ABSTRACT
Neurons are highly polarized cells that exhibit one of the more complex morphology and function. Neuronal intracellular trafficking plays a key role in dictating the directionality and specificity of vesicle formation, transport and fusion, allowing the transmission of information in sophisticate cellular network. Thus, the integrity of protein trafficking and spatial organization is especially important in neuronal cells. RAB proteins, small monomeric GTPases belonging to the RAS superfamily, spatially and temporally orchestrate specific vesicular trafficking steps.
In this review we summarise the known roles of RAB GTPases involved in the maintenance of neuronal vesicular trafficking in the central nervous system. In particular, we discriminate the axonal pre-synaptic trafficking and dendritic post-synaptic trafficking, to better underlie how a correct orchestration of vesicle movement is necessary to maintain neuronal polarity and then, to permit an accurate architecture and functionality of synaptic activity.
KEYWORDS: axon, dendrite, neuronal cells, neuronal trafficking, post-synaptic, pre-synaptic, RAB GTPases, synaptic function, vesicle transport
Introduction
Neurons are the most sophisticated cells able to discriminate and process an enormous variety of events in the environment, forming a complex network in the nervous system. The complex morphology and the specialized compartments, in the form of cell body, multiple dendrites, one axon and pre- and post-synaptic buttons, are necessary for synaptic transmission.1,2 The formation and conservation of the asymmetric domains as well as the generation and the maintenance of connectivity need a continuous membrane supply, a correct distribution of membrane receptors, adhesion molecules and intracellular mediators.3 Thus, the integrity of protein trafficking and spatial organization are especially important in neuronal cells, because they crucially determine morphogenesis and connectivity, information processing and synaptic plasticity.4,5
RAB proteins, small monomeric GTPases belonging to the RAS superfamily, are involved in correct vesicle sorting, fission, transport, tethering, docking and fusion, by the interaction with effector proteins.6-8
RAB proteins count for more than 60 members in eukaryotes and act as a network to orchestrate the transport of specific vesicles both spatially and temporally.9-11 They coordinate this function from the ability to switch from an inactive Guanosine-5′-DiPhosphate (GDP)-bound state to an active Guanosine-5′-TriPhosphate (GTP)-bound state. To ensure the correct coordination between GDP release and GTP hydrolysis, accessory regulatory proteins are necessary to guarantee and accelerate the switch:12 RAB GDP dissociation inhibitor (GDI),13,14 Guanine nucleotide exchange factor (GEF) and GTPase-activating protein (GAP).15 Additionally, RAB GTPases are physically associated with specific organelles,9,16 becoming the first true molecular markers to discriminate the membrane compartments of the endocytic and secretory pathway.17 However, the exact cellular localization and tissue expression profile of many RABs remain unidentified. To date, 24 RAB proteins are documented to play a role in brain, driving different and sequentially steps of neuronal trafficking.18
In this review we focus on a status update on the role played by RAB GTPases in intracellular neuronal vesicular trafficking in the central nervous system. The intrinsic complexity of RAB family proteins and the sophisticate neuronal morphology and specialized compartmentalization, require a systematic description of RAB GTPases-mediated trafficking, from the cell body, to reach, by traveling through the axon and dendrites, the final destination, the pre- and post-synaptic compartments for synaptic function. Indeed, a complete and overall perspective of how RAB GTPases operate in neurons is crucial for a better comprehension of multiple aspects of neuronal physiology and pathophysiology.
RAB GTPases involved in the first steps of neuronal trafficking: at the cell body
The importance of the entire neuronal trafficking, from the cell body to the synaptic sites, for synaptic activity was well described by Waller during a lecture he delivered at the Royal Institution of Great Britain in 1861: “A nerve cell would be to its effluent nerve fibers what a fountain is to the rivulet which trickles from it – a center of nutritive energy.” The nutritive energy comes from the protein synthesis that initially occurs in the cell body where they start their route.
Initially, proteins take on secretory pathway where a series of RAB GTPases work together regulating anterograde, such as RAB1,19 RAB2,20 RAB8,21 RAB39B22 and retrograde, such as RAB7,23 RAB6,24 RAB33B,25 RAB226 vesicular trafficking/transport. Here, impairments in the activity of RAB proteins perturb Endoplasmic Reticulum (ER) and Golgi structures20 leading to alterations in axonal and dendritic outgrowth,2 as well as impacting on a correct synaptic formation.27-29
Then, traffic though the Golgi compartment is responsible for secretion of proteins targeted to the membrane. In particular neurons count several types of secreted vesicles that are differentially targeted to dendrites and/or to the axon to maintain cellular polarity. Dendritic transport polarization is well established because vesicles containing dendritic proteins are bi-directionally transported via microtubules in dendrites but not in axons. Axonal polarization is more complicated because some vesicles containing axonal proteins undergo toward axon but also dendrites; the polarity is guaranteed by endocytosis that avoid the accumulation of axonal proteins of dendritic surface.30,31
For neuronal cells it is important to maintain a well-regulated dendritic and axonal transport to establish and preserve the correct polarized morphology. A right cell polarization is in turn essential for the organization and maintenance of synaptic activity.5
RAB GTPases involved in axonal trafficking
The secretory process in neurons is strictly similar to that in other cells but the axon-terminal, the primary target of secretion, is greatly distant from the cell body (up to a meter in length). Two types of vesicle traffic exist in the axon: slow and fast transport.32 Cytosolic and cytoskeletal proteins move in the anterograde direction by the slow axonal transport with kinetics of 0.01-0.001 µm/sec. Instead membranous organelles move in the anterograde and retrograde direction by fast axonal transport at 1-5 µm/sec.33 Several RAB GTPases control protein transport in the axon to guarantee, at first, the right axonal development and then, a proper pre-synaptic functionality.
RAB proteins-mediated trafficking during neuronal development for axonal polarization
Some RAB proteins are key masters of axonal polarization and elongation through membrane precursor vesicles or neurotrophic receptors transport.
RAB10 is involved in regulating activities to induce axonal polarization and elongation. Following RAB10 release from GDI by Lgl1,34 RAB10 mediates trafficking from trans-Golgi network (TGN) to the plasma membrane of membrane precursor vesicles by MyosinVb35 and kinesin-depending movement.28 Finally RAB10 binds to Myristoylated Alanin-Rich C-Kinase Substrate (MARCKS) at the plasma membrane to make the docking and fusion to the plasma membrane of RAB10-exocytic carriers, resulting in axonal outgrowth.36 It has been demonstrated that the expression of wild type (WT) or constitutively active form of RAB10 (RAB10-Q68L) leads to axonal arborization; in contrast the dominant negative form of RAB10 (RAB10-T23N) inhibits this process.34,37 Moreover in Rab10 – null mouse, target disruption of Rab10 leads abnormal endosomes and ER hyperplasia disturbing the general balance of membrane trafficking, resulting to early embryonic lethality (from E9.5).38
RAB33A, a TGN-related RAB protein, mediates anterograde synaptophysin-positive vesicles trafficking impacting on axonal outgrowth. In particular, in hippocampal neurons RAB33A downregulation negatively affects neuronal polarization, at contrary the overexpression of the constitutively active mutant (RAB33A-Q95L) leads to multi-axonic neurons.39
RAB4 and RAB11 are involved in axonal outgrowth by working in endosome recycling, which can be classified in fast and slow routes depending on the presence of RAB4- and RAB11-positive recycling endosomes, respectively.8
In Xenopus retinal ganglion cells, RAB4 is recruited to the endosomes in the growth cone, and RAB4 downregulation or the dominant negative RAB4 mutant (RAB4-N121I) decrease axonal elongation.40 RAB4 upregulation was found in patients with Alzheimer's disease and mild cognitive disorder,41,42 indicating that deficits in neuronal endosomal sorting may establish these disorders.43
In rat cortical neurons, RAB11 improves axonal elongation following the upstream activation of signaling cascade controlled by cyclin-dependent protein kinase 5 (CDK5).44 Overexpression of RAB11 WT and the RAB11 constitutively active mutant (RAB11-Q70L) promote axonal elongation in rat cultured cortical neurons. In contrast RAB11 downregulation45 or dominant negative expression form of RAB11 (RAB11-S25N) in the striatum and cortex of normal mice lead to decreased axonal length causing neuropathology and motor dysfunctions.46 Moreover, alteration in RAB11 GDP to GTP conversion was described in a mouse model for Huntington's disease, supporting the idea that defects in vesicle formation impact on early stages of the synaptic dysfunctions in this disorder.47,48 Indeed Huntington's disease phenotype was partially rescued upon enhancing RAB11 activity.49,50 However, in Nerve Growth Factor (NGF)-stimulated PC12 cells it was described an increase in the interaction between phosphorylated protrudin and the inactive GDP-bound form of RAB11.51 This evidence raises some question on which form of RAB11, the GDP- or GTP-bound form, impacts on axonal outgrowth. Given that the majority of RAB11 studies have found that RAB11-GTP enhances axonal elongation, it is conceivable that protrudin promotes RAB11-GDP to the cell periphery before its activation.52 Recently, it was showed that RAB11 works together with RAB25 and RAB14 in the N2A cells53 in regulating axonal outgrowth, however additional work is necessary to clarify this mechanism.
RAB5 and RAB7 have been shown to sequentially mediate the retrograde transport of NGF and neurotrophin receptors.40,54,55 After internalisation by clathrin-mediated endocytosis or micropinocytosis, neurotrophin receptors are clustered into RAB5-positive early endosomes56 thanks to Phosphatidil Inositol 3-kinase (PI3K) activity. Indeed, PI3K allows a production of Phosphatidil Inositol 3-Phospate (PI(3)P)57 which binds the Early Endosome Antigen 1 (EEA1) tethering factor, which in turn permits the RAB5-positive endosomes fusion in order to concentrate cargos58 and consequently the advance into the RAB7-controlled late endosomal pathway.55 RAB7 is considered a marker for the axonal retrograde transport.54 The expression of RAB7 dominant negative mutant (RAB7-T22N) allows an accumulation and a prolonged persistence of internalized neurotrophin receptors, leading to axonal degeneration.59 Misregulation of both RAB5 and RAB7 were also found associated with human neurodegenerative diseases, such as Alzheimer's disease and Down Syndrome, leading an enlargment of the endosomes in neuronal cells, finally causing the cell death.60-62 In particular they were upregulated in specific human post-mortem brain regions, as basal forebrain, frontal cortex, and hippocampus but not in cerebellum and striatum. Upregulation of endosomal vesicle trafficking is also associated to Parkinson's disease where an increased RAB5 was found in the striatal neurons.63 These evidences raised the hypothesis that a tight link between protein-compartment-marker and the vulnerability of cell types within selective brain regions exists.42
A new role played by RAB7 is in neurite outgrowth, by interconnecting secretory and endocytic pathway by permitting repeated late endomoses (LE)-ER contacts. RAB7, binding the ER protein prodrutin, mediates transfer of vesicles to LE and promotes the microtubule-dependent translocation of LEs to the cell periphery and subsequently synaptotagmin-VII-dependent fusion with the plasma membrane in PC12 cell lines.64 Moreover expression of mutated forms of RAB7, mutations causing Charco-Marie-Tooth type 2B (CMT2B) neuropathy, leads a marked inhibition of neurite outgrowth in PC12 and N2A cell lines.65
The anterograde transport of neurotrophin receptors, in particular of TrkB, is regulated by RAB27B. Given to the generation of LacZ-Rab27b knockout mouse, RAB27B was found to be the isoform of RAB27 higher expressed in mammalian neurons.66 RAB27B together with Slp1/CRMP-2 complex directly moves TrkB-containing vesicles to the neuronal membrane, by Kinesin-1 dependent transport. Downregulation of RAB27B decreases the axonal membrane targeting of TrkB,67 however the Rab27b mouse model shows a normal development and behavior.66
RAB35 has a role also in neurite outgrowth in PC12 cell line. It determines the localization of MICAL-L1, a multiple RAB-binding protein, to Arf6-positive recycling endosomes. In turn MICAL-L1 functions as a scaffold for the recruitment of RAB8, RAB13 and RAB26.68 In particular RAB35 permits axonal elongation in rat primary neurons thanks to Microtubule-associated protein 1B (MAP1B) which, interacting with p53-related protein kinase (PRPK), protects RAB35 from ubiquitin-proteasome degradation pathway.69
RAB13, as mentioned before, supports neurite outgrowth in PC12 and DRG cell lines, regulating the transport of membrane-containing vesicles from TGN to recycling endosomes.70 In cultured Dorsal Root Ganglion (DRG) neurons, RAB13 co-localizes with the Growth Associated Protein 43 (GAP43) in neurites and in the growth cones. Expression of the constitutive active form of RAB13 (RAB13-Q67L) promotes neurite extension in the PC12 cell line when stimulated with NGF.71 At contrary the downregulation of RAB13 by RNA interference inhibits neurite outgrowth in NGF-treated PC12 cells.72 In particular RAB13, together with Corinin1b, is required for axonal regeneration in mice following the transcriptional regulation control of the tumor suppressor p53.70 Indeed after facial nerve axotomy, a model for neuronal regeneration,73 p53 null mice did not show expression of Coronin1b and RAB13 in axonal sprouts in regenerating facial nuclei as in WT mice.72
RAB proteins-mediated trafficking for pre-synaptic function
At last, a proper pre-synaptic functionality is ensured by the organelles trafficking including vesicles of the constitutive secretory pathway, synaptic vesicle precursor membranes, mitochondria and elements of smooth ER and in particular neurotransmitters. Neurotransmitters are packaged into synaptic vesicles (SVs) and clustered at the pre-synaptic membrane in the axon-terminal, on the pre-synaptic side of a synapse. In response to a local transient increase in Ca2+ concentration at the active zone following the arrival of an action potential, SVs, that enclose neurotransmitters and are properly docked and primed, fuse with the pre-synaptic membrane and release the content in the synaptic cleft.74 The recycling of SVs by endocytosis ensures the refilling of SV reserve. For any SV cycling step, there are specific RAB GTPases that guarantee the timing and the correct directionality for vesicles.
SV docking and exocytosis are controlled by RAB3 subfamily and RAB27B.
RAB3 subfamily is composed of RAB3A, RAB3B, RAB3C and RAB3D. They are highly expressed in the murine brain74 and it is the first group of RAB GTPases associated with a neuronal specific pathway.27 RAB3A, the most investigated RAB of RAB3 subfamily, is required for the assembly and transport of vesicles in fast anterograde axonal transport by a Kinesin1-dependent transport.75 One of the RAB3 known effector protein is RIM1, considered the core component of the active zone. It forms a trimeric complex with RAB3 and MUNC1376 recruiting SVs to the active zone and organizing them for release.77 Single, double, triple and quadruple Rab3 KO mice were generated and they succumb when one of deleted RAB3 is RAB3A. In particular quadruple Rab3 KO mice, that are born alive and die to respiratory failure, show no apparent changes in synapse structure or brain composition except for a mild reduction of 30% in neurotransmitter release.78 This may be due to role played by RAB27B, which is structurally tight related to RAB3. RAB27B localization partially overlaps with RAB3 wherewith shares regulator and effector proteins.79,80 Overexpression of constitutive active (RAB27B-Q78L) or inactive (RAB27B-N133I) RAB27B mutants in murine neurons causes a strong reduction in SV recycling,79 however the mechanism has to be further investigated.
Recycling of SVs by endocytosis is directed through several pathways. One is identified as kiss-and-run and vesicles are undocked and recycled locally. Three mechanisms of SV retrieval consist in full fusion of vesicles with the plasma membrane: clathrin-mediated endocytosis (CME), which retrieves SVs during mild synaptic stimulation,81 clathrin-independent bulk endocytosis (CIE), which permits invagination of a large region of the plasma membrane during an intense stimulus,82 and ultra-fast endocytosis, which retrieves single, large endocytic vesicles next to the pre-synaptic densities (speed: 50-100 ms) in response to a single stimulus or during mild stimulation.83,84
Endocytic events are regulated by RAB4, RAB5, RAB10, RAB11, RAB14, RAB35 and RAB7.29,85
RAB5 localizes to a subset of SVs and it is principally involved in SV retrieval, recycling and SV uniformity size control: it orchestrates early endosomes by step-wise recruitment of effector proteins (Rabaptin5/Rabex5/PI(3)P/Vps34/EEA1/Rabenosyn5) to endosomal micro-domains.86-88 Alterations in RAB5 affect formation and composition of endosomal compartment at the nerve terminal and impair SV recycling leading to altered synaptic transmission in Drosophila.87 In rat hippocampal neurons overexpression of RAB5A reduced the recycling pool size by 50%.89
A functional screen on Drosophila90 and C. elegans91,92 models to assess the impact of a battery of constitutively active RABs on SV cycling, identified RAB35, RAB7, RAB11 and RAB10 as putative regulators of post-endosomal trafficking of SVs. RAB4, RAB5 and RAB11 collaborate in regulating different steps of the endosome recycling80 RAB7, driving LE-positive vesicles from RAB5-positive EE, participates in several steps of the autophagic pathway: from maturation to the trafficking process of autophagosomes and amphisomes.93 RAB10 and RAB14 are implicated in clathrin-coated traffic and recycling pathways.80,94,95 RAB35, previously described having a role in axonal and neurite outgrowth, is important for regeneration of new SVs, indeed the expression of the constitutively active RAB35 mutant or loss-of-function of its GAP, TBC1D24/Skywalker, allows a recovery of SVs via endosomal intermediates and increases synaptic transmission.2,90 However, how these RABs operate in mammalian pre-synaptic buttons remains to be investigated.85
RAB35 also plays a role in SV degradation and turnover. In cultured rat hippocampal neurons GTP-RAB35 recruits the Endosomal Sorting Complex Required for Transport (ESCRT)-0 protein Hsr to SV pools to initiate ESCRT complex formation, then mediating the degradation of SV integral membrane proteins SV2 and VAMP2.96
Recent studies provided the link between SV-associated RAB26 and the autophagic pathway. RAB26 binds the autophagosomal markers ATG16L1, the complex was found in a subset of Synaptobrevin and RAB3A-positive vesicles suggesting that RAB26 covers the gap between recycling and autophagic pathway.29,85,97 Moreover, RAB26 overexpression leads to SV clustering and engulfment by autophagosomes. RAB33B, ubiquitously presents in murine organs, shares the binding to ATG16L1 with RAB26 regulating autophagosomes formation.39,98,99 If RAB26 and RAB33B overlap their role or sequentially work in regulating SV recycling and autophagy, remain to be demonstrated.
All the RAB GTPases described to be involved in axonal and pre-synaptic trafficking are essential for a correct synaptic activity formation and maintenance. Indeed, mutations and physiological abnormalities affecting synaptic RABs, and their regulator and effector proteins, lead to neurodevelopmental and neurodegenerative disorders.
RAB GTPases involved in dendritic trafficking
Neuronal dendrites play a critical role in integrating synaptic inputs, so that they may determine an action potential. For these reasons it is important, at first, to study how the dendritic outgrowth is regulated and maintained. Dendrites are specialized compartments that receive signals from the axonal termini of other neurons; this occurs where pre-synaptic buttons contact dendritic spines, situated through the dendritic tree.100,101 Indeed, dendrites are strewed with spines that exhibit different types of Ligand-Gated Ion Channels classified into three families: Cys-loop receptors, ionotropic receptors and Adenosine Tri-Phosphate (ATP)-gated channels.102 In the brain, most excitatory transmission is mediated by α-Amino-3-hydroxy-5-Methyl-4-isoxazolePropionic Acid (AMPA)-type ionotropic glutamate receptors; they have the highest influence on the strength of the synaptic response and are crucially involved in synaptic plasticity and learning and memory processes.103
RAB proteins-mediated trafficking during neuronal development for dendritic polarization
One of the first studies on this issue described the role of RAB8 in dendrite-specific transport. It has two different isoforms, RAB8A and RAB8B, which compensates for each other.104 RAB8 localizes in neurites of cultured hippocampal neurons in early developmental stages; in mature neurons it localizes in dendrites, but not in axon.27,105,106 RAB8 regulates membrane precursor-vesicle trafficking from TGN to the plasma membrane playing a crucial role in neurite outgrowth. In neuronal cells, downregulation of RAB8 inhibits anterograde formation and transport of vesicles and avoids neurite outgrowth.106,107 However Rab8 knockout mouse model dies prematurely, not for alterations in its role in neurons but, for its role played in the development of intestinal epithelial cells triggering its ubiquitously tissue expression.104 A recent study described that RAB8 shares its GEF Rabin8 with RAB10 and RAB11 in regulation of neurite outgrowth. However Rabin8 regulates Rab10 and Rab11 by a GEF-dependent and -independent mechanism, respectively.108
It was reported that RAB11 also controls dendritic arborisation in rat hippocampal neurons, regulating the trafficking of TrkB via MyosinVb. In particular, it permits the TrKB retention in dendrites, increasing the local signal needed for arborisation. Indeed, the overexpression of the constitutively active form of RAB11 (RAB11-Q70L) leads to increase dendritic branching, with an accumulation of TrkB in dendrites.109
Dendritic outgrowth is also regulated by RAB17 and RAB21. RAB17 is specifically expressed in dendrites of mouse hippocampal neurons, it localizes at dendritic growth cones, shafts, filopodia and mature spines.110 RAB17 mediates the dendrite growth and branching thanks to its GEF Rabex-5,111 found to be also one of RAB5 GEF, suggesting an inter-play between these RABs. Downregulation of RAB17, by shRNA technology, decreases synaptic branching in mouse hippocampal neurons.111
RAB21 controls the exocytic vesicle transport to the cell periphery in mouse hippocampal neurons through its GEF protein VARP and the non-canonical SNARE Vesicle-Associated Membrane Protein 7 (VAMP7).112
RAB proteins-mediated trafficking for post-synaptic function
RAB8, RAB11 and RAB17, previously described to play a role in dendrite outgrowth, participate also in receptor trafficking and recycling.
RAB8 is involved in GluA1-AMPA receptors trafficking from ER to the Golgi complex, and their delivery at the post-synapse.113,114 In central neurons RAB8 works in accordance with RAB11, present at the base of the dendritic spines.115 RAB11 is well known as the mediator of recycling endosomes: interestingly it also mediates the activity-dependent delivery of GluA1-containing AMPA receptors to synapses.116 Neuronal activity-dependent insertion or removal of AMPA receptors from the post-synaptic plasma membrane triggers the phenomenon of synaptic plasticity, translated in the experimental manifestation of long-term Potentiation (LTP) and long-term Depression (LTD).27 Dominant negative form of RAB11 (RAB11-S25N) is able to block AMPA receptor recycling and LTP.116
RAB17 promotes GluK2-kinate receptor surface expression thanks to syntaxin-4 in mouse hippocampal neurons. Indeed, RAB17 downregulation leads to syntaxin-4 redistribution away from dendrites and reduction of surface expression of GluK2-kinate receptors, while overexpression of the constitutively active form of RAB17 (RAB17-Q77L) endorses an accumulation of syntaxin-4 in dendrites and an increase of dendritic surface insertion of GluK2-kainate receptors.117
AMPA receptor internalization is controlled by RAB5 in Cornus Ammonis 1 (CA1) hippocampal neurons: it drives the internalization of AMPARs in a clathrin-dependent manner. In fact RAB5 is rapidly and transiently activated during N-Methyl-D-Aspartate (NMDA)-dependent LTD induction.118 AMPARs recycling is controlled by RAB4. It binds the neuronal specific GRIP-associated proteins 1 (GRASP1) performing a key role in the coordination of recycling endosome maturation in dendrites: in particular it plays a role in AMPARs recycling and in connecting early and late recycling endosomal compartments.119,120 Conversely, the transport of AMPA receptors, via RAB7-dependent late endosomes, ensures receptor removal from the synaptic membrane toward lysosomes.121 Another RAB GTPase involved in AMPA receptor trafficking is the neuronal specific protein RAB39B. It coordinates the secretory pathway of the AMPA receptor hetero-tetramer formed by GluA2-GluA3 subunits, leading to a correct AMPA receptor composition at the post-synaptic site. The downregulation of RAB39B in murine hippocampal neurons leads to an increase in Ca2+-permeable GluA2-lacking AMPA receptors at the neuronal surface, allowing impairments in excitatory post-synaptic currents.22 Moreover loss- or gain-of-function mutations in RAB39B cause Intellectual Disability122-124 and early onset Parkinson's disease.125-127
Finally, a correct orchestration by RAB proteins of dendritic and post-synaptic trafficking is essential for the architecture and maintenance of synaptic plasticity. In fact mutations in human RAB GTPases are described to cause neurodevelopmental and neurodegenerative disorders.
Conclusive remarks
The knowledge about the expression profile and the consequent role of RAB GTPases has considerably grown in recent years. In particular the isolation of RAB regulators and their effector proteins is providing the opportunity to understand, at first, their subcellular localization and consequently how RAB proteins work together.
In this review it well emerges how RAB GTPases communicate with each other thanks to common effectors, even if they localize in different neuronal compartments, and then how they efficiently coordinate the several steps of vesicular trafficking building up the cellular conditions for a correct synaptic function. A summary of described RAB proteins, that play a role in formation and maintenance of polarized neuronal synaptic architecture in the central nervous system, is schematically showed in Fig. 1.
Figure 1.
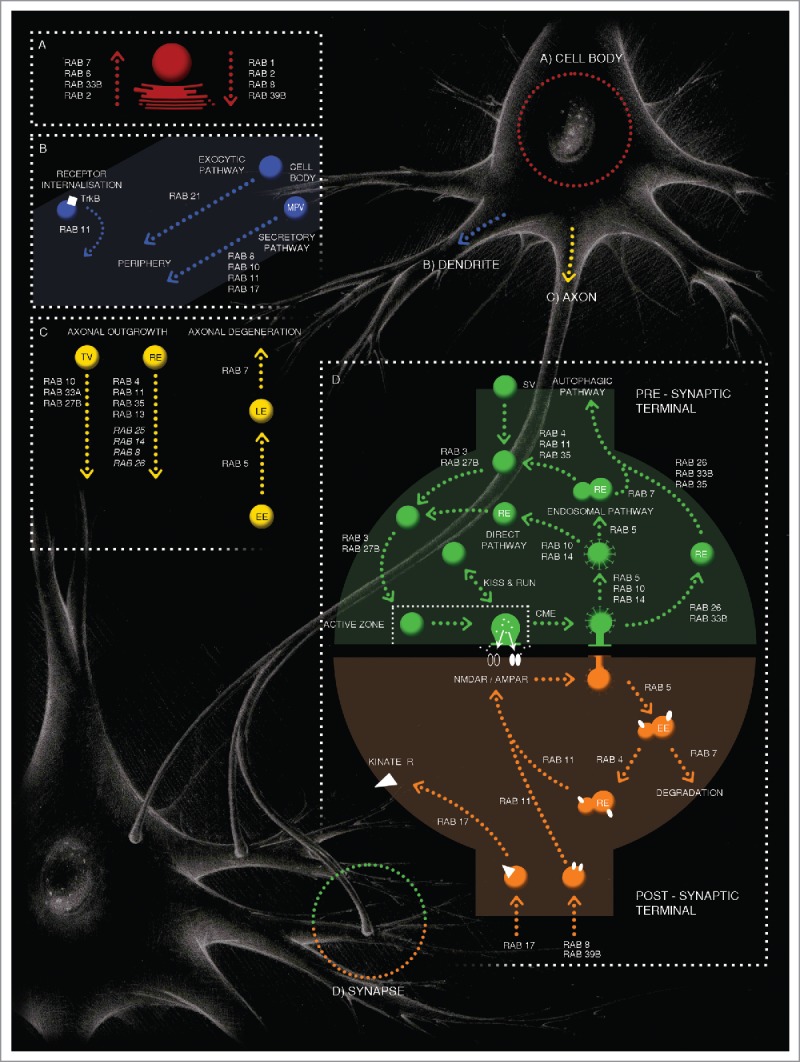
RAB GTPases orchestrate different neuronal trafficking steps. Graphical representation of RAB GTPase-mediating intracellular vesicular trafficking steps in different neuronal cell compartments. Section A (in red) represents the cell body compartment with RAB GTPases that mediate ER-Golgi pathway and vice versa. RAB1, RAB2, RAB8, RAB39B are involved in anterograde traffic and RAB7, RAB6, RAB33B, RAB2 play a role in retrograde transport. Section B (in blue) shows RAB proteins involved in dendritic vesicle trafficking. RAB8, RAB10, RAB11 controls constitutive Membrane Precursors Vesicle (MPV) trafficking in secretory pathway from cell body to the cell periphery. RAB17 acts at early neuronal developmental stage in secretory pathway through MPV for dendritic morphogenesis. RAB21 controls the exocytic vesicle transport to the cell periphery. A specialized function is showed for RAB11 in TrkB receptor (white rectangle) internalisation leading to local receptor signal increase for dendritic arborisation. Section C (in yellow) recapitulates RAB GTPases involved in axonal vesicle trafficking. Transport vesicles (TV) and recycling endosomes (RE) are involved in axonal outgrowth. RAB10, RAB33A and RAB27B mediate TV trafficking. RAB10 controls the vesicle transport from TGN to the plasma membrane. RAB27B regulates the anterograde transport of neurotrophin receptors and RAB33A mediates anterograde synaptophysin-positive vesicles. RAB4, RAB11, RAB35 and RAB13 are involved in RE pathway. RAB4 and RAB11 control RE fast and slow recycling route, respectively. RAB25 and RAB14 collaborate with RAB11. RAB35 permits neurite and axon elongation together with RAB8, RAB13 and RAB26. RABs reported in italic, represent the finding of their presence on RE without a well-defined role in neuron. RAB5 and RAB7 have been shown to sequentially mediate the retrograde transport of NGF and neurotrophin receptors from RAB5-positive Early Endosomes (EE) to RAB7-positive Late Endosomes (LE), leading to axonal degeneration. Section D recapitulates RAB proteins involved in pre- (green) and post- (orange) synaptic functions. At pre-synaptic site, RAB3 and RAB27B play a role in specific steps of SV docking and exocytosis at the pre-synapse. Recycling of SVs by endocytosis is directed through several pathways, represented are kiss-and-run and clathrin-mediated endocytosis (CME). The first step of CME is mediated by RAB5, RAB10 and RAB14. Then, RAB10 and RAB14 control the SVs direct pathway and RAB5 directs the endosomal pathway via RE. RAB5-mediated RE trafficking involves at the end, RAB4, RAB11 and RAB35 in SV regeneration. RAB7, driving LE-positive vesicles from RAB5-positive EE, links endosomal-recycling pathway to the autophagic process. RAB26, RAB33B and RAB35 are involved in SV degradation via the autophagic pathway. At the post-synaptic site, RAB17 controls kinate receptor (white triangle) surface expression. RAB39B and RAB8 are involved respectively, in GluA2/GluA3- and GluA1-AMPA receptor (white ellipse) trafficking and delivery. RAB11 is the mediator of recycling endosomes and contributes with RAB8 to AMPAR delivery. RAB5, controlling EE, is involved in a clathrin-dependent receptor internalization. Through RAB4 and RAB11receptors are recycled to the plasma membrane, and through RAB7-dependent late endosomes they are transported toward lysosomes.
The models used from different work-groups gave the opportunity to start to explore the role of several RAB GTPases in intracellular trafficking of central neurons, where the complexity of cellular morphology and activity is translated in a sophisticated and intricate coordination of vesicular traffic. It remains essential to generate animal models of different RAB proteins to better comprehend, not only the expression profile but also, the specific role of RABs in different tissues of the central nervous system. In particular the exact definition of multiple intracellular trafficking steps in neurons raises the groundwork in understanding the mechanisms involved in several neuropathological conditions. These open the way to the development of effective therapeutic strategies.
Abbreviations
- AMPA
α-Amino-3-hydroxy-5-Methyl-4-isoxazole Propionic Acid
- ATP
Adenosine Tri-Phospate
- CA1
Cornus Ammonis 1
- CDK5
Cyclin-Dependent protein Kinase 5
- CIE
Clathrin-independent bulk endocytosis
- CME
Clathrin-mediated endocytosis
- CMT2B
Charco-Marie-Tooth type 2B
- DRG
Dorsal Root Ganglion
- EEA1
Early Endosome Antigen 1
- ER
Endoplasmic Reticulum
- ESCRT
Endosomal Sorting Complex Required for Transport
- GAP
GTPase-Activating Protein
- GAP43
Growth Associated Protein 43
- GDI
RAB GDP Dissociation Inhibitor
- GDP
Guanosine-5′-DiPhosphate
- GEF
Guanine Nucleotide Exchange Factor
- GRASP1
GRIP-ASsociated Protein 1
- GTP
Guanosine-5′-TriPhosphate
- LE
Late Endosome
- LTD
long-term Depression
- LTP
long-term Potentiation
- MAP1B
Microtubule-Associated Protein 1B
- MARCKS
Myristoylated Alanin-Rich C-Kinase Substrate
- NGF
Nerve Growth factor
- NMDA
N-Methyl-D-Aspartate
- PI3K
Phosphatidyl Inositol 3-kinase
- PRPK
p53-Related ProteinKinase
- PtdIns(3)P
Phosphatidil Inositol 3-Phospate
- SV
Synaptic vesicle
- TGN
trans-Golgi network
- VAMP7
Vesicle-Associated Membrane Protein 7
- WT
wild type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank S. Mignogna for graphic support. M.L. Mignogna is supported by Fondazione Umberto Veronesi fellowship program 2016.
Funding
This work was supported by Ministero della Salute RF-2013-02355326.
References
- [1].Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci 2005; 6:201-14. PMID:15711600 [DOI] [PubMed] [Google Scholar]
- [2].Villarroel-Campos D, Bronfman FC, Gonzalez-Billault C. Rab GTPase signaling in neurite outgrowth and axon specification. Cytoskeleton (Hoboken) 2016; 73(9):498-507; PMID:27124121; http://dx.doi.org/22715881 10.1002/cm.21303 [DOI] [PubMed] [Google Scholar]
- [3].Cheng PL, Poo MM. Early events in axon/dendrite polarization. Annu Rev Neurosci 2012; 35:181-201. PMID:22715881; http://dx.doi.org/ 10.1146/annurev-neuro-061010-113618 [DOI] [PubMed] [Google Scholar]
- [4].Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 2008; 9:846-59. PMID:18946474; http://dx.doi.org/ 10.1038/nrm2521 [DOI] [PubMed] [Google Scholar]
- [5].Ramirez OA, Couve A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol 2011; 21:219-27. PMID:21215635; http://dx.doi.org/ 10.1016/j.tcb.2010.12.003 [DOI] [PubMed] [Google Scholar]
- [6].Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics 2008; 7:1031-42. PMID:18256213; http://dx.doi.org/ 10.1074/mcp.M700569-MCP200 [DOI] [PubMed] [Google Scholar]
- [7].Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci 2006; 29:325-62. PMID:16776589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25. PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- [9].Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107-17. PMID:11252952 [DOI] [PubMed] [Google Scholar]
- [10].Hutagalung AH, Novick PJ. Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol Rev 2011; 91:119-49. PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59. PMID:22463690; http://dx.doi.org/ 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309. PMID:23303910; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- [13].Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 2006; 103:11821-7. PMID:16882731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 2004; 5:886-96. PMID:15520808 [DOI] [PubMed] [Google Scholar]
- [15].Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol 2010; 22:461-70. PMID:20466531; http://dx.doi.org/ 10.1016/j.ceb.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gurkan C, Lapp H, Alory C, Su AI, Hogenesch JB, Balch WE. Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol Biol Cell 2005; 16:3847-64. PMID:15944222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 2013; 25:414-9. PMID:23639309; http://dx.doi.org/ 10.1016/j.ceb.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].D'Adamo P, Masetti M, Bianchi V, More L, Mignogna ML, Giannandrea M, Gatti S. RAB GTPases and RAB-interacting proteins and their role in the control of cognitive functions. Neurosci Biobehav Rev 2014; 46 Pt 2:302-14. PMID:24412241; http://dx.doi.org/ 10.1016/j.neubiorev.2013.12.009 [DOI] [PubMed] [Google Scholar]
- [19].Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis–Golgi tethering. Traffic 2001; 2:268-76. PMID:11285137 [DOI] [PubMed] [Google Scholar]
- [20].Ortiz Sandoval C, Simmen T. Rab proteins of the endoplasmic reticulum: functions and interactors. Biochem Soc Trans 2012; 40:1426-32. PMID:23176493; http://dx.doi.org/ 10.1042/BST20120158 [DOI] [PubMed] [Google Scholar]
- [21].Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell 2008; 19:2059-68. PMID:18287531; http://dx.doi.org/ 10.1091/mbc.E07-09-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, et al.. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat Commun 2015; 6:6504. PMID:25784538; http://dx.doi.org/ 10.1038/ncomms7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell 2005; 16:5294-303. PMID:16120649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Young J, Stauber T, del Nery E, Vernos I, Pepperkok R, Nilsson T. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A′. Mol Biol Cell 2005; 16:162-77. PMID:15483056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valsdottir R, Hashimoto H, Ashman K, Koda T, Storrie B, Nilsson T. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett 2001; 508:201-9. PMID:11718716 [DOI] [PubMed] [Google Scholar]
- [26].Ayala J, Touchot N, Zahraoui A, Tavitian A, Prochiantz A. The product of rab2, a small GTP binding protein, increases neuronal adhesion, and neurite growth in vitro. Neuron 1990; 4:797-805. PMID:2111712 [DOI] [PubMed] [Google Scholar]
- [27].Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev 2008; 58:236-46. PMID:18485483 [DOI] [PubMed] [Google Scholar]
- [28].Bucci C, Alifano P, Cogli L. The role of rab proteins in neuronal cells and in the trafficking of neurotrophin receptors. Membranes (Basel) 2014; 4:642-77. PMID:25295627; http://dx.doi.org/ 10.3390/membranes4040642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Binotti B, Jahn R, Chua JJ. Functions of Rab Proteins at Presynaptic Sites. Cells 2016; 5:pii: E7; PMID:26861397; http://dx.doi.org/27511065 10.3390/cells5010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bentley M, Banker G. The cellular mechanisms that maintain neuronal polarity. Nature Reviews Neuroscience 2016; 17:611-22. PMID:27511065; http://dx.doi.org/ 10.1038/nrn.2016.100 [DOI] [PubMed] [Google Scholar]
- [31].van Beuningen SF, Hoogenraad CC. Neuronal polarity: remodeling microtubule organization. Current Opinion Neurobiology 2016; 39:1-7. [DOI] [PubMed] [Google Scholar]
- [32].Black MM. Axonal transport: The orderly motion of axonal structures. Methods Cell Biol 2016; 131:1-19. PMID:26794507; http://dx.doi.org/ 10.1016/bs.mcb.2015.06.001 [DOI] [PubMed] [Google Scholar]
- [33].Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol 2003; 160:817-21. PMID:12642609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu J, Fu XQ, He M, Luo ZG. Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell 2011; 21:431-44. PMID:21856246; http://dx.doi.org/ 10.1016/j.devcel.2011.07.007 [DOI] [PubMed] [Google Scholar]
- [35].Liu Y, Xu XH, Chen Q, Wang T, Deng CY, Song BL, Du JL, Luo ZG. Myosin Vb controls biogenesis of post-Golgi Rab10 carriers during axon development. Nat Commun 2013; 4:2005. PMID:23770993; http://dx.doi.org/ 10.1038/ncomms3005 [DOI] [PubMed] [Google Scholar]
- [36].Xu XH, Deng CY, Liu Y, He M, Peng J, Wang T, Yuan L, Zheng ZS, Blackshear PJ, Luo ZG. MARCKS regulates membrane targeting of Rab10 vesicles to promote axon development. Cell Res 2014; 24:576-94. PMID:24662485; http://dx.doi.org/ 10.1038/cr.2014.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deng CY, Lei WL, Xu XH, Ju XC, Liu Y, Luo ZG. JIP1 Mediates Anterograde Transport of Rab10 Cargos during Neuronal Polarization. Journal of Neuroscience 2014; 34:1710-23. PMID:24478353; http://dx.doi.org/ 10.1523/JNEUROSCI.4496-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lv P, Sheng Y, Zhao Z, Zhao W, Gu L, Xu T, Song E. Targeted disruption of Rab10 causes early embryonic lethality. Protein Cell 2015; 6:463-7. PMID:25860786; http://dx.doi.org/ 10.1007/s13238-015-0150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nakazawa H, Sada T, Toriyama M, Tago K, Sugiura T, Fukuda M, Inagaki N. Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J Neurosci 2012; 32:12712-25. PMID:22972995; http://dx.doi.org/ 10.1523/JNEUROSCI.0989-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Falk J, Konopacki FA, Zivraj KH, Holt CE. Rab5 and Rab4 regulate axon elongation in the Xenopus visual system. J Neurosci 2014; 34:373-91. PMID:24403139; http://dx.doi.org/ 10.1523/JNEUROSCI.0876-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 2000; 157:277-86. PMID:10880397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S. Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer's disease. J Chem Neuroanat 2011; 42:102-10. PMID:21669283; http://dx.doi.org/ 10.1016/j.jchemneu.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peric A, Annaert W. Early etiology of Alzheimer's disease: tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol 2015; 129:363-81. PMID:25556159; http://dx.doi.org/ 10.1007/s00401-014-1379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Takano T, Tomomura M, Yoshioka N, Tsutsumi K, Terasawa Y, Saito T, Kawano H, Kamiguchi H, Fukuda M, Hisanaga S. LMTK1/AATYK1 Is a Novel Regulator of Axonal Outgrowth That Acts via Rab11 in a Cdk5-Dependent Manner. Journal of Neuroscience 2012; 32:6587-99. PMID:22573681; http://dx.doi.org/ 10.1523/JNEUROSCI.5317-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takano T, Xu C, Funahashi Y, Namba T, Kaibuchi K. Neuronal polarization. Development 2015; 142:2088-93. PMID:26081570; http://dx.doi.org/ 10.1242/dev.114454 [DOI] [PubMed] [Google Scholar]
- [46].Li X, Sapp E, Chase K, Comer-Tierney LA, Masso N, Alexander J, Reeves P, Kegel KB, Valencia A, Esteves M, et al.. Disruption of Rab11 activity in a knock-in mouse model of Huntington's disease. Neurobiol Dis 2009; 36:374-83. PMID:19699304; http://dx.doi.org/ 10.1016/j.nbd.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li X, Valencia A, Sapp E, Masso N, Alexander J, Reeves P, Kegel KB, Aronin N, Difiglia M. Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington's disease. J Neurosci 2010; 30:4552-61. PMID:20357106; http://dx.doi.org/ 10.1523/JNEUROSCI.5865-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McClory H, Williams D, Sapp E, Gatune LW, Wang P, DiFiglia M, Li X. Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington's disease mice. Acta Neuropathol Commun 2014; 2:179. PMID:25526803; http://dx.doi.org/ 10.1186/s40478-014-0178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Richards P, Didszun C, Campesan S, Simpson A, Horley B, Young KW, Glynn P, Cain K, Kyriacou CP, Giorgini F, et al.. Dendritic spine loss and neurodegeneration is rescued by Rab11 in models of Huntington's disease. Cell Death Differ 2011; 18:191-200. PMID:21217767; http://dx.doi.org/ 10.1038/cdd.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Steinert JR, Campesan S, Richards P, Kyriacou CP, Forsythe ID, Giorgini F. Rab11 rescues synaptic dysfunction and behavioural deficits in a Drosophila model of Huntingtons disease. Hum Mol Genet 2012; 21:2912-22. PMID:22466800; http://dx.doi.org/ 10.1093/hmg/dds117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science 2006; 314:818-21. PMID:17082457 [DOI] [PubMed] [Google Scholar]
- [52].Matsuzaki F, Shirane M, Matsumoto M, Nakayama KI. Protrudin serves as an adaptor molecule that connects KIF5 and its cargoes in vesicular transport during process formation. Mol Biol Cell 2011; 22:4602-20. PMID:21976701; http://dx.doi.org/ 10.1091/mbc.E11-01-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lall P, Lindsay AJ, Hanscom S, Kecman T, Taglauer ES, McVeigh UM, Franklin E, McCaffrey MW, Khan AR. Structure-Function Analyses of the Interactions between Rab11 and Rab14 Small GTPases with Their Shared Effector Rab Coupling Protein (RCP). J Biol Chem 2015; 290:18817-32. PMID:26032412; http://dx.doi.org/ 10.1074/jbc.M114.612366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006; 52:293-305. PMID:17046692 [DOI] [PubMed] [Google Scholar]
- [55].Zhang K, Fishel Ben Kenan R, Osakada Y, Xu W, Sinit RS, Chen L, Zhao X, Chen JY, Cui B, Wu C. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J Neurosci 2013; 33:7451-62. PMID:23616551; http://dx.doi.org/ 10.1523/JNEUROSCI.4322-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Curr Opin Neurobiol 2005; 15:40-8. PMID:15721743 [DOI] [PubMed] [Google Scholar]
- [57].Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992; 70:715-28. PMID:1516130 [DOI] [PubMed] [Google Scholar]
- [58].Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005; 122:735-49. PMID:16143105 [DOI] [PubMed] [Google Scholar]
- [59].Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci 2005; 25:10930-40. PMID:16306406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA. App gene dosage modulates endosomal abnormalities of Alzheimer's disease in a segmental trisomy 16 mouse model of down syndrome. J Neurosci 2003; 23:6788-92. PMID:12890772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Laifenfeld D, Patzek LJ, McPhie DL, Chen Y, Levites Y, Cataldo AM, Neve RL. Rab5 mediates an amyloid precursor protein signaling pathway that leads to apoptosis. J Neurosci 2007; 27:7141-53. PMID:17611268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, Jiang Y, Nixon RA. Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer's disease. Mol Psychiatry 2016; 21:707-16. PMID:26194181; http://dx.doi.org/ 10.1038/mp.2015.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mingazov ER, Ugrumov MV. Gene expression of proteins of the vesicle cycle in dopaminergic neurons in modeling of Parkinson's disease. Dokl Biochem Biophys 2016; 468:206-8. PMID:27417722; http://dx.doi.org/ 10.1134/S1607672916030133 [DOI] [PubMed] [Google Scholar]
- [64].Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, et al.. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 2015; 520:234-+. PMID:25855459; http://dx.doi.org/ 10.1038/nature14359 [DOI] [PubMed] [Google Scholar]
- [65].Cogli L, Progida C, Lecci R, Bramato R, Kruttgen A, Bucci C. CMT2B-associated Rab7 mutants inhibit neurite outgrowth. Acta Neuropathol 2010; 120:491-501. PMID:20464402 [DOI] [PubMed] [Google Scholar]
- [66].Gomi H, Mori K, Itohara S, Izumi T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell 2007; 18:4377-86. PMID:17761531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, et al.. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell 2009; 16:675-86. PMID:19460344; http://dx.doi.org/ 10.1016/j.devcel.2009.03.005 [DOI] [PubMed] [Google Scholar]
- [68].Kobayashi H, Etoh K, Ohbayashi N, Fukuda M. Rab35 promotes the recruitment of Rab8, Rab13 and Rab36 to recycling endosomes through MICAL-L1 during neurite outgrowth. Biol Open 2014; 3:803-14. PMID:25086062; http://dx.doi.org/ 10.1242/bio.20148771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Villarroel-Campos D, Henriquez DR, Bodaleo FJ, Oguchi ME, Bronfman FC, Fukuda M, Gonzalez-Billault C. Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B. J Neurosci 2016; 36:7298-313. PMID:27383602; http://dx.doi.org/ 10.1523/JNEUROSCI [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Di Giovanni S, De Biase A, Yakovlev A, Finn T, Beers J, Hoffman EP, Faden AI. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. Journal of Biological Chemistry 2005; 280:2084-91. PMID:15522871 [DOI] [PubMed] [Google Scholar]
- [71].Sakane A, Honda K, Sasaki T. Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2. Mol Cell Biol 2010; 30:1077-87. PMID:20008558; http://dx.doi.org/ 10.1128/MCB.01067-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. Embo J 2006; 25:4084-96. PMID:16946709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al.. The AP-1 transcription factor c-jun is required for efficient axonal regeneration. Neuron 2004; 43:57-67. PMID:15233917 [DOI] [PubMed] [Google Scholar]
- [74].Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 2012; 4:a005645. PMID:22763746; http://dx.doi.org/ 10.1101/cshperspect.a005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, et al.. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci 2009; 29:14534-44. PMID:19923287; http://dx.doi.org/ 10.1523/JNEUROSCI.1546-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron 2011; 69:317-31. PMID:21262469; http://dx.doi.org/ 10.1016/j.neuron.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? Embo J 2005; 24:2839-50. PMID:16052212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci 2004; 24:6629-37. PMID:15269275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pavlos NJ, Gronborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, Urlaub H, Rizzoli SO, Jahn R. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci 2010; 30:13441-53. PMID:20926670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pavlos NJ, Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases 2011; 2:77-81. PMID:21776405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci 2004; 27:509-47. PMID:15217342 [DOI] [PubMed] [Google Scholar]
- [82].Park J, Cho OY, Kim JA, Chang S. Endosome-mediated endocytic mechanism replenishes the majority of synaptic vesicles at mature CNS synapses in an activity-dependent manner. Sci Rep-Uk 2016; 6:31807; PMID:27534442; http://dx.doi.org/24305055 10.1038/srep31807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Watanabe S, Rost BR, Camacho-Perez M, Davis MW, Sohl-Kielczynski B, Rosenmund C, Jorgensen EM. Ultrafast endocytosis at mouse hippocampal synapses. Nature 2013; 504:242-+. PMID:24305055; http://dx.doi.org/ 10.1038/nature12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhou LJ, McInnes J, Verstreken P. Ultrafast Synaptic Endocytosis Cycles to the Center Stage. Dev Cell 2014; 28:5-6. PMID:24434135; http://dx.doi.org/ 10.1016/j.devcel.2013 [DOI] [PubMed] [Google Scholar]
- [85].Fassio A, Fadda M, Benfenati F. Molecular Machines Determining the Fate of Endocytosed Synaptic Vesicles in Nerve Terminals. Front Synaptic Neurosci 2016; 8:10. PMID:27242505; http://dx.doi.org/ 10.3389/fnsyn.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci 2000; 113 Pt 2:183-92. PMID:10633070 [DOI] [PubMed] [Google Scholar]
- [87].Shimizu H, Kawamura S, Ozaki K. An essential role of Rab5 in uniformity of synaptic vesicle size. J Cell Sci 2003; 116:3583-90. PMID:12876219 [DOI] [PubMed] [Google Scholar]
- [88].Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol 2003; 161:609-24. PMID:12743108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Star EN, Newton AJ, Murthy VN. Real-time imaging of Rab3a and Rab5a reveals differential roles in presynaptic function. J Physiol 2005; 569:103-17. PMID:16141272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell 2011; 145:117-32. PMID:21458671; http://dx.doi.org/ 10.1016/j.cell.2011.02.039 [DOI] [PubMed] [Google Scholar]
- [91].Liu O, Grant BD. Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes. PLoS Genet 2015; 11:e1005514. PMID:26393361; http://dx.doi.org/ 10.1371/journal.pgen.1005514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Taylor CA, Yan J, Howell AS, Dong X, Shen K. RAB-10 Regulates Dendritic Branching by Balancing Dendritic Transport. PLoS Genet 2015; 11:e1005695. PMID:26633194; http://dx.doi.org/ 10.1371/journal.pgen.1005695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hyttinen JMT, Niittykoski M, Salminen A, Kaarniranta K. Maturation of autophagosomes and endosomes: A key role for Rab7. Bba-Mol Cell Res 2013; 1833:503-10; http://dx.doi.org/ 10.1016/j.bbamcr.2012.11.018 [DOI] [PubMed] [Google Scholar]
- [94].Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, Klumperman J, Scheller RH. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell 2004; 15:2218-29. PMID:15004230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell 2006; 17:3156-75. PMID:16641372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sheehan P, Zhu M, Beskow A, Vollmer C, Waites CL. Activity-Dependent Degradation of Synaptic Vesicle Proteins Requires Rab35 and the ESCRT Pathway. Journal of Neuroscience 2016; 36:8668-86. PMID:27535913; http://dx.doi.org/ 10.1523/JNEUROSCI.0725-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbruggen G, Schalk AM, Kuhnel K, Boyken J, Erck C, Martens H, et al.. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. Elife 2015; 4:e05597. PMID:25643395; http://dx.doi.org/ 10.7554/eLife.05597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Fukuda M, Itoh T. Direct link between Atg protein and small GTPase Rab. Autophagy 2008; 4:824-6. PMID:18670194 [DOI] [PubMed] [Google Scholar]
- [99].Szatmari Z, Sass M. The autophagic roles of Rab small GTPases and their upstream regulators: a review. Autophagy 2014; 10:1154-66. PMID:24915298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Jan YN, Jan LY. Dendrites. Genes Dev 2001; 15:2627-41. PMID:11641269 [DOI] [PubMed] [Google Scholar]
- [101].Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol 2008; 10:1164-71. PMID:18758452; http://dx.doi.org/ 10.1038/ncb1776 [DOI] [PubMed] [Google Scholar]
- [102].Tasneem A, Iyer LM, Jakobsson E, Aravind L. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 2005; 6:R4. PMID:15642096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].van der Sluijs P, Hoogenraad CC. New insights in endosomal dynamics and AMPA receptor trafficking. Semin Cell Dev Biol 2011; 22:499-505. PMID:21843653 [DOI] [PubMed] [Google Scholar]
- [104].Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, et al.. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci 2014; 127:422-31. PMID:24213529; http://dx.doi.org/ 10.1242/jcs.136903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Huber LA, de Hoop MJ, Dupree P, Zerial M, Simons K, Dotti C. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. Journal of Cell Biology 1993; 123:47-55. PMID:8408204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Huber LA, Dupree P, Dotti CG. A deficiency of the small GTPase rab8 inhibits membrane traffic in developing neurons. Mol Cell Biol 1995; 15:918-24. PMID:7823956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpaa K, Laakkonen P, Peranen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci 2006; 119:4866-77. PMID:17105768 [DOI] [PubMed] [Google Scholar]
- [108].Homma Y, Fukuda M. Rabin8 regulates neurite outgrowth in both GEF activity-dependent and -independent manners. Mol Biol Cell 2016; 27:2107-18. PMID:27170183; http://dx.doi.org/ 10.1091/mbc.E16-02-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci 2013; 33:6112-22. PMID:23554492; http://dx.doi.org/ 10.1523/JNEUROSCI.4630-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mori Y, Matsui T, Furutani Y, Yoshihara Y, Fukuda M. Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons. J Biol Chem 2012; 287:8963-73. PMID:22291024; http://dx.doi.org/ 10.1074/jbc.M111.314385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mori Y, Matsui T, Fukuda M. Rabex-5 protein regulates dendritic localization of small GTPase Rab17 and neurite morphogenesis in hippocampal neurons. J Biol Chem 2013; 288:9835-47. PMID:23430262; http://dx.doi.org/ 10.1074/jbc.M112.427591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Burgo A, Proux-Gillardeaux V, Sotirakis E, Bun P, Casano A, Verraes A, Liem RK, Formstecher E, Coppey-Moisan M, Galli T. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev Cell 2012; 23:166-80. PMID:22705394; http://dx.doi.org/ 10.1016/j.devcel.2012.04.019 [DOI] [PubMed] [Google Scholar]
- [113].Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem 2004; 279:43870-8. PMID:15297461 [DOI] [PubMed] [Google Scholar]
- [114].Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci 2007; 27:13311-5. PMID:18045925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 2006; 52:817-30. PMID:17145503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science 2004; 305:1972-5. PMID:15448273 [DOI] [PubMed] [Google Scholar]
- [117].Mori Y, Fukuda M, Henley JM. Small GTPase Rab17 regulates the surface expression of kainate receptors but not alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in hippocampal neurons via dendritic trafficking of Syntaxin-4 protein. J Biol Chem 2014; 289:20773-87. PMID:24895134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 2005; 45:81-94. PMID:15629704 [DOI] [PubMed] [Google Scholar]
- [119].Ginsberg SD, Mufson EJ, Counts SE, Wuu J, Alldred MJ, Nixon RA, Che S. Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2010; 22:631-9. PMID:20847427; http://dx.doi.org/ 10.3233/JAD-2010-101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, et al.. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol 2010; 8:e1000283. PMID:20098723; http://dx.doi.org/ 10.1371/journal.pbio.1000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fernandez-Monreal M, Brown TC, Royo M, Esteban JA. The Balance between Receptor Recycling and Trafficking toward Lysosomes Determines Synaptic Strength during Long-Term Depression. Journal of Neuroscience 2012; 32:13200-5. PMID:22993436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D'Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, et al.. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet 2010; 86:185-95. PMID:20159109; http://dx.doi.org/ 10.1016/j.ajhg.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, van Lier B, Arts P, Wieskamp N, del Rosario M, et al.. A de novo paradigm for mental retardation. Nat Genet 2010; 42:1109-12. PMID:21076407; http://dx.doi.org/ 10.1038/ng.712 [DOI] [PubMed] [Google Scholar]
- [124].Vanmarsenille L, Giannandrea M, Fieremans N, Verbeeck J, Belet S, Raynaud M, Vogels A, Mannik K, Ounap K, Jacqueline V, et al.. Increased dosage of RAB39B affects neuronal development and could explain the cognitive impairment in male patients with distal Xq28 copy number gains. Hum Mutat 2014; 35:377-83. PMID:24357492 [DOI] [PubMed] [Google Scholar]
- [125].Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Riseley JR, Stephenson SE, Fitzpatrick E, Haas SA, Pope K, et al.. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am J Hum Genet 2014; 95:729-35. PMID:25434005; http://dx.doi.org/ 10.1016/j.ajhg.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L, Guerreiro R, Majounie E, Federoff M, Heutink P, et al.. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet 2015; 1:e9. PMID:27066548; http://dx.doi.org/ 10.1212/NXG.0000000000000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mata IF, Jang Y, Kim CH, Hanna DS, Dorschner MO, Samii A, Agarwal P, Roberts JW, Klepitskaya O, Shprecher DR, et al.. The RAB39B p.G192R mutation causes X-linked dominant Parkinson's disease. Mol Neurodegener 2015; 10:50. PMID:26399558; http://dx.doi.org/ 10.1186/s13024-015-0045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


