 ± 5%) and warm (40.2 ± 0.3°C, RH: 20 ± 0%) rooms when they felt ‘too cool’ (C→W) or ‘too warm’ (W→C). Mean skin and intestinal temperatures and blood pressure were measured. Data were analyzed as a change from pretest baseline. Changes in mean skin temperature were not different between groups at C→W (Younger: +0.2 ± 0.8°C, ‘At Risk’: +0.7 ± 1.8°C, P = 0.51) or W→C (Younger: +2.7 ± 0.6°C, ‘At Risk’: +2.9 ± 1.9°C, P = 0.53). Changes in intestinal temperature were not different at C→W (Younger: 0.0 ± 0.1°C, ‘At Risk’: +0.1 ± 0.2, P = 0.11), but differed at W→C (-0.1 ± 0.2°C vs. +0.1 ± 0.3°C, P = 0.02). Systolic pressure at C→W increased (Younger: +10 ± 9 mmHg, ‘At Risk’: +24 ± 17 mmHg) and at W→C decreased (Younger: −4 ± 13 mmHg, ‘At Risk’: -23 ± 19 mmHg) to a greater extent in ‘At Risk’ (P ≤ 0.05). Differences were also apparent for diastolic pressure at C→W (Younger: −2 ± 4 mmHg, ‘At Risk’: +17 ± 23 mmHg, P < 0.01), but not at W→C (Younger Y: +4 ± 13 mmHg, ‘At Risk’: −1 ± 6 mmHg, P = 0.29). Despite little evidence for differential control of thermal behavior, the initiation of behavior in ‘at risk’ older adults is preceded by exaggerated blood pressure responses.
KEYWORDS: blood pressure, cutaneous vascular conductance, metabolic heat production, skin blood flow, sweat rate, thermal discomfort, thermoregulatory behavior
Introduction
The risk of cardiovascular events is elevated during cold and heat exposure.1 This risk is particularly pronounced in older adults,1 and even more so in those presenting with cardiovascular co-morbidities (e.g., hypertension, type II diabetes, hypercholesteremia, etc.).2 This risk is likely due to the relative hyper- or hypotensive challenges induced by cold or heat exposure,3 which can acutely increase the chance of cardiovascular events.4 Notably, healthy older adults exhibit altered hemodynamic responses to heat5,6 and cold7,8 exposure, and there is evidence that these responses are further exacerbated in older adults with cardiovascular co-morbidities, such as hypertension.9,10 Thus, there is a need to understand interactions between temperature regulation and hemodynamic responses in this population of older adults ‘at risk’ of cardiovascular events during thermal stress.
Autonomic thermoregulatory responses (i.e., sweating, shivering, skin blood flow) are impaired in healthy older adults,11 and the presence of cardiovascular co-morbidities, such as hypertension,12 type II diabetes13 and hypercholesteremia,14 further impairs aspects of autonomic thermoeffector activation. Notably, however, body temperature is regulated by both autonomic and behavioral responses,15 with behavior being the most efficient and effective thermoregulatory modality.15,16 Thermal behavior in young, healthy adults is elicited primarily by changes in skin temperature.17-21 The use of behavioral responses prevents changes in internal temperature18-21 and sweating or changes in metabolism.18,21 However, utilizing thermal behavior does not eliminate transient changes in blood pressure, and corresponding hemodynamic responses, prior to initiating behavior.20,21 Compared to younger adults, thermal behavior in healthy older adults is initiated after greater changes in body temperature.22-26 Whether thermal behavior is similarly affected and whether the hemodynamic responses upon the initiation of thermal behavior are altered in older adults with cardiovascular co-morbidities (i.e., ‘at risk’ older adults) is unknown. Therefore, the purpose of this study was to test the hypotheses that older adults with cardiovascular co-morbidities will demonstrate: i) greater changes in body temperature and ii) exaggerated changes in blood pressure before initiating thermal behavior.
Methods
Subjects
Twelve younger adults and six older adults with cardiovascular co-morbidities participated in this study. The subject characteristics are presented in Table 1. Subjects were physically active, non-smokers, cognitively normal, and reported to be free from any signs or symptoms related to neurological, including peripheral neuropathy, or psychological diseases. Younger and older subjects were matched for anthropometric characteristics. A portion of these data from the younger group of subjects has been presented in a previously published manuscript that tested a unique hypothesis.21
Table 1.
Subject characteristics.
| Younger | At Risk | |
|---|---|---|
| Sex (M/F) | 6 / 6 | 3 / 3 |
| Age (y) | 25 ± 3 (19 – 32) | 67 ± 4 Y (62 – 74) |
| Height (cm) | 173 ± 13 (157 – 204) | 167 ± 9 (158 – 183) |
| Weight (kg) | 77.6 ± 13.1 (59.5 – 104.0) | 78.1 ± 12.8 (57.4 – 97.2) |
| Body surface area (m2) | 1.9 ± 0.2 (1.6 – 2.4) | 1.9 ± 0.1 (1.7 – 2.0) |
| Sum of skinfolds (mm) | 137 ± 53 (61 – 210) | 156 ± 54 (100 – 230) |
| Body fat (%) | 26 ± 13 (8 – 47) | 30 ± 13 (20 – 50) |
| Prescription medications (n) | 0 | 6 Y |
| Hypertension (ANGII antagonists/ACE inhibitors) | 0 | 3 (2/1) |
| Type II Diabetes (metformin) | 0 | 2 |
| Hypercholesteremia (statins) | 0 | 5 |
| Screening heart rate (bpm) | 64 ± 11 (52 – 88) | 62 ± 10 (48 – 72) |
| Screening systolic blood pressure (mmHg) | 118 ± 9 (106 – 138) | 136 ± 7 Y (130 – 148) |
| Screening diastolic blood pressure (mmHg) | 72 ± 9 (60 – 90) | 78 ± 15 (58 – 102) |
| Screening mean arterial pressure (mmHg) | 88 ± 8 (76 – 101) | 97 ± 11 Y (77 – 117) |
| Physical activity (high/moderate/low) a | 4 / 7 / 1 | 3 / 3 / 0 |
| Montreal Cognitive Assessment Score b | 29 ± 2 (26 – 30) | 27 ± 1 Y (26 – 27) |
Younger subjects were not taking any medications except oral contraceptives (n = 2 females) and were free of any cardiovascular or metabolic disease. Younger female subjects were eumenorrheic and were not pregnant, which was confirmed via a urine pregnancy test. ‘At risk’ older subjects were permitted to participate if they had pharmacologically controlled hypercholesteremia (statin therapy, n = 5), type II diabetes (metformin, n = 2), or hypertension (ANGII antagonist, n = 2 or ACE inhibitor, n = 1). All of these subjects were taking medications for at least two of these conditions. All subjects taking medication were in a stable disease state, as indicated by no change in medication dosage in more than three months. All medications were reported to be taken as prescribed and taken at the same time every day. Each of these prescription drugs exerts primary or secondary effects that either augment14,27,28 or impair27,29 cardiovascular and/or thermoregulatory function. To our knowledge, the effect of combined therapies on cardiovascular and/or thermoregulatory function is unknown. All older female subjects were postmenopausal. These conditions were chosen because they are common in older adults and because all three are risk factors for cardiovascular events, but they can be well controlled via prescription medication. This was deemed important from the perspective of subject safety during study participation. Moreover, the use of pharmacologically controlled subjects conferred a high external validity compared to these subjects withholding their medications prior to the study.
Each subject was fully informed of the experimental procedures and possible risks before giving informed written consent. The study was approved by the Institutional Review Board at the University at Buffalo, and performed in accordance with the standards set by the latest revision of the Declaration of Helsinki. Subjects visited the laboratory on two occasions. Visit one was a screening and familiarization visit and visit two was the experimental trial.
Instrumentation and measurements
Height and weight were measured with a stadiometer and scale (Sartorius Corp. Bohemia, NY, USA), and body surface area was calculated accordingly.30 Skinfold thickness was measured in triplicate at the chest, axilla, triceps, subscapula, abdomen, suprailliac, and thigh (Harpenden, Baty International, UK), and percent body fat was estimated from body density,31 which was calculated from the sum of skinfolds for males32 and females.33 Urine specific gravity was measured in duplicate using a refractometer (Atago USA, Inc., Bellevue, WA, USA). Physical activity level was estimated using the validated International Physical Activity Questionnaire34 and cognitive ability was measured using the Montreal Cognitive Assessment.35
At least 60 min prior to any experimental testing, subjects swallowed a telemetry pill (HQ Inc., Palmetto, FL, USA) for the measurement of internal temperature. One younger male and one ‘at risk’ older male had contraindications for taking the telemetry pill. In these subjects, rectal temperature was measured at a depth of 10 cm past the anal sphincter using a general purpose thermistor (Mon-a-therm, Mallinckrodt Medical, Inc., St. Louis, MO, USA). Mean skin temperature was measured as the weighted average of six thermocouples (Omega Engineering, Inc. Stamford, CT, USA) attached to the following locations: abdomen (14%), calf (11%), chest (22%), lower back (19%), thigh (14%), and upper back (19%).36 Mean skin and internal temperatures are evenly weighted for producing thermal discomfort during resting conditions.37,38 Thus, because thermal discomfort is the perceptual mediator of thermal behavior,39 mean body temperature was calculated as 0.5 x internal temperature + 0.5 x mean skin temperature (Tbody1:1), as has been recently employed.40 Mean body temperature was also calculated as 0.9 x internal temperature + 0.1 x mean skin temperature while in a warm environment (Tbody9:1) and 0.67 x internal temperature + 0.33 x mean skin temperature in a cool environment (Tbody3:1). These weightings reflect the relative contributions of mean skin and internal body temperatures to autonomic thermoeffector activation during exposure to warm41 and cool42 environments, whereas Tbody1:1 reflects the contributions of these variables to thermal behavior.40
Heart rate was measured continually from a three lead electrocardiogram (DA100C, Biopac Systems, Inc. Goleta, CA, USA). Beat-to-beat blood pressure was measured via the Penaz method (Finometer Pro, FMS, Amsterdam, The Netherlands). Finometer derived blood pressure data were corrected to a manual blood pressure taken by an experienced member of the research team during the pre-testing period. Finometer derived blood pressure waveforms were maintained throughout all experimental testing. Stroke volume was estimated from the blood pressure waveform using Modelflow.43 Cardiac output was calculated as the product of stroke volume and heart rate, while total peripheral resistance (TPR) was calculated as the quotient of cardiac output and mean arterial pressure. Stroke work (stroke volume x mean arterial pressure), rate pressure product (RPP, systolic pressure x heart rate), and cardiac power output [mean arterial pressure x cardiac output x (2.22 × 10−3)] were calculated as indices of left ventricular work,44 myocardial oxygen demand,45 and left ventricular power.46
Skin blood flow (SkBF) was measured via integrated laser Doppler flowmetry (Periflux System 5010, Perimed, Stockholm, Sweden) at two locations: One on the dorsal surface of the left forearm, the other on the pad of the index fingertip of the left hand. At both locations, the laser Doppler probe was inserted into a thin plastic holder and the local temperature was allowed to drift with changes in skin temperature. The accuracy of the skin blood flow measurement was ensured based upon the observation of a clear pulsatile signal that coincided with the pulse wave. The laser Doppler system was regularly calibrated according to the manufacturers specifications. Skin blood flow data are reported as absolute values and normalized to mean arterial pressure, providing an index of cutaneous vascular conductance (CVC), which is indicative of cutaneous vasomotor tone.
Local sweat rate was measured by securing a plastic capsule that covered 3.9 cm2 of skin on the dorsal surface of the left forearm and on the midline of the chest beneath the sternal notch. These capsules were perfused with dry nitrogen at a flow rate of 0.6 L/min. The water vapor of the gas exiting the capsules was measured by capacitance hygrometry (HMT130, Vaisala, Woburn, WA, USA), and local sweat rate was calculated by multiplying the absolute humidity output (sensitive to 0.1 g/m3) by flow rate and dividing that value by the surface area of the capsule.47 The measurement of local sweat rate was sensitive to 0.01 mg/cm2/min.
Metabolic data were obtained via a facemask and three-way non-rebreathing valve (Han Rudolph, Inc., Shawnee, KS, USA) that was worn throughout the study. The rate of metabolic heat production was calculated from oxygen uptake and the respiratory exchange ratio (RER) and normalized to body surface area using a standard equation.48 Oxygen uptake and carbon dioxide production (sensitive to 0.1 L/min) were calculated from minute ventilation and the fraction of expired oxygen and carbon dioxide using the Haldane Transformation. Minute ventilation was calculated from expired airflow that was measured via a heated pneumotachometer (Hans Rudolph, Inc. Shawnee, KS, USA), which was continually integrated over 30 s and corrected to STPD. The fraction of expired oxygen and carbon dioxide (Vacumed, Ventura, CA, USA) was continually measured from a 3 L mixing chamber. The calculation of the rate of metabolic heat production was sensitive to 1 W/m2.
Thermal sensation [to the nearest 0.5, 1 = cold, 4 = neutral, 7 = hot49], thermal discomfort [to the nearest 0.5, 1 = comfortable, 4 = very uncomfortable49], and perceptions of sweating and shivering [to the nearest 0.5, 0 = none, 10 = most ever20] were measured on subjective scales.
Experimental protocol
Subjects arrived at the laboratory euhydrated, confirmed via urine specific gravity ≤1.02050 (Younger: 1.013 ± 0.007, ‘At Risk’: 1.007 ± 0.003), and having refrained from strenuous exercise, alcohol and caffeine for 12 h, and food for 2 h. Subjects in the ‘At Risk’ group were instructed to take their medication as prescribed the day of testing. To control for menstrual cycle hormones, younger females were tested during the first 10 days following self-identified menstruation (n = 4) or during the placebo phase of their oral contraceptives (n = 2), a period in which estrogen and progesterone are at their lowest levels.51 All experimental testing was conducted during the winter months in Buffalo, NY, USA (outdoor temperature on day and time of experimental protocol: 1 ± 7°C). Time of day was not controlled. Subjects wore a cotton t-shirt, underwear, athletic shorts, and light sandals [estimated insulation of clothing ensemble: 0.3 Clo52].
Following instrumentation, subjects rested quietly, seated on a mesh chair in a 25.5 ± 0.7°C (24 ± 6% relative humidity) environment for 20 min. Following this rest period, the thermostat in room in which the subjects were seated was lowered. This marked the beginning of the thermal behavior assessment, which was 90 min in duration. The temperature of this room gradually decreased to 17.6 ± 1.2°C (36 ± 12% relative humidity, Fig. 1). At any time during the room cooling, and throughout the thermal behavior assessment, subjects could move between the cool room and the adjacent warm room, which was maintained at 40.0 ± 0.6°C (20 ± 0% relative humidity, Fig. 1). The decision to behaviorally thermoregulate was defined as the decision to move from cool to warm (C→W) or from warm to cool (W→C).18-21 Subjects passively moved between the two rooms by pressing a button on a remote control. This shuttled them between the two rooms without exertion and allowed for continual data collection. The amount of time spent in each environment prior to behaving was recorded. Subjects watched from a selection of non-stimulating documentaries and maintained the same seated posture throughout the assessment. During the baseline period, subjects were read an instructional script that reviewed the experimental procedures and instructed them to exit the warm room when they became ‘too warm’ and exit the cool room when they became ‘too cool’.18-20 This ‘shuttle box’ thermal behavioral model provides a reliable index of thermoregulatory behavior in humans,19 and is considered better than other models (e.g., thermal gradient, self-paced exercise53,54) because it allows for the determination of the exact moment at which a decision to behaviorally thermoregulate is made.55
Figure 1.
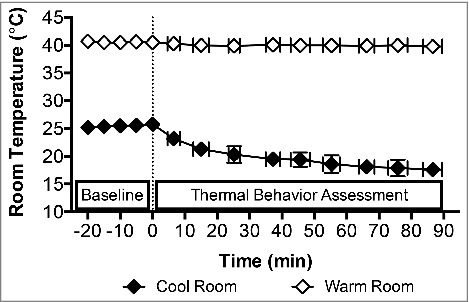
Temperature dynamics of the cool and warm rooms. Baseline measurements were taken at 0 min in the cool room after 20 min seated rest in a 25.5 ± 0.7°C (24 ± 6% relative humidity) environment. After the Baseline period, the cool room was set to 17.6 ± 1.2°C (36 ± 12% relative humidity) and the 90 min behavioral assessment commenced (dashed line) in the midst of this progressive room cooling. The warm room was maintained at 40.0 ± 0.6°C (20 ± 0% relative humidity) throughout, while the average temperature in the cool room during the thermal behavioral assessment was 18.1 ± 1.8°C (29 ± 5% relative humidity). Mean ± SD, n = 18 (Younger: n = 12, ‘At Risk’: n = 6).
Data and statistical analyses
Physiological data were sampled continuously at 100 Hz via a data acquisition system (Biopac MP150, Goleta, CA, USA). These data were analyzed at Baseline, which was a 60 s average at the end of the 20 min pre-protocol resting period and as a 30 s average immediately prior to C→W and W→C. Perceptual data were collected at Baseline and upon C→W and W→C. Each subject behaved a different number of times during the thermal behavior protocol. Thus, data were averaged across behaviors for a given subject, as we have done previously.18-21 To account for any age-related differences at Baseline, data at C→W and W→C were analyzed as a change from Baseline.
Subject characteristics and data at Baseline were analyzed using unpaired t-tests. Data at C→W and W→C were analyzed using mixed-model two-way repeated measures ANOVA (group x behavior, 2 × 2). The exceptions are changes in metabolic heat production (C→W), sweat rate (W→C), Tbody3:1 (C→W) and Tbody9:1 (W→C), which were only analyzed at C→W or W→C as noted. These data were analyzed via unpaired t-tests. All data were assessed for approximation to a normal distribution and sphericity, and no corrections were necessary. Where appropriate, post hoc pairwise comparisons were made using unpaired t-tests. Data were analyzed using Prism software (Version 6, GraphPad Software Inc. La Jolla, CA, USA). A priori statistical significance was set at P ≤ 0.05. Actual P-values are reported where possible. Data are reported as mean ± SD.
Results
Baseline
Mean skin (P = 0.06) and intestinal (P = 0.08) temperatures did not differ between groups at Baseline, while Tbody1:1 was higher in the Younger group (P = 0.02, Table 2). Tbody3:1 and Tbody9:1 were also higher in the Younger group (P ≤ 0.03, Table 2). Mean arterial pressure and systolic pressure were both higher in the ‘At Risk’ group compared to the Younger group at Baseline (P ≤ 0.03), while diastolic pressure did not differ between groups during this time (P = 0.08, Table 2). Likewise, heart rate (P = 0.13), cardiac output (P = 0.43), TPR (P = 0.08), RPP (P = 0.12), and cardiac power output (P = 0.19) did not differ between groups at Baseline, whereas stroke volume (P = 0.03) and stroke work (P = 0.01) were higher in the ‘At Risk’ group (Table 2). There were no differences between groups in activation of the autonomic thermoeffectors (e.g., SkBF, CVC, sweat rate, and metabolic heat production) at Baseline (P ≥ 0.15, Table 2). Subjects generally felt thermally comfortable, perceived neutral thermal sensation and did not perceive to be sweating or shivering, and there were no differences between groups (P ≥ 0.10, Table 2).
Table 2.
Baseline values.
| Younger | ‘At Risk’ | |
|---|---|---|
| Body Temperatures | ||
| Mean skin temperature (°C) | 31.9 ± 0.7 | 31.2 ± 1.0 |
| Internal temperature (°C) | 37.2 ± 0.3 | 37.0 ± 0.3 |
| Tbody1:1 (°C) | 34.5 ± 0.3 | 34.1 ± 0.6 Y |
| Tbody3:1 (°C) | 35.4 ± 0.2 | 35.1 ± 0.4 Y |
| Tbody9:1 (°C) | 36.7 ± 0.3 | 36.4 ± 0.3 Y |
| Hemodynamics | ||
| Mean arterial pressure (mmHg) | 83 ± 8 | 90 ± 6 Y |
| Systolic pressure (mmHg) | 113 ± 12 | 126 ± 10 Y |
| Diastolic pressure (mmHg) | 68 ± 7 | 73 ± 6 |
| Heart rate (bpm) | 75 ± 9 | 67 ± 10 |
| Stroke volume (mL/beat) | 81 ± 8 | 90 ± 12 Y |
| Cardiac output (L/min) | 6.0 ± 1.1 | 6.0 ± 0.7 |
| TPR (mmHg/L/min) | 16.4 ± 2.8 | 18.2 ± 2.2 |
| RPP (mmHg x bpm / 1000) | 8.4 ± 1.6 | 8.4 ± 1.4 |
| Cardiac power output (W) | 1.1 ± 0.3 | 1.2 ± 0.2 |
| Stroke work (mL / beat x mmHg) | 6.6 ± 1.1 | 8.2 ± 1.2 Y |
| Autonomic Thermoeffectors | ||
| Fingertip SkBF (PU) | 232 ± 167 | 173 ± 150 |
| Fingertip CVC (PU/mmHg) | 1.8 ± 1.1 | 1.6 ± 1.3 |
| Forearm SkBF (PU) | 28 ± 19 | 31 ± 8 |
| Forearm CVC (PU/mmHg) | 0.32 ± 0.24 | 0.29 ± 0.08 |
| Chest sweat rate (mg/min/cm2) | 0.05 ± 0.02 | 0.06 ± 0.02 |
| Forearm sweat rate (mg/min/cm2) | 0.06 ± 0.01 | 0.07 ± 0.01 |
| Metabolic heat production (W/m2) | 60 ± 10 | 56 ± 13 |
| Perceptions | ||
| Thermal discomfort (a.u.) | 1.1 ± 0.2 | 1.2 ± 0.4 |
| Thermal sensation (a.u.) | 3.9 ± 0.4 | 3.7 ± 0.4 |
| Sweating (a.u.) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Shivering (a.u.) | 0.2 ± 0.4 | 0.0 ± 0.0 |
Mean ± SD, Younger: n = 12, ‘At Risk’: n = 6, Tbody1:1: mean body temperature with internal and mean skin temperatures weighted 1:1, Tbody3:1: mean body temperature with internal and mean skin temperatures weighted 3:1, Tbody9:1: mean body temperature with internal and mean skin temperatures weighted 9:1, TPR: total peripheral resistance, RPP: rate pressure product, SkBF: skin blood flow, CVC: cutaneous vascular conductance,
different from younger (P ≤ 0.03)
Thermal behavior characteristics
The time before behaving and the number of behaviors did not differ between the Younger and ‘At Risk’ groups at either C→W or W→C (P ≥ 0.59, Table 3). However, the time before behaving was longer W→C compared to C→W in the ‘At Risk’ group (P = 0.02, Table 3).
Table 3.
Thermal behavior characteristics and perceptual responses.
| Younger | ‘At Risk’ | |
|---|---|---|
| C → W | ||
| Time before behaving (min) | 9.8 ± 4.3 | 8.4 ± 2.8 |
| Number of behaviors (#) | 4 ± 2 | 5 ± 5 |
| Thermal discomfort (a.u.) | 2.0 ± 0.2 | 2.2 ± 0.6 Y |
| Thermal sensation (a.u.) | 2.5 ± 0.5 | 2.1 ± 0.6 |
| Sweating perception (a.u.) | 0.0 ± 0.1 | 0.0 ± 0.0 |
| Shivering perception (a.u.) | 1.3 ± 1.1 | 2.5 ± 2.4 |
| W → C | ||
| Time before behaving (min) | 14.5 ± 4.3 | 18.9 ± 18.4 * |
| Number of behaviors (#) | 4 ± 2 | 4 ± 2 |
| Thermal discomfort (a.u.) | 2.1 ± 0.2 | 2.7 ± 0.5 * |
| Thermal sensation (a.u.) | 5.7 ± 0.4 * | 5.8 ± 0.4 * |
| Sweating perception (a.u.) | 1.2 ± 1.1 * | 0.9 ± 0.9 * |
| Shivering perception (a.u.) | 0.0 ± 0.0 * | 0.0 ± 0.1 * |
Mean ± SD, Younger: n = 12, ‘At Risk’: n = 6, C→W: decision to move from cool-to-warm, W→C: decision to move from warm-to-cool,
different from younger (P < 0.01),
different from C→W (P ≤ 0.02)
Body temperatures
The change in internal temperature was not different between the Younger and ‘At Risk’ groups at C→W (P = 0.11). Internal temperature changed to a greater extent at W→C compared to C→W in the Younger group (P = 0.02) such that the change in internal temperature was greater in the Younger group compared to the ‘At Risk’ group (P = 0.02, Fig. 2). Changes in mean skin temperature and Tbody1:1 were not different between groups at C→W or W→C (P ≥ 0.38), while the mean skin temperature and Tbody1:1 at the decision to behave were higher at W→C compared to C→W (P < 0.01). At C→W, changes in Tbody3:1 did not differ between groups (P = 0.11, Fig. 2). However, changes in Tbody9:1 differed between groups at W→C (P = 0.04, Fig. 2).
Figure 2.
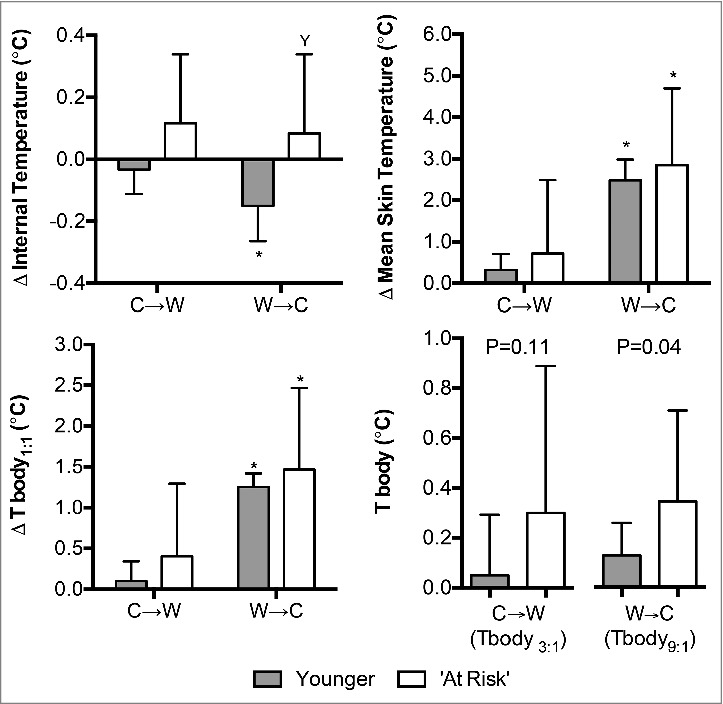
Changes (Δ) in intestinal, mean skin, and mean body temperatures upon the decision to move from cool-to-warm (C→W) and from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, * different from C→W (P ≤ 0.02), Y different from younger (P = 0.02). Tbody1:1: mean body temperature with internal and mean skin temperatures weighted 1:1, Tbody3:1: mean body temperature with internal and mean skin temperatures weighted 3:1, Tbody9:1: mean body temperature with internal and mean skin temperatures weighted 9:1.
Autonomic thermoeffector activation
The change in forearm and fingertip SkBF and CVC did not differ between the Younger and ‘At Risk’ groups at C→W (P ≥ 0.38, Fig. 3). The change in forearm and fingertip SkBF and CVC at W→C was greater than at C→W in both groups (P < 0.01). The magnitude of increase in fingertip SkBF and CVC was greater in the ‘At Risk’ compared to the Younger group (P≤0.04), but there were no differences between groups at the forearm location (P ≥ 0.40, Fig. 3).
Figure 3.
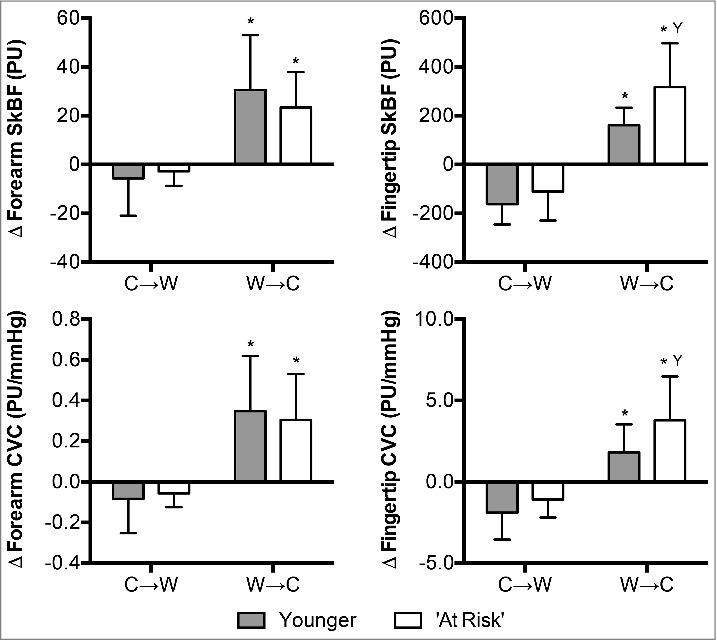
Changes (Δ) in forearm and fingertip skin blood flow (SkBF) and cutaneous vascular conductance (CVC) upon the decision to move from cool-to-warm (C→W) and from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, * different from C→W (P < 0.01), Y different from younger (P ≤ 0.04).
Changes in metabolic heat production at C→W did not differ between the Younger and ‘At Risk’ groups (P = 0.18, Fig. 4). Changes in both chest (P = 0.058) and forearm (P = 0.055) sweat rate at W→C were greater in the ‘At Risk’ group (Fig. 4).
Figure 4.
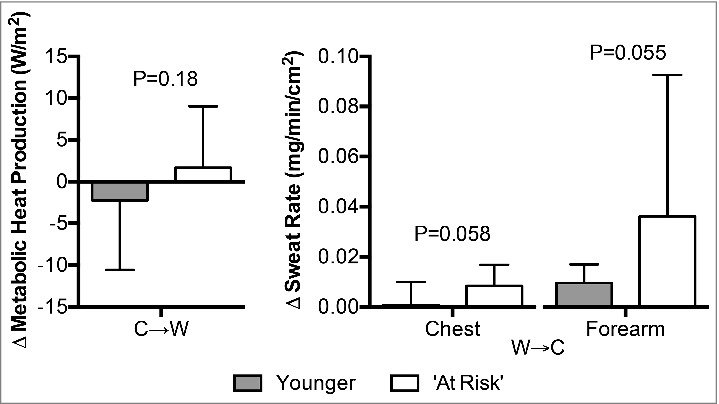
Changes (Δ) in metabolic heat production upon the decision to move from cool-to-warm (C→W), and chest and forearm sweat rate upon the decision to move from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, actual p-values are reported.
Hemodynamic responses
The change in mean arterial pressure was not different between groups at C→W (P = 0.35, Fig. 5). However, the change in both systolic and diastolic pressure was greater at C→W in the ‘At Risk’ group compared to the Younger group (P ≤ 0.05, Fig. 5). Mean arterial pressure and systolic pressure were lower at W→C in both groups (P < 0.01, Fig. 5), while diastolic pressure at W→C only decreased in the ‘At Risk’ group (P < 0.01, Fig. 5). The magnitude of changes in mean arterial pressure and systolic pressure at W→C were greater in the ‘At Risk’ group compared to the Younger group (P≤0.03, Fig. 5). Changes in diastolic pressure at W→C did not differ between groups (P = 0.29, Fig. 5).
Figure 5.
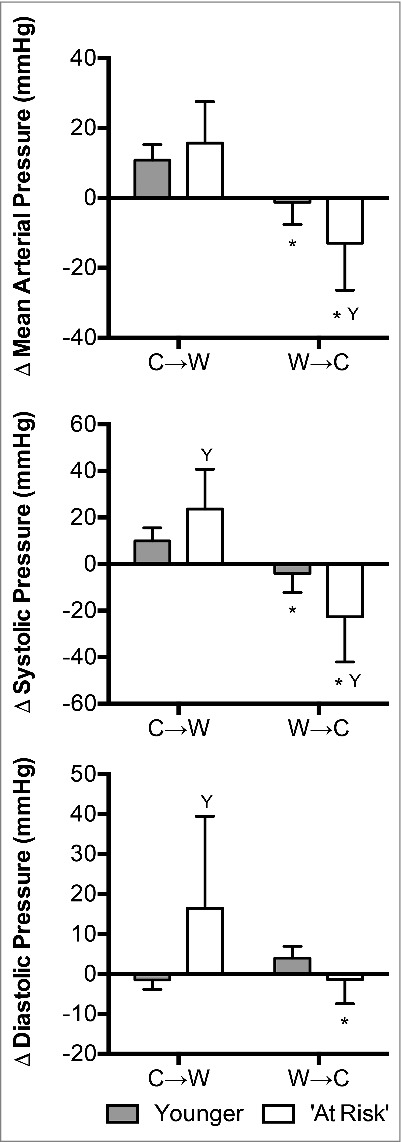
Changes (Δ) in mean arterial pressure, systolic pressure and diastolic pressure upon the decision to move from cool-to-warm (C→W) and from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, * different from C→W (P < 0.01), Y different from younger (P ≤ 0.05).
Changes in heart rate (P = 0.82), cardiac output (P = 0.12), and TPR (P = 0.51) were not different between groups at C→W (Fig. 6). However, at C→W the change in stroke volume differed between groups; stroke volume increased in the ‘At Risk’ group and decreased in the Younger group (P = 0.02, Fig. 6). Heart rate was higher at W→C compared to C→W in both groups (P < 0.01) and there were no differences between groups (P = 0.46, Fig. 6). The change in stroke volume differed between C→W and W→C in the ‘At Risk’ group (P = 0.05), but not in the Younger group (P = 0.52, Fig. 6). However, the change in stroke volume did not differ at W→C between groups (P = 0.84, Fig. 6). The change in cardiac output differed between C→W and W→C in the Younger group (P = 0.02), but not in the ‘At Risk’ group (P = 0.39, Fig. 6). However, the change in cardiac output did not differ at W→C between groups (P = 0.45, Fig. 6). TPR was lower at W→C compared to C→W in both groups (P < 0.01) and there were no differences between groups (P = 0.13, Fig. 6).
Figure 6.
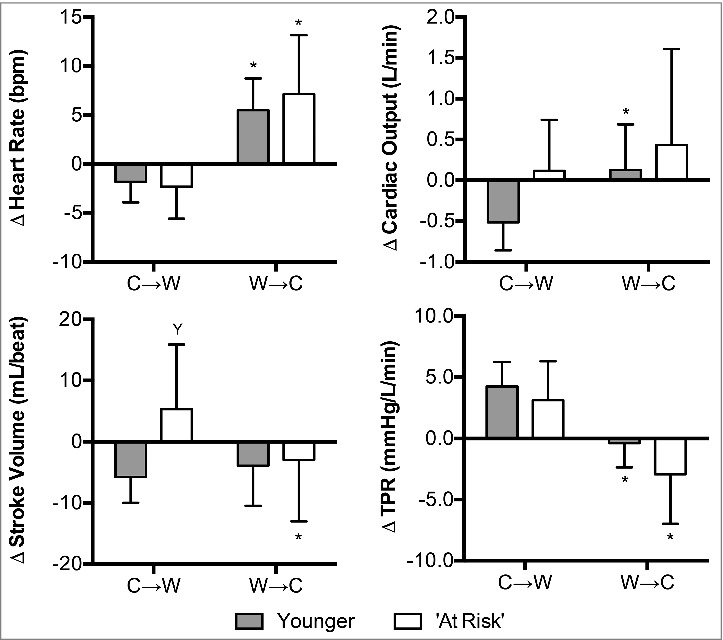
Changes (Δ) in heart rate, stroke volume, cardiac output, and total peripheral resistance (TPR) upon the decision to move from cool-to-warm (C→W) and from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, * different from C→W (P ≤ 0.05), Y different from younger (P ≤ 0.02).
The change in RPP at C→W did not differ between groups (P = 0.11, Fig. 7). However, changes in cardiac power output (P < 0.01) and stroke work (P < 0.01) were greater in the ‘At Risk’ group compared to the Younger group (Fig. 7). Change in RPP (P < 0.01), cardiac power output (P < 0.01) and stroke work (P < 0.01) differed between C→W and W→C in the ‘At Risk’ group, but not in the Younger group (P ≥ 0.07, Fig. 7). While the change in RPP (P = 0.06) and cardiac power output (P = 0.11) between groups at W→C did not reach statistical significance, stroke work decreased to a greater extent at W→C in the ‘At Risk’ group compared to the Younger group (P < 0.01, Fig. 7).
Figure 7.
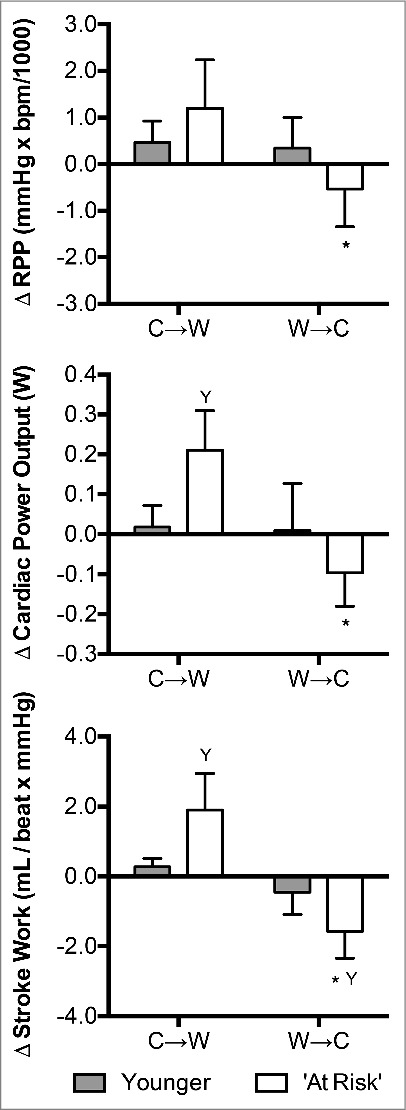
Changes (Δ) in rate pressure product (RPP), cardiac power output, and stroke work upon the decision to move from cool-to-warm (C→W) and from warm-to-cool (W→C) in younger adults (n = 12) and ‘at risk’ older adults (n = 6). Mean ± SD, * different from C→W (P < 0.01), Y different from younger (P < 0.01).
Perceptual responses
Younger subjects felt slightly thermally uncomfortable at both C→W and W→C (P = 0.64, Table 3). However, the ‘At Risk’ subjects were more thermally uncomfortable at C→W (P = 0.02) and was more uncomfortable at C→W compared to the Younger group (P < 0.01, Table 3). Subjects felt cooler at C→W compared to W→C (P < 0.01) and there were no differences between groups (P = 0.49, Table 3). At C→W subjects perceived to be very slightly shivering and at W→C they perceived to be very slightly sweating, with no differences between groups (P ≥ 0.40, Table 3).
Discussion
In contrast to our first hypothesis, we observed no differences in the time before initiating thermal behavior (Table 3), the total number of behaviors during the thermal behavioral assessment (Table 3), or changes in Tbody1:1 (Fig. 2) between groups at C→W or W→C. As a result, at C→W there was no evidence for differential changes in SkBF/CVC (Fig. 3) or metabolic rate (Fig. 4) between groups. Interestingly, at W→C the ‘At Risk’ group exhibited greater increases in fingertip SkBF (Fig. 3) and sweat rate (Fig. 4) compared to the Younger group. In support of our second hypothesis, we observed greater increases in systolic and diastolic pressure at C→W and greater reductions in systolic, diastolic and mean arterial pressure at W→C in the ‘At Risk’ group (Fig. 5). These data obtained from a relative small sample of ‘at risk’ older adults demonstrate that utilization of thermal behavior in older adults with cardiovascular co-morbidities does not likely prevent hemodynamic changes that can challenge cardiovascular function, despite that thermal behavior is generally not altered in this population.
Thermal behavior in older adults with cardiovascular co-morbidities
Thermal behavior in healthy older adults is initiated after greater changes in body temperature compared to younger adults.22-26 In the present study, we found no evidence that the utilization of thermal behavior differed between ‘at risk’ older adults and young healthy adults. Specifically, the time before initiating thermal behavior and the total number of behaviors during the 90-min thermal behavioral assessment did not differ between groups (Table 3). Furthermore, the magnitude of changes in mean skin temperature and Tbody1:1 (i.e., the stimulus for behavioral thermoeffector activation37-39) did not differ between groups at C→W or W→C (Fig. 2). Consistent with our previous data,18,20,21 however, we observed lower internal temperatures at W→C compared to C→W in the Younger group (Fig. 2). This is not likely to be causal in the decision to behaviorally thermoregulate because the direction of the changes are counter to that which would be expected,18 but is probably a function of the redistribution of blood flow in the body owing to changes in mean skin temperature.56 Interestingly, internal temperature did not differ between C→W or W→C in the ‘At Risk’ group (Fig. 2). Because internal temperature was elevated at C→W (Fig. 2), we do not believe that these differences in internal temperature contributed to the initiation of thermal behavior. Rather, the observed changes in internal temperature in the ‘At Risk’ group likely reflect an age and/or disease related attenuation in blood flow redistribution during exposure to cool and warm environments.6
Tbody3:1 (i.e., the stimulus for autonomic thermoeffector activation in a cool environment42) upon the initiation of thermal behavior did not differ between groups (Fig. 2). Thus, it was not surprising that at C→W changes in forearm or fingertip SkBF/CVC (Fig. 3) and metabolic rate (Fig. 4) were not different between the ‘At Risk’ and Younger groups. Interestingly, despite that increases in forearm SkBF/CVC did not differ between groups at W→C (Fig. 3), the ‘At Risk’ group exhibited greater increases in fingertip SkBF/CVC (Fig. 3) and sweat rate (Fig. 4) at W→C. This is likely because of the greater thermal stimulus at W→C in the ‘At Risk’ group, such that Tbody9:1 (i.e., the stimulus for autonomic thermoeffector activation in a warm environment41) was higher than in the Younger group (Fig. 2). This finding might suggest differential control of thermal behavior in a warm environment in ‘at risk’ older adults, compared to their young, healthy counterparts.
In contrast to non-glabrous (i.e., hairy) skin, vasoconstrictor responsiveness in glabrous (i.e., non-hairy) skin is well maintained in older adults.57 Thus, it was surprising that differences in SkBF/CVC at W→C were observed in glabrous skin (i.e., fingertip) and not non-glabrous skin (i.e., forearm) (Fig. 3). Glabrous skin is innervated by only sympathetic vasoconstrictor nerves.58 Therefore, the observation that fingertip SkBF/CVC was higher at W→C in the ‘At Risk’ group compared to the Younger group (Fig. 3), suggests that for a given increase in body temperature ‘at risk’ older adults exhibit a greater withdrawal of cutaneous vasoconstrictor tone to this vascular bed. Notably, the role that glabrous skin and the thermal status of the hands and feet play in the initiation of thermal behavior is largely unknown. However, we have previously identified that extremity (i.e., fingertip) temperatures, and the thermal perceptions thereof, may contribute to the decision to behaviorally thermoregulate.20 Unfortunately, in the current study extremity temperatures were not measured. However, it is likely that fingertip skin temperature was higher at W→C in the ‘At Risk’ group given that a rise in SkBF promotes increases in skin temperature.59 Thus, we speculate that a greater change in extremity skin temperature occurred prior to the initiation of thermal behavior in the ‘At Risk’ group compared to the Younger group. This speculation for a greater thermal stimulus prior to W→C is supported by greater increases in Tbody9:1 and sweat rate at W→C in the ‘At Risk’ group (Fig. 4). This is contrasted by data indicating that young healthy adults utilize thermal behavior before the activation of sweating.21 The increases in sweat rate in the ‘At Risk’ group at W→C may reflect a reliance upon the activation of sweating in the decision to behaviorally thermoregulate. That is, thermal discomfort, and thus the decision to behaviorally thermoregulate,39 can be brought about or exacerbated by skin wetness,60 which occurs subsequent to sweating.61 Thus, it is possible that the ‘At Risk’ group was using the activation of sweating, and resulting perception of skin wetness, in the decision to behaviorally thermoregulate. Unfortunately, however, we did not measure skin wetness or wetness perception in the current study. Nevertheless, it is clear that further research is required to elucidate the signals underlying the initiation of thermal behavior in a warm environment in older adults with cardiovascular co-morbidities.
Hemodynamic responses upon the initiation of thermal behavior in older adults with cardiovascular co-morbidities
We have previously identified that the initiation of thermal behavior in young healthy adults coincides with elevations in blood pressure at C→W and reductions in blood pressure at W→C.20 This occurred despite modest changes in body temperature and was likely due to changes in vascular resistance occurring largely because of changes in cutaneous vasomotor tone.20,21 In the present study, thermal behavior was initiated after relatively similar changes in body temperature between groups (Fig. 2). However, we observed greater relative hypertension at C→W and relative hypotension at W→C in the ‘At Risk’ group compared to the Younger group (Fig. 5). Such findings at C→W were expected given that hypertensive older adults demonstrate greater increases in blood pressure for a given reduction in body temperature.10 However, the greater hypotension in the ‘At Risk’ group at W→C was somewhat unexpected given data indicating that blood pressure during heat exposure is maintained equally as well in healthy older and younger adults.62 The reason for this observation is likely related to age dependent delays in baroreflex responsiveness during baroreceptor unloading induced by heat stress.63 Thus, we speculate that had the exposure to the warm environment been more prolonged or severe any differences between groups would have been diminished. Admittedly, however, this speculation ignores any impact of cardiovascular co-morbidities and/or medications on baroreflex function during heat exposure. Notably, while baroreflex function in individuals with hypertension, type II diabetes and hypercholesteremia are impaired,64-67 to our knowledge, any such interactions during heat exposure are unknown.
Our data indicate that ‘at risk’ older adults do not likely utilize thermal behavior in such a manner that protects them against temperature induced changes in blood pressure. Such a finding suggests that during thermal stress the utilization of behavioral strategies to thermoregulate does not fully alleviate the risk of cardiovascular events in older adults with cardiovascular co-morbidities, despite that they are medicated to alleviate this risk. This is indirectly reflected in our indices of cardiac stress, which generally demonstrated greater changes in myocardial oxygen demand (RPP), left ventricular power (cardiac power output), and left ventricular work (stroke work) in the ‘At Risk’ group compared to the Younger group upon the decision to behaviorally thermoregulate (Fig. 7). Particularly striking are the differential increases in cardiac stress at C→W (Fig. 7) because these changes can readily challenge cardiovascular function.4 Importantly, at W→C it could be argued that the ‘At Risk’ group was better off than the Younger group, owing to greater reductions in left ventricular work (stroke work) (Fig. 7). That said, the ramifications of the greater swings in hemodynamic and cardiac stress indices observed in the ‘At Risk’ group between C→W and W→C remains to be seen. Furthermore, despite these observations, it is likely that the utilization of thermal behavior successfully attenuates the risk of cardiovascular events in ‘At Risk’ older adults compared to if behavioral thermoregulation was not employed. Although this is a logical conclusion, direct evidence is required.
Given that there were no differences in TPR (Fig. 6) or SkBF/CVC (Fig. 3) at C→W between groups, the reasons underlying the differential changes in blood pressure at C→W are unclear from the present study. However, this may be due to subtle differences in cardiac output that failed to reach statistical significance in the current study (P = 0.12) and/or that there were differences in vascular resistance in areas not assessed (e.g., in the viscera68). Furthermore, despite no differences between groups regarding changes in heart rate, cardiac output, and TPR at C→W, the ‘At Risk’ group demonstrated increases in stroke volume (Fig. 6). This finding is supported by previous data indicating that during more severe cold stress stroke volume is largely unchanged in healthy young adults, but increases in older adults.8 The present study extends these findings to indicate that the modest thermal stress incurred prior to initiating thermal behavior in a cool environment is sufficient to evoke differential changes in stroke volume that are not buffered by reductions in heart rate (Fig. 6). Collectively, this contributed to the exacerbated increases in stroke work at C→W (Fig. 7).
The reasons for the greater hypotension observed at W→C in the ‘At Risk’ group are not likely due to differential changes in cardiac output (Fig. 6), TPR (Fig. 6), or forearm SkBF/CVC (Fig. 3). It is tempting to suggest that the greater hypotension may be due to greater increases in glabrous SkBF/CVC (Fig. 3). However, this is unlikely given that the modest increases in body temperature at W→C would have only increased glabrous skin blood flow in the hands and feet by a combined ∼0.1 L/min,69 which comprises a very small proportion of cardiac output (less than ∼2%). Thus, it is likely that the greater hypotension observed at W→C in the ‘At Risk’ group is due to the aforementioned delays in baroreflex responsiveness during baroreceptor unloading induced by heat stress.63 However, direct evidence is required.
Considerations
There are a number of methodological considerations that warrant attention. First, we included a relatively low number of subjects in our ‘At Risk’ older group. This may have limited our statistical power and challenged our ability to identify statistical significance in some instances. In the interest of full disclosure, therefore, we have presented our data as mean ± SD and have reported actual p-values throughout the manuscript. We believe this best allows the reader to independently interpret our data. Second, our ‘At Risk’ group was relatively heterogeneous and medications were not withheld. This was done to improve the external validity of the study. However, it should be noted that the independent effect of the disease process, in the absence of prescription medication, on behavioral thermoregulation and associated hemodynamic responses in older adults with cardiovascular co-morbidities remains unknown. Moreover, further studies should aim to delineate the independent impact of each cardiovascular co-morbidity independently. Nevertheless, to our knowledge this is the first study of its kind. Thus, this study should be considered as a first step towards targeted studies aiming to examine the independent and combined effects of cardiovascular co-morbidities on thermoregulatory behavior and the cardiovascular consequences in older adults. Third, we assumed that a lack of change in dosage was indicative of a stable disease state. This does not take into account compliance with taking the prescription medications and we do not have blood data to support if the drugs were appropriate at controlling cholesterol and/or glucose (e.g., HbA1c) levels. Fourth, the control group in the present study was comprised of young, healthy adults. As a result, the independent contribution of aging on the primary dependent variables in the present study remains unknown. Fifth, Modelflow underestimates stroke volume during more severe whole-body heat stress.70 The use of Modelflow in the present study was deemed acceptable because there are no other methods that can provide a beat-to-beat measure of stroke volume and because it is unlikely that moderate changes in skin temperature modify aortic impedance and compliance, factors proposed as the reason why Modelflow is inaccurate during passive heat stress.70 Notably, however, whether this holds true in older adults with cardiovascular co-morbidities during thermal stress is unknown. Sixth, because intestinal temperature is slower to change to alterations in body heat loss or gain compared to esophageal temperature,71 our measurement of intestinal temperature may not have been sensitive enough to detect small changes in body heat content between groups. Thus, future studies should employ the measurement of esophageal temperature. Finally, given that the accuracy of mean skin temperature measurements is generally improved as the number of measurement locations increases,72,73 our 6 site mean skin temperature measurement may not have been as robust as the measurement of ≥10 sites.72
Perspectives
It is predicted that the frequency and severity of heat events will increase in the coming decades, while periodic cold events will persist.74 Cardiovascular health is particularly susceptible to hot and cold ambient temperatures.1 Such deleterious health outcomes have been suggested to be driven by the cardiovascular adjustments that occur secondary to changes in body temperature.75,76 These adjustments can acutely increase cardiovascular risk,4 particularly in populations with cardiovascular co-morbidities. The present study demonstrates exaggerated hemodynamic adjustments upon the initiation of thermoregulatory behavior in older adults with cardiovascular co-morbidities. These differential hemodynamic changes occurred despite that thermal behavior was elicited by relatively modest changes in body temperature that did not differ from young, healthy adults and that these ‘at risk’ older adults were appropriately medicated for their conditions. These findings suggest that ‘at risk’ older adults do not likely utilize thermal behavior in such a manner that protects them against thermal induced hemodynamic changes that can provoke cardiovascular events. However, it is likely that the utilization of thermal behavior attenuates the risk of cardiovascular events compared to if behavioral thermoregulation was not employed and body temperatures deviated to a greater extent.
Conclusions
The present study demonstrates that the initiation of thermal behavior in older adults with cardiovascular co-morbidities is preceded by exaggerated reductions in blood pressure in a warm environment and increases in blood pressure in a cool environment. These divergent blood pressure responses generally resulted in exacerbated changes in indices of cardiac stress in these ‘at risk’ older adults. Notably, these hemodynamic differences occurred despite that the utilization of thermal behavior did not differ between this population and healthy, younger adults. Collectively, these findings suggest that the appropriate utilization of thermal behavior in older adults with cardiovascular co-morbidities does not protect them against thermal induced hemodynamic changes that can challenge cardiovascular function.
Funding Statement
This study was not grant funded.
Abbreviations
- Δ
Change from baseline
- C→W
Decision to move from the cool to the warm room
- CVC
Cutaneous vascular conductance
- PU
Perfusion units
- RER
Respiratory exchange ratio
- RPP
Rate pressure product
- SkBF
Skin blood flow
- TPR
Total peripheral resistance
- Tbody1:1
mean body temperature with internal and mean skin temperatures weighted 1:1
- Tbody3:1
mean body temperature with internal and mean skin temperatures weighted 3:1
- Tbody9:1
mean body temperature with internal and mean skin temperatures weighted 9:1
- W→C
Decision to move from the warm to the cool room
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the subjects for participating in our study. We would also like to thank Lindsey Russo, MS, for her technical assistance.
Author contributions
| ZJS | GLC | JRS | SS | CLC | DH | BDJ | |
|---|---|---|---|---|---|---|---|
| Conception and design | X | X | X | ||||
| Performed experiments | X | X | X | X | X | X | X |
| Analyzed data | X | X | X | ||||
| Interpreted results | X | X | X | ||||
| Prepared figures | X | ||||||
| Drafted manuscript | X | ||||||
| Edited and revised manuscript | X | X | X | X | X | X | X |
| Approved final version of manuscript | X | X | X | X | X | X | X |
References
- 1.Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiol. 2009;20:205. doi: 10.1097/EDE.0b013e318190ee0810.1097/01.ede.0000362325.91185.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ. 2010;182:1053-60. doi: 10.1503/cmaj.081050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson TE, Klabunde RE, Monahan KD. Using thermal stress to model aspects of disease states. J Therm Biol. 2014;43:24-32. doi: 10.1016/j.jtherbio.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863-72. doi: 10.1161/CIRCULATIONAHA.105.596189 [DOI] [PubMed] [Google Scholar]
- 5.Lucas RA, Sarma S, Schlader ZJ, Pearson J, Crandall CG. Age‐related changes to cardiac systolic and diastolic function during whole‐body passive hyperthermia. Exp Physiol. 2015;100:422-34. doi: 10.1113/expphysiol.2014.083014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323-32 [DOI] [PubMed] [Google Scholar]
- 7.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076-82. doi: 10.1152/japplphysiol.00605.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1627-R33. doi: 10.1152/ajpregu.00099.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greaney JL, Kenney WL, Alexander LM. Neurovascular mechanisms underlying augmented cold‐induced reflex cutaneous vasoconstriction in human hypertension. J Physiol. 2017;595:1687-98. doi: 10.1113/JP273487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greaney JL, Kenney WL, Alexander LM. Sympathetic function during whole-body cooling is altered in hypertensive adults. FASEB J. 2017;31:847.1-.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney WL, Munce TA. Aging and human temperature regulation. J Appl Physiol. 2003;95:2598-603. doi: 10.1152/japplphysiol.00202.2003 [DOI] [PubMed] [Google Scholar]
- 12.Kenney W, Kamon E, Buskirk E. Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J Applied Physiol. 1984;56:930-5. [DOI] [PubMed] [Google Scholar]
- 13.Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature. 2016;3:119-45. doi: 10.1080/23328940.2015.1131506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol. 2011;589:4787-97. doi: 10.1113/jphysiol.2011.212100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlader ZJ, Stannard SR, Mundel T. Human thermoregulatory behavior during rest and exercise – a prospective review. Physiol Behav. 2010;99:269-75. doi: 10.1016/j.physbeh.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Hardy JD. Thermal comfort and health. Ashrae J. 1971;13:43-51. [Google Scholar]
- 17.Cabanac M, Bleichert R, Massonne B. Preferred skin temperature as a function of internal and mean skin temperature. J Appl Physiol. 1972;33:699-703 [DOI] [PubMed] [Google Scholar]
- 18.Schlader ZJ, Perry BG, Che Jusoh MR, Hodges LD, Stannard SR, Mundel T. Human temperature regulation when given the opportunity to behave. Eur J Appl Physiol. 2013;113:1291-301. doi: 10.1007/s00421-012-2544-0 [DOI] [PubMed] [Google Scholar]
- 19.Schlader ZJ, Prange HD, Mickleborough TD, Stager JM. Characteristics of the control of human thermoregulatory behavior. Physiol Behav. 2009;98:557-62. doi: 10.1016/j.physbeh.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 20.Schlader ZJ, Sarker S, Mundel T, Coleman G, Chapman CL, Sackett JR, Johnson BD. Hemodynamic responses upon the intiation of thermoregulatory behavior in young healthy adults. Temperature. 2016;3:271-85. doi: 10.1080/23328940.2016.1148938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlader ZJ, Coleman GL, Sackett JR, Sarker S, Chapman CL, Johnson BD. Activation of autonomic thermoeffectors preceding the decision to behaviorally thermoregulate in resting humans. Exp Physiol. 2016;101:1218-29. doi: 10.1113/EP085837. [DOI] [PubMed] [Google Scholar]
- 22.Taylor NAS, Allsopp NK, Parkes DG. Preferred room temperature of young vs aged males – the influence of thermal sensation, thermal comfort, and affect. J Gerontol A – Biol. 1995;50:M216-M21. doi: 10.1093/gerona/50A.4.M216. [DOI] [PubMed] [Google Scholar]
- 23.Collins KJ, Extonsmith AN, Dore C. Urban hypothermia – preferred temperature and thermal perception in old-age. Brit Med J. 1981;282:175-7. doi: 10.1136/bmj.282.6259.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe JP, Moore RE. Physiological and behavoural responses of aged men to passive heating. J Physiol. 1974;236 (Proceedings):43-5 [PubMed] [Google Scholar]
- 25.Ohnaka T, Tochihara Y, Tsuzuki K, Nagai Y, Tokuda T, Kawashima Y. Preferred temperature of the elderly after cold and heat exposures determined by individual self-selection of air temperature. J Therm Biol. 1993;18:349-53. doi: 10.1016/0306-4565(93)90058-2. [DOI] [Google Scholar]
- 26.Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K. Preferred ambient temperature for old and young men in summer and winter. Int J Biometeorol. 1992;36:1-4. doi: 10.1007/BF01208726 [DOI] [PubMed] [Google Scholar]
- 27.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am College Cardiol. 2001;37:1344-50. doi: 10.1016/S0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 28.B-q Zhu, RE Sievers, Browne AE, Lee RJ, Chatterjee K, Grossman W, Karliner JS, Parmley WW. Comparative effects of aspirin with ACE inhibitor or angiotensin receptor blocker on myocardial infarction and vascular function. J Renin-Angiotensin-Aldosterone Sys. 2003;4:31-7. doi: 10.3317/jraas.2003.005. [DOI] [PubMed] [Google Scholar]
- 29.Westaway K, Frank O, Husband A, McClure A, Shute R, Edwards S, Curtis J, Rowett D. Medicines can affect thermoregulation and accentuate the risk of dehydration and heat‐related illness during hot weather. J Clin Pharmacy Therapeutics. 2015;40:363-7. doi: 10.1111/jcpt.12294. [DOI] [PubMed] [Google Scholar]
- 30.Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;73:863-71. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 31.Siri WE. Body composition from fluid spaces and density: analysis of methods In: Brozek J, Henschel A, eds. Techniques for measuring body composition. Washington DC: National Academy of Sciences, National Research Council, 1961:223-43. [Google Scholar]
- 32.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497-504. doi: 10.1079/BJN19780152 [DOI] [PubMed] [Google Scholar]
- 33.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175-81. doi: 10.1249/00005768-198023000-00009 [DOI] [PubMed] [Google Scholar]
- 34.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al.. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-9. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 36.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586-92 [DOI] [PubMed] [Google Scholar]
- 37.Frank SM, Raja SN, Bulcao CF, Goldstein DS. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol. 1999;86:1588-93 [DOI] [PubMed] [Google Scholar]
- 38.Bulcao CF, Frank SM, Raja SN, Tran KM, Goldstein DS. Relative contribution of core and skin temperatures to thermal comfort in humans. J Therm Biol. 2000;25:147-50. doi: 10.1016/S0306-4565(99)00039-X. [DOI] [Google Scholar]
- 39.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav. 2011;103:217-24. doi: 10.1016/j.physbeh.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 40.Guéritée J, Redortier B, House JR, Tipton MJ. Thermal comfort following immersion. Physiol Behavior. 2015;139:474-81. doi: 10.1016/j.physbeh.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol. 2006;100:1692-701. doi: 10.1152/japplphysiol.01124.2005 [DOI] [PubMed] [Google Scholar]
- 42.Hardy JD, Dubois EF. Basal metabolism, radiation, convection, and vapourization at temperatures 22°C to 35°C. J Nutr. 1938;15:477. [Google Scholar]
- 43.Wesseling K, Jansen J, Settels J, Schreuder J. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566-73 [DOI] [PubMed] [Google Scholar]
- 44.Wilson T, Brothers R, Tollund C, Dawson E, Nissen P, Yoshiga C, Jons C, Secher NH, Crandall C. Effect of thermal stress on Frank–Starling relations in humans. J Physiol. 2009;587:3383-92. doi: 10.1113/jphysiol.2009.170381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gobel FL, Norstrom L, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549-56. doi: 10.1161/01.CIR.57.3.549 [DOI] [PubMed] [Google Scholar]
- 46.Schlader ZJ, Mundel T, Barnes MJ, Hodges LD. Peak cardiac power output in healthy, trained males. Clin Physiol Funct Imaging. 2010;30:480-4. doi: 10.1111/j.1475-097X.2010.00959.x [DOI] [PubMed] [Google Scholar]
- 47.Graichen H, Rascati R, Gonzalez RR. Automatic dew-point temperature sensor. J Appl Physiol. 1982;52:1658-60 [DOI] [PubMed] [Google Scholar]
- 48.Cramer MN, Jay O. Biophysical aspects of human thermoregulation during heat stress. Autonomic Neuroscience. 2016;196:3-13. doi: 10.1016/j.autneu.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 49.Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1:1-20. doi: 10.1016/0013-9351(67)90002-3 [DOI] [PubMed] [Google Scholar]
- 50.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377-90 [DOI] [PubMed] [Google Scholar]
- 51.Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol. 2012;590:5963-73. doi: 10.1113/jphysiol.2012.240739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCullough EA, Jones BW, Huck J. A comprehensive data base for estimating clothing insulation. publisher not identified; 1985. ASHRAE Report No. 2888 (RP-411). [Google Scholar]
- 53.Schlader ZJ, Stannard SR, Mundel T. Evidence for thermoregulatory behavior during self-paced exercise in the heat. J Therm Biol. 2011;36:390-6. doi: 10.1016/j.jtherbio.2011.07.002. [DOI] [Google Scholar]
- 54.Mundel T, Raman A, Schlader ZJ. Head temperature modulates thermal behaviour in the cold in humans. Temperature. 2016;3:298-306. doi: 10.1080/23328940.2016.1156214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barber BJ, Crawford EC. Dual threshold control of peripheral temperature in the lizard dipsosaurus-dorsalis. Physiol Zool. 1979;52:250-63. doi: 10.1086/physzool.52.2.30152568. [DOI] [Google Scholar]
- 56.Cranston W, Gerbrandy J, Snell E. Oral, rectal and oesophageal temperatures and some factors affecting them in man. J Physiol. 1954;126:347. doi: 10.1113/jphysiol.1954.sp005214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kingma BR, Frijns AJ, Saris WH, Van Steenhoven A, van Marken Lichtenbelt W. Cold-induced vasoconstriction at forearm and hand skin sites: the effect of age. European J Applied Physiol. 2010;109:915-21. doi: 10.1007/s00421-010-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson J, Pergola P, Liao F, Kellogg D, Crandall C. Skin of the dorsal aspect of human hands and fingers possesses an active vasodilator system. J Applied Physiol. 1995;78:948-54. [DOI] [PubMed] [Google Scholar]
- 59.Romanovsky A. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210:498-507. doi: 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukazawa T, Havenith G. Differences in comfort perception in relation to local and whole body skin wettedness. Eur J Appl Physiol. 2009;106:15-24. doi: 10.1007/s00421-009-0983-z [DOI] [PubMed] [Google Scholar]
- 61.Hardy JD. Thermal comfort: skin temperature and physiological thermoregulation In: Hardy JD, Gagge AP, Stolwijk JA, eds. Physiological and Behavioral Temperature Regulation. Springfield, IL, USA: Charles C. Thomas Publisher, 1970. [Google Scholar]
- 62.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol. 2015;593:2225-35. doi: 10.1113/JP270162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucas RA, Cotter JD, Morrison S, Ainslie PN. The effects of ageing and passive heating on cardiorespiratory and cerebrovascular responses to orthostatic stress in humans. Exp Physiol. 2008;93:1104-17. doi: 10.1113/expphysiol.2008.042580 [DOI] [PubMed] [Google Scholar]
- 64.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68-72. doi: 10.1161/01.HYP.31.1.68 [DOI] [PubMed] [Google Scholar]
- 65.Pikkujämsä SM, Huikuri HV, Airaksinen KJ, Rantala AO, Kauma H, Lilja M, Savolainen MJ, Kesäniemi YA. Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertension. 1998;11:523-31. doi: 10.1016/S0895-7061(98)00035-1. [DOI] [PubMed] [Google Scholar]
- 66.Madden KM, Lockhart C, Potter TF, Cuff D. Aerobic training restores arterial baroreflex sensitivity in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Clin J Sport Med. 2010;20:312. doi: 10.1097/JSM.0b013e3181ea8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewandowski J, Siński M, Bidiuk J, Abramczyk P, Dobosiewicz A, Ciarka A, Gaciong Z. Simvastatin reduces sympathetic activity in men with hypertension and hypercholesterolemia. Hypertension Res. 2010; 33:1038-43. doi: 10.1038/hr.2010.137. [DOI] [PubMed] [Google Scholar]
- 68.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol. 2007;103:1257-62. doi: 10.1152/japplphysiol.00401.2007 [DOI] [PubMed] [Google Scholar]
- 69.Caldwell JN, Matsuda-Nakamura M, Taylor NA. Three-dimensional interactions of mean body and local skin temperatures in the control of hand and foot blood flows. Eur J Appl Physiol. 2014;114:1679-89. doi: 10.1007/s00421-014-2894-x [DOI] [PubMed] [Google Scholar]
- 70.Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol. 2011;300:R486-R91. doi: 10.1152/ajpregu.00505.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mündel T, Carter JM, Wilkinson DM, Jones DA. A comparison of rectal, oesophageal and gastro‐intestinal tract temperatures during moderate‐intensity cycling in temperate and hot conditions. Clin Physiol Funct Imaging. 2014;36:11-6. [DOI] [PubMed] [Google Scholar]
- 72.Liu W, Lian Z, Deng Q, Liu Y. Evaluation of calculation methods of mean skin temperature for use in thermal comfort study. Build Environ. 2011;46:478-88. doi: 10.1016/j.buildenv.2010.08.011. [DOI] [Google Scholar]
- 73.Mitchell D, Wyndham CH. Comparison of weighting formulas for calculating mean skin temperature. J Appl Physiol. 1969;26:616-22 [DOI] [PubMed] [Google Scholar]
- 74.IPCC. Climate Change 2013 – The Physical Science Basis. New York, NY, USA: Cambridge University Press, 2013. [Google Scholar]
- 75.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging and human cardiovascular health. Med Sci Sport Exer. 2014;46:1891-9. doi: 10.1249/MSS.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Blois J, Kjellstrom T, Agewall S, Ezekowitz J, Armstrong P, Atar D. The effects of climate change on cardiac health. Cardiology. 2015;131:209-17. doi: 10.1159/000398787 [DOI] [PubMed] [Google Scholar]
- 77.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal cognitive assessment (MoCA) in a population-based sample. Neurology. 2012;77:1272-5. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]


