ABSTRACT
Viruses are obligate intracellular parasites that utilize cellular machinery for many aspects of their replication cycles. Enveloped viruses generally rely upon host vesicular trafficking machinery to direct their structural proteins and genomes to sites of virus replication, assembly, and budding. Rab GTPases have been implicated in the replication of many important viral pathogens infecting humans. This review provides a summary of virus-Rab protein interactions, with a particular focus on the role of Rab-related trafficking pathways on late events in the lifecycle of herpesviruses and of HIV-1.
KEYWORDS: CMV, HIV-1, HSV-1, Rab GTPases, RSV, viral glycoprotein, virus assembly
Introduction
Eukaryotic cells contain a variety of membranous organelles of defined lipid and protein composition. An essential requirement of the cell is to regulate the trafficking of cellular cargo between membranous organelles, delivering proteins and lipids to the precise site where the cargo carries out its function. The vesicular transport machinery of the eukaryotic cell has developed to regulate this process. Small GTPases are important regulators of vesicular transport. Rab GTPases are the largest family of small GTPases engaged in vesicular transport, with almost 70 Rab family members identified.1,2 Rab proteins localize to specific membrane compartments, where they contribute to membrane identity, and act in a general sense to ensure that membrane-associated cargoes are transported to their precise destination within a cell. Because of their prominent role in determining the specificity and directionality of vesicular transport, Rab GTPases are known as master regulators of intracellular membrane trafficking.2,3
The molecular control of Rab activity has been recently reviewed in this journal,4 and will be outlined here only briefly. Newly synthesized Rab proteins first bind to the Rab escort protein (REP), and are subsequently prenylated at C-terminal cysteine residues by Rab geranylgeranyltransferase. Prenylation is required for Rab protein membrane interaction and for subsequent functions in vesicular trafficking. Prenylated, membrane-bound Rab proteins act as molecular switches, cycling between a GDP-bound, inactive state and a GTP-bound, active state. Activation is catalyzed by guanine nucleotide exchange factors (GEFs), and the predominant action of GEFs to enhance GTP binding is due to the much higher abundance of GTP as compared with GDP in the cytosol.4,5 Individual GEFs mediate nucleotide exchange for only a limited subset of Rab proteins, adding complexity to the regulation of Rab activity. In the GTP-bound state, Rab proteins interact with effector proteins that mediate specific cellular functions. Rab effectors include motor proteins, tethers, sorting adaptors, regulators, kinases, phosphatases, and other types of effectors.6 GTPase activating proteins (GAPs) hydrolyze GTP to GDP, resulting in reversion of an active Rab protein to the inactive state. GDP dissociation inhibitors (GDIs) can bind to inactive Rab proteins and extract them from the membrane, allowing a cytosolic pool of Rabs to be available for subsequent recruitment to specific cellular membranes.
General principles of virus-Rab interactions
Viruses depend upon host cell machinery to carry out many facets of their replication cycles. The variety of viruses infecting eukaryotic cells is large, so that the specific manner by which each virus engages with host cell machinery will necessarily have unique features. Here we will discuss some of the ways in which viruses engage with Rab-related cellular trafficking pathways that may be applicable to multiple virus families. Figure 1 illustrates 3 common areas of virus-Rab pathway interaction. Viral entry is frequently mediated by endocytic pathways, and utilizes early endosomes marked by Rab5, with some viruses progressing to late endosomes marked by Rab7 before cytosolic entry (Figure 1, pathway I). Another major segment of the viral lifecycle that intersects frequently with Rab-related vesicular trafficking is during late assembly/release steps (Figure 1, pathway II). Outward sorting of assembled virions from the recycling endosome mediated by Rab11 and its adaptors, and in some cases from the late endosome, marked by Rab9, have been implicated for several enveloped viruses. A third major intersection with Rab-directed vesicular trafficking pathways is in the sorting of viral envelope glycoproteins (Figure 1, pathway III). Glycoproteins may be sorted on transport vesicles from the trans-golgi network (TGN) to the plasma membrane (PM), and can be re-directed to intracellular sites by endocytosis to early endosomes or the recycling endosome. A fourth general mechanism used by viruses is the modification of cellular membranes, in which case Rab proteins may end up in replication or envelopment/assembly compartments that do not represent their natural subcellular organelle. This mechanism is not pictured in Figure 1 but will be discussed with regard to the replication of human cytomegalovirus later in this review.
Figure 1.
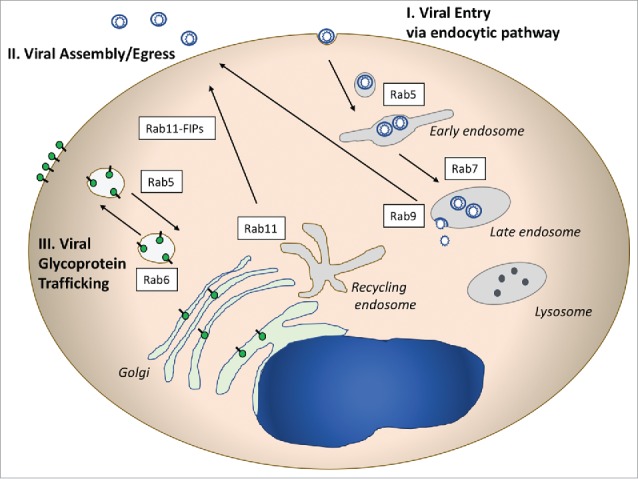
Illustrative Rab-related pathways used by viruses. I. Rab proteins involved in entry pathways are pictured on the right side of the diagram. Numerous viruses utilize the endocytic pathway to enter cells, and may deliver their capsids to the cytoplasm through Rab5+ or Rab7+ endosomes. II. Rab proteins play critical roles in the assembly and egress of enveloped viruses from cells. Rab11 and Rab 11 FIPs (adaptor proteins) have been implicated in RSV, CMV, and HIV assembly. Rab9 plays a role in late events for several viruses by an as yet unclear mechanism. III. Viral glycoprotein trafficking follows Rab-directed vesicular trafficking pathways, and may involve both outward movement to the plasma membrane and/or endocytosis to intracellular sites of assembly.
This review focuses on the intersection of viruses with Rab-directed vesicular trafficking pathways, focusing principally on the role of Rab proteins in the replication of enveloped viruses. The goal of the review is not to be completely comprehensive, but rather to point out the intimate relationships between viruses and Rab GTPases where they are known, providing specific examples that illustrate the principles outlined above. We will start with a discussion of the role of Rab GTPases in viral entry events, and then will focus more extensively on Rab GTPase contributions to virus assembly and egress. Examples where viruses co-opt Rab-related vesicular trafficking pathways to deliver their glycoproteins to the site of virus assembly are included. We note that some of the examples below were included in a prior review of this topic from 2006.7
Rab proteins involved in early phases of virus replication
Most enveloped viruses engage endocytic pathways to enter cells, while a subset fuse with the plasma membrane. Influenza virus is a well-studied example of an enveloped virus that enters cells following uptake into early endosomes. Influenza viruses are members of the family Orthomyxoviridae, viruses bearing a segmented, negative-sense RNA genome. The influenza virus entry process begins with attachment of the viral hemagglutinin (HA) protein to sialic acid residues on cell surface glycoproteins,8,9 followed by internalization of particles by clathrin-mediated endocytosis or by alternative routes such as micropinocytosis.10,11 Influenza virus must then pass through different endosomes before fusion and entry, and it is here that Rab proteins are implicated in early steps of influenza replication. Influenza viruses are observed within early endosomes (EEs), as marked by Rab5, before progressing to late endosomes (LEs) marked by Rab7 where fusion and cytoplasmic entry occur.12 The lower pH of LEs is required for triggering a conformational change in HA that exposes the fusion peptide and allows subsequent fusion events to move forward. Both Rab5 and Rab7 have been implicated as important for influenza entry,13 reflecting the need for particle endocytosis and subsequent endosomal maturation/trafficking steps. Dominant-negative forms of Rab5 or Rab7 were shown to disrupt influenza virus entry, while 2 other enveloped viruses (Semliki Forest virus [SFV] and vesicular stomatitis virus [VSV]) were sensitive to Rab5 dominant-negative inhibition but not Rab7 dominant-negative inhibition.13 There are suggestions that other viruses interact with Rab-related pathways during endocytosis, including evidence for entry of mouse polyomavirus through Rab11-positive early/recycling endosomes,14,15 and evidence that Rab5 is required for entry of the flaviviruses dengue virus, West Nile virus,16 and hepatitis C virus (HCV).17 This role may extend to numerous other viruses where the specific role of Rab GTPases has not been examined, as most animal viruses enter cells through endocytic uptake.18 The role of Rab5 extends to endocytic uptake of non-enveloped viruses as well, including adenovirus19 and potentially rotavirus.20 Thus, in a general sense, Rab GTPases required for the function and makeup of early endosomes play a role in the entry of viruses that utilize endocytic pathways, and for some viruses such as influenza those Rabs involved in formation and function of late endosomes are also implicated.
Rab proteins involved in late phases of virus replication
A growing body of literature implicates specific Rab-related trafficking events in late stages of viral replication such as virus assembly and budding. Here we will first focus on the specific roles of Rab GTPases in late events of the lifecycle of herpesviruses, and describe a connection between Rab9 and the egress of several unrelated enveloped viruses. We will then concentrate on the role of Rab GTPases in retrovirus assembly, with a particular focus on an evolving story implicating Rab-related trafficking and the Rab11-Family interacting proteins (FIPs) in trafficking of the HIV-1 envelope glycoprotein.
Rab GTPases and herpesvirus envelopment
Herpesviruses are large, enveloped, double-stranded DNA viruses that are widespread in human and animal populations. Herpes simplex virus type 1 (HSV-1) is an important human pathogen responsible for cold sores, genital ulcer disease, and severe disease in neonates and immunocompromised individuals. The HSV-1 particle includes a capsid surrounded by a complex structure known as the tegument that links the capsid to the envelope. The budding and envelopment of HSV-1 is complex, and the source of the final viral membranous envelope has been somewhat controversial. The model of HSV-1 morphogenesis known as the envelopment-de-envelopment-re-envelopment model posits that capsids assembled in the nucleus first bud and acquire their envelope from the inner nuclear membrane, lose their envelope when fusing with the outer nuclear membrane, and finally regain an envelope from the TGN.21-23 Hollinshead and colleagues used confocal and electron microscopic approaches to show that Rab5 and Rab11 are involved in HSV-1 envelopment.24 In this work, HSV-1 capsids were enveloped by tubular membranes derived from the plasma membrane in a process dependent on dynamin and Rab5. Viral glycoprotein gD was initially transported through the secretory pathway to the PM, then retrieved by endocytosis to become incorporated in tubular membranes surrounding the viral capsids. Although they concentrated in this work on HSV-1 glycoprotein D (gD), the investigators proposed that the same process holds for other HSV-1 glycoproteins. Depletion of Rab5 or Rab11 markedly reduced particle production in this study. This provides a unique if somewhat counterintuitive model for HSV-1 envelopment and envelope glycoprotein incorporation, including a prominent role for the recycling compartment in the acquisition of the envelope. Rab6 appears to play an additional role in HSV-1 assembly processes, as depletion of this Rab profoundly diminished HSV-1 infectious particle yield.25 The influence of Rab6 was shown to be on TGN-to-PM trafficking of HSV-1 glycoproteins, and the investigators concluded that this is an essential step that precedes endocytosis and envelopment. The role of Rab6 in HSV-1 glycoprotein TGN-to-PM transit, Rab5 in glycoprotein endocytosis, and Rab11 in HSV-1 particle envelopment is depicted schematically in Figure 2A. As will be outlined for HIV-1 later in this review, the transport of viral glycoproteins to the PM followed by an internalization step required to achieve particle incorporation is not unique to HSV-1.
Figure 2.
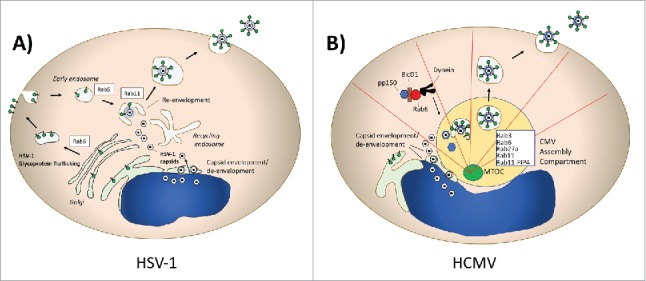
Herpesviruses and Rab Proteins. (A) Herpes simplex virus Type 1 (HSV-1) assembly and Rab proteins. HSV-1 utilizes Rab6-dependent TGN-to-PM transport of its glycoproteins, which are subsequently endocytosed in a Rab5-dependent process. Rab11 is essential for re-envelopment of capsids. (B) Human Cytomegalovirus (HCMV) utilization of Rab protein trafficking. Pictured schematically is the CMV assembly compartment, where a series of Rab proteins are recruited and contribute to assembly. Rab6 interacts with the adaptor BicD1 to bring the tegument protein pp150 to the assembly compartment in a dynein-dependent transport process.
Additional Rab GTPases have been implicated in the assembly of HSV-1. Rab27a has been found to colocalize with HSV-1 glycoproteins gH and gD in the TGN, and depletion of Rab27a reduces HSV-1 particle production.26 Rab1a/b and Rab43 depletion created defects in HSV-1 envelopment.27 The Enquist group has used pseudorabies virus, an alphaherpesvirus and veterinary pathogen, as a model organism to study the dynamics of herpesvirus particle egress by live cell fluorescence microscopy.28 They observed the movement of virus particles to the PM inside of small, acidified secretory vesicles. Rab6a, Rab8a, and Rab11a were present on the secretory vesicles, data that is consistent with the roles suggested for Rab6a and Rab11a in assembly and envelopment discussed above. Altogether, the role of Rab GTPases in regulating enveloped virus assembly is well-illustrated by the depth of investigation that has been performed for HSV-1 and related alphaherpesviruses.
Human cytomegalovirus (HCMV) assembly provides some similarities and some substantial differences with regard to herpesvirus interaction with Rab-regulated pathways. HCMV is also an important human pathogen, causing serious diseases in the setting of congenital infection and in immunocompromised hosts.29 HCMV modifies the host secretory pathway in infected cells, creating a perinuclear compartment termed the assembly compartment (AC) that is enriched in virion tegument and envelope proteins. The AC is formed around the microtubule organizing center of the cell (MTOC), with microtubules radiating out from the AC.30 This compartment serves as a site of intersection of viral tegument proteins, envelope proteins, and capsids and is the site of tegumentation and envelopment of viruses before viral shedding.31,32 The formation of the AC is also dependent upon the molecular motor dynein.30 In contrast to the pathway described for HSV-1, human cytomegalovirus (HCMV) has been shown to be enveloped into vacuoles containing Rab3, the HCMV glycoprotein gB, and the golgi marker mannosidase II.31 Rab5 is lacking in the viral vesicles, suggesting that the process of envelopment is very different than that outlined above for HSV-1. Further work on mechanisms of HCMV assembly identified Bicaudal D1 (BicD1), an effector protein that links Rab6 to dynein, as an interactor for the HCMV tegument protein pp150.33 Depletion of BicD1 led to diminished virus yield and defective trafficking of pp150 to the HCMV assembly compartment. This work suggests a model in which Rab6-mediated trafficking of pp150 is important for its delivery to the AC, which also depends upon linkage to dynein. The importance of Rab6 in HCMV assembly was further supported by the identification of the ternary complex composed of Rab6, BicD1, and pp150.34 Expression of a dominant-negative form of Rab6 (Rab6AT27N) in cells infected with HCMV reduced infectious yield by approximately 1 log10. Taken together, this body of evidence links Rab6-related trafficking strongly to HCMV assembly. The role of Rab6 in HCMV assembly is markedly different than in its role in HSV-1 assembly, as BicD1 and movement of tegument proteins to the MTOC-associated AC is important for HCMV, while BicD1 is dispensable in the case of Rab6-mediated outward transport of HSV-1 glycoproteins.25
Additional Rab proteins play significant roles in HCMV assembly. Rab27a localizes to the AC, and depletion markedly inhibits HCMV production.35 Rab11a and a member of the Rab11 family interacting proteins (FIPS), FIP4, are also required for mature AC formation.36 FIP4 is an adaptor protein that links Rab11 family members to cellular cargo, in this case interacting with the cytoplasmic tail of HCMV glycoprotein M. This finding implicates the endosomal recycling complex (ERC) in AC formation and HCMV assembly. The HCMV AC is schematically depicted in Figure 2B, with the variety of implicated Rabs and the site of envelopment indicated. Note that the very distinct roles of Rab6 in HSV-1 vs. CMV assembly can be compared in Figure 2A vs. Figure 2B. HCMV illustrates the general principle of viral re-organization of cellular membranous compartments and vesicular trafficking machinery, and is a good illustration of the complex ways in which viruses interact with Rab proteins.
Role of Rab9 in viral egress
Murray and coworkers used gene trap mutagenesis to identify host factors required for viral replication, and identified Rab9 as an important component of the replication cycles of HIV-1, the filoviruses Ebola virus and Marburg virus, and measles virus.37 Although the mechanism in common that led to inhibition of these diverse viruses was not entirely clear, the investigators suggested that a late endosome-to-PM vesicular transport step was important. Rab9 has also been implicated in release of HCV particles.38 A peculiar aspect of HCV assembly is its assembly within lipid droplets of hepatocytes.39 Tail interacting protein of 47 kDa (TIP47) has been associated with lipid droplets and plays a role in their formation.40 Ploen and coworkers found a connection between TIP47 and HCV particle release, and this finding was mapped to the Rab9 binding domain of TIP47.38 They concluded that the Rab9-TIP47 interaction was required for correct TIP47 targeting, and that properly-targeted TIP47 is required for viral release. Indeed, in the absence of this interaction, the de novo synthesized particles were directed to the autophagosome. Rab9 has thus been implicated in late events for multiple enveloped viruses, but a common mechanism has not been clearly elucidated.
Rab proteins involved in late events of the HIV-1 lifecycle
Multiple Rab protein-related pathways have been implicated in the replication of HIV-1. The importance of Rab-related pathways in late events of the HIV lifecycle makes intuitive sense, as HIV assembly is intimately linked to host vesicular trafficking pathways. In T lymphocytes, particle assembly occurs on specific plasma membrane microdomains enriched in phospholipids that are regulated by Rab-related trafficking.41,42 In HIV-1-infected human macrophages, viral particles accumulate in an intracellular compartment termed the virus-containing compartment or VCC that is enriched in late endosomal or multivesicular body markers such as CD9, CD81, and CD63.43,44 The nature of this compartment as an assembly or holding compartment has been debated, and dynamic tubular connections to the plasma membrane demonstrated.45-48 The HIV-1 envelope glycoprotein traverses the secretory pathway and utilizes endocytic and recycling pathways to direct its incorporation into budding particles.49 Thus, numerous compartments in the cell that are themselves defined and regulated by Rab proteins are intimately involved in the late events of the HIV-1 lifecycle. A role for Rab9 in HIV-1 egress has been mentioned above, and others including Rab7a, Rab27a, Rab14, and the Rab-related adaptor protein Rab11-FIP1C will be discussed individually below.
Rab7a plays a role in HIV-1 envelope maturation and particle egress
In addition to the suggestive role of Rab9 in late events of the HIV-1 lifecycle discussed above, Rab7a has been implicated in HIV-1 assembly and release. Caillet and colleagues performed siRNA depletions of 8 individual Rab proteins involved in endocytic and exocytic pathways and measured particle release and infectivity.50 These investigators found in a multiple-round assay that depletion of Rab4a, Rab8a, Rab9a, and Rab11a resulted in a moderate reduction of viral replication. Rab6a depletion resulted in a more substantial reduction in HIV-1 replication. However, the most profound inhibition of replication was observed following Rab7a knockdown. The investigators then further pursued the role of Rab7a in the HIV lifecycle, demonstrating that Rab7a knockdown impaired cleavage of the envelope glycoprotein, resulting in reduced infectivity of released particles. Furthermore, Rab7a knockdown reduced particle release by interfering with Vpu-induced degradation of the host restriction factor tetherin.50 Thus, in this study multiple Rab proteins were implicated in replication of HIV-1, and Rab7a played a role in 2 distinct aspects of viral particle assembly and release.
Rab27a influences HIV-1 assembly through effects on phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)
The HIV-1 Pr55Gag polyprotein directs the process of HIV-1 particle assembly and forms the structural core of the virus. Pr55Gag is a myristoylated protein that interacts with the inner leaflet of the plasma membrane.51-53 The matrix (MA) region of Gag interacts specifically with PtdIns(4,5)P2-enriched membranes through a patch of basic residues on its surface and the exposed myristyl group.42,54 Gerber and colleagues found that Rab27a regulates HIV-1 particle assembly through effects on production of PtdIns(4,5)P2 at the membrane.41 Silencing of Rab27a was accompanied by reduced PtdIns(4,5)P2 levels at the PM of CD4+ T cells. Levels of phosphoinositide 4-phosphate, a PtdIns(4,5)P2 precursor, were reduced at the PM, and the mechanism was determined to be reduced trafficking of late endosomes containing PI4-kinase to the PM. Thus the effect on HIV assembly was due to reduced Rab27a-directed trafficking of endosomes to the PM, reduced PI4-kinase activity, and reduced membrane PtdIns(4,5)P2 to interact with Gag.
The incorporation of HIV-1 envelope glycoprotein complex (Env) into developing particles is dependent upon Rab11-FIP1C and Rab14
The Rab11 family interacting proteins (FIPs) were first discovered by a yeast 2-hybrid screens using either dominant active Rab11a S20V or Rab4Q67L as bait.55,56 The Rab11-FIP family of adaptor proteins includes 5 family members (reviewed in57,58). Each family member features a highly conserved 20 amino acid Rab binding domain (RBD) at its C-terminus. Rab11-FIP1, FIP2, and FIP5 contain phospholipid binding C2-domains at their N termini, while FIP3 and FIP4 possess ezrin-radixin-moiesin (ERM) domains and 2 EF-hand domains. The Rab11-FIPs serve as effectors of Rab11 GTPases and regulate trafficking through recycling endosomes. Rab11-FIP1C (also known as Rab coupling protein, RCP) has been implicated in endocytic sorting, trafficking of epidermal growth factor receptor (EGFR) and endosome-to-TGN transport.59-61 Rab11-FIP1C forms parallel homodimers that bind to 2 members of the Rab11 GTPase subfamily (Rab11a, Rab11b, Rab25), Rab4, or to Rab14 through their RBD domains.57,62
The HIV-1 envelope glycoprotein complex (Env) is translated as a gp160 precursor on the rough ER. Env trimerizes in the ER, and is transported through the golgi stacks. In the TGN a furin-like protease cleaves gp160 into its subunit gp120 (SU) and gp41 (TM) cleavage products. HIV-1 Env contains a 150 amino acid cytoplasmic domain that includes numerous tyrosine- and dileucine-based motifs that are potential interaction sites for host cell trafficking factors (reviewed in63). Although the long cytoplasmic tail is dispensable for Env incorporation into particles in certain transformed cell lines such as HEK 293T cells, the full length tail is required for particle incorporation in T cell lines such as Jurkat and H9 cells and in primary T cells and macrophages.64 One explanation for cell type-dependent incorporation of HIV-1 Env is differential availability/expression of trafficking factors required for particle incorporation. Consistent with this idea, our laboratory found that Rab11-FIP1C depletion led to a loss of HIV-1 envelope glycoprotein incorporation into developing particles.49 FIP1C dependence was most prominent in certain T cell lines (Jurkat, H9) and in primary cells. We then evaluated which Rab GTPase was required for Env incorporation. Rab14 depletion led to a marked defect in Env incorporation into HIV-1 particles, while depletion of Rab11a or 11b had little effect.49 The Rab14S25N mutant that maintains a GDP-bound conformation acted as a dominant-negative for Env incorporation, supporting an important role for Rab14 in concert with Rab11-FIP1C in mediating Env trafficking to the particle assembly site. From these data, we proposed the Env trafficking model pictured in Figure 3. According to this model, HIV-1 Env first traverses the secretory pathway and reaches the cell surface. Env trimers are then endocytosed by an interaction of the membrane-proximal Yxxϕ motif of Env with the clathrin adaptor AP-2 complex. Env subsequently reaches the endosomal recycling compartment (ERC), where subsequent outward sorting from tubular endosomes occurs on vesicles bearing Rab11-FIP1C and Rab14. This outward sorting event is required for particle incorporation, potentially through delivery to a specialized microdomain on the PM where the HIV-1 Gag protein initiates particle formation.
Figure 3.
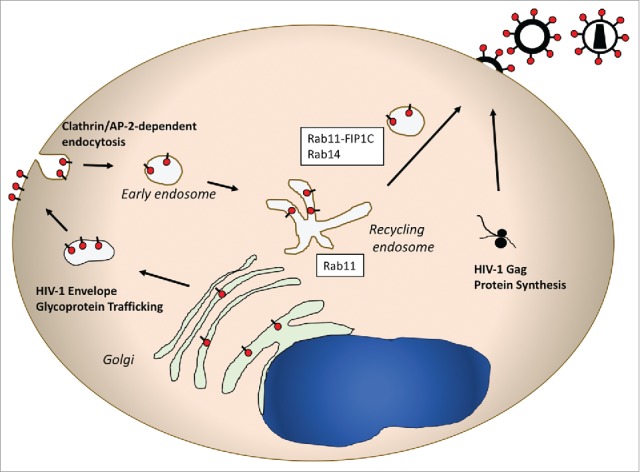
Schematic representation of HIV-1 envelope trafficking and assembly. This pathway features a prominent role for Rab14 and Rab11-FIP1C in directing the viral envelope to the plasma membrane site where the Gag lattice forms. Note that in a manner similar to HSV-1 glycoprotein trafficking, the HIV envelope glycoprotein is transported from TGN-to-PM, followed by endocytosis to the endosomal recycling compartment. From there, Rab11-FIP1C and Rab14 are required for outward sorting to the particle assembly site on the PM.
The model of tail-dependent trafficking of HIV-1 Env by a FIP1C-directed vesicular trafficking was further explored by evaluation of Env cytoplasmic tail mutants. GFP-tagged FIP1C moves out of the perinuclear ERC to the cellular periphery upon expression of HIV-1 Env, which provides a surrogate measure for its role in Env interaction and trafficking. Using this outward movement of GFP-FIP1C from the perinuclear ERC of HeLa cells as a marker, we identified a mutation in a tyrosine-based motif (YW795SL) that eliminated Rab11-FIP1C redistribution.65 The YW795SL mutant reproduced the cell type-specific defects in Env particle incorporation observed with Env bearing a near complete tail truncation, suggesting that this motif plays an important role in normal Env trafficking and incorporation. A second-site revertant in the tail of Env (YW795SL; L850S) restored particle incorporation and dependence on Rab11-FIP1C, suggesting that the loss-of-function had somehow eliminated interaction with Rab11-FIP1C. Together, these studies demonstrate specific, directional transport of HIV Env by Rab11-FIP1C and Rab14 that is dependent upon specific signals in the Env cytoplasmic tail. It is not yet clear why HIV-1 has evolved to use such a complex strategy involving the exocytic pathway, followed by endocytosis, and subsequently by recycling to the PM to achieve particle incorporation. As outlined previously, the delivery of glycoproteins to the cell surface followed by endocytosis and recycling is not unique to HIV, but is a pathway very similar to that followed by HSV-1 glycoproteins.
Rab11-FIP2 and respiratory syncytial virus (RSV) budding
Rab11-FIP2 is another member of the Rab11-FIPs that has been implicated in late events in virus replication. RSV is a negative-sense RNA virus belonging to the Paramyxoviridae family, and is a leading cause of serious lower respiratory tract infection in infants and young children. RSV buds preferentially from the apical surface of polarized epithelial cells, implicating the apical recycling endosome (ARE). Remarkably, expression of a fragment of the myosin Vb tail that functions as a dominant negative inhibitor of ARE sorting in polarized Madin-Darby canine kidney (MDCK) cells led to a >9000-fold reduction in viral yield.66 Subsequent work demonstrated that expression of a Rab11-FIP2 protein lacking the N-terminal C2 domain also significantly diminished virus particle yield.67 Scanning EM revealed long RSV filaments attached to the cell surface in the presence of FIP2-ΔC2, suggesting that viruses assembled on the plasma membrane normally but lacked the cellular machinery necessary for the fission process. The investigators concluded that RSV is released from cells via an ESCRT-independent mechanism that requires Rab11-FIP2.
Conclusions
It should not be surprising that viruses as obligate cellular parasites should evolve to effectively utilize intracellular trafficking pathways. The interactions described here are clearly just examples of a much more widespread phenomenon of viral interactions with Rab-related proteins and Rab-regulated trafficking pathways. Many viruses co-opt essential cellular trafficking pathways to perform key steps in their lifecycles, as illustrated by the numerous viruses that enter cells via interactions with endocytic machinery. Rab adaptor proteins add a layer of complexity to virus-host trafficking interactions, such as the role of RAb11-FIP1C in directing HIV-1 Env trafficking and particle incorporation. The specific interaction of viral proteins with Rab proteins or Rab-related adaptors represents a unique target for the development of antiviral therapies. New knowledge of viral interactions with Rab GTPases is certain to arise in the future given the central role of Rab proteins in regulating trafficking events in the cell.
Funding
This work was supported by NIH R01 GM111027.
References
- [1].Delevoye C, Goud B. Rab GTPases and kinesin motors in endosomal trafficking. Methods Cell Biol 2015; 130:235-46; PMID:26360038 [DOI] [PubMed] [Google Scholar]
- [2].Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; https://doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- [3].Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 2014; 6:a022616; PMID:25341920; https://doi.org/ 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Muller MP, Goody RS. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases 2017:1-17; https://doi.org/ 10.1080/21541248.2016.1276999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 1994; 140:1-22; PMID:7877593; https://doi.org/ 10.1007/BF00928361 [DOI] [PubMed] [Google Scholar]
- [6].Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128:3171-6; PMID:26272922; https://doi.org/ 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- [7].Hodge TW, Murray JL. Rab-GTPases: dual roles in vesicular transport and viral replication. Future Virol 2006; 1:811-22; https://doi.org/ 10.2217/17460794.1.6.811 [DOI] [Google Scholar]
- [8].Edinger TO, Pohl MO, Stertz S. Entry of influenza A virus: host factors and antiviral targets. J Gen Virol 2014; 95:263-77; PMID:24225499; https://doi.org/ 10.1099/vir.0.059477-0 [DOI] [PubMed] [Google Scholar]
- [9].Luo M. Influenza virus entry. Adv Exp Med Biol 2012; 726:201-21; PMID:22297515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Vries E, Tscherne DM, Wienholts MJ, Cobos-Jimenez V, Scholte F, Garcia-Sastre A, Rottier PJ, de Haan CA. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog 2011; 7:e1001329; PMID:21483486; https://doi.org/ 10.1371/journal.ppat.1001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rossman JS, Leser GP, Lamb RA. Filamentous influenza virus enters cells via macropinocytosis. J Virol 2012; 86:10950-60; PMID:22875971; https://doi.org/ 10.1128/JVI.05992-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu SL, Wu QM, Zhang LJ, Wang ZG, Sun EZ, Zhang ZL, Pang DW. Three-dimensional tracking of Rab5- and Rab7-associated infection process of influenza virus. Small 2014; 10:4746-53; PMID:24976105; https://doi.org/ 10.1002/smll.201400944 [DOI] [PubMed] [Google Scholar]
- [13].Sieczkarski SB, Whittaker GR. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 2003; 4:333-43; PMID:12713661; https://doi.org/ 10.1034/j.1600-0854.2003.00090.x [DOI] [PubMed] [Google Scholar]
- [14].Liebl D, Difato F, Hornikova L, Mannova P, Stokrova J, Forstova J. Mouse polyomavirus enters early endosomes, requires their acidic pH for productive infection, and meets transferrin cargo in Rab11-positive endosomes. J Virol 2006; 80:4610-22; PMID:16611921; https://doi.org/ 10.1128/JVI.80.9.4610-4622.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mannova P, Forstova J. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J Virol 2003; 77:1672-81; PMID:12525601; https://doi.org/ 10.1128/JVI.77.3.1672-1681.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol 2007; 81:4881-5; PMID:17301152; https://doi.org/ 10.1128/JVI.02210-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol 2006; 80:11571-8; PMID:17005647; https://doi.org/ 10.1128/JVI.01717-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem 2010; 79:803-33; PMID:20196649; https://doi.org/ 10.1146/annurev-biochem-060208-104626 [DOI] [PubMed] [Google Scholar]
- [19].Rauma T, Tuukkanen J, Bergelson JM, Denning G, Hautala T. rab5 GTPase regulates adenovirus endocytosis. J Virol 1999; 73:9664-8; PMID:10516081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Enouf V, Chwetzoff S, Trugnan G, Cohen J. Interactions of rotavirus VP4 spike protein with the endosomal protein Rab5 and the prenylated Rab acceptor PRA1. J Virol 2003; 77:7041-7; PMID:12768023; https://doi.org/ 10.1128/JVI.77.12.7041-7047.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol 2011; 9:382-94; PMID:21494278; https://doi.org/ 10.1038/nrmicro2559 [DOI] [PubMed] [Google Scholar]
- [22].Mettenleiter TC, Minson T. Egress of alphaherpesviruses. J Virol 2006; 80:1610-1; author reply 1–2; PMID:16415038; https://doi.org/ 10.1128/JVI.80.3.1610-1612.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC. Egress of alphaherpesviruses: comparative ultrastructural study. J Virol 2001; 75:3675-84; PMID:11264357; https://doi.org/ 10.1128/JVI.75.8.3675-3684.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hollinshead M, Johns HL, Sayers CL, Gonzalez-Lopez C, Smith GL, Elliott G. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J 2012; 31:4204-20; PMID:22990238; https://doi.org/ 10.1038/emboj.2012.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johns HL, Gonzalez-Lopez C, Sayers CL, Hollinshead M, Elliott G. Rab6 dependent post-Golgi trafficking of HSV1 envelope proteins to sites of virus envelopment. Traffic 2014; 15:157-78; PMID:24152084; https://doi.org/ 10.1111/tra.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bello-Morales R, Crespillo AJ, Fraile-Ramos A, Tabares E, Alcina A, Lopez-Guerrero JA. Role of the small GTPase Rab27a during herpes simplex virus infection of oligodendrocytic cells. BMC Microbiol 2012; 12:265; PMID:23164453; https://doi.org/ 10.1186/1471-2180-12-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zenner HL, Yoshimura S, Barr FA, Crump CM. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol 2011; 85:8012-21; PMID:21680502; https://doi.org/ 10.1128/JVI.00500-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hogue IB, Bosse JB, Hu JR, Thiberge SY, Enquist LW. Cellular mechanisms of alpha herpesvirus egress: live cell fluorescence microscopy of pseudorabies virus exocytosis. PLoS Pathog 2014; 10:e1004535; PMID:25474634; https://doi.org/ 10.1371/journal.ppat.1004535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vora SB, Englund JA. Cytomegalovirus in immunocompromised children. Curr Opin Infect Dis 2015; 28:323-9; PMID:26098503; https://doi.org/ 10.1097/QCO.0000000000000174 [DOI] [PubMed] [Google Scholar]
- [30].Alwine JC. The human cytomegalovirus assembly compartment: a masterpiece of viral manipulation of cellular processes that facilitates assembly and egress. PLoS Pathog 2012; 8:e1002878; PMID:23028305; https://doi.org/ 10.1371/journal.ppat.1002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Homman-Loudiyi M, Hultenby K, Britt W, Soderberg-Naucler C. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II. J Virol 2003; 77:3191-203; PMID:12584343; https://doi.org/ 10.1128/JVI.77.5.3191-3203.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sanchez V, Greis KD, Sztul E, Britt WJ. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol 2000; 74:975-86; PMID:10623760; https://doi.org/ 10.1128/JVI.74.2.975-986.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Indran SV, Ballestas ME, Britt WJ. Bicaudal D1-dependent trafficking of human cytomegalovirus tegument protein pp150 in virus-infected cells. J Virol 2010; 84:3162-77; PMID:20089649; https://doi.org/ 10.1128/JVI.01776-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Indran SV, Britt WJ. A role for the small GTPase Rab6 in assembly of human cytomegalovirus. J Virol 2011; 85:5213-9; PMID:21411515; https://doi.org/ 10.1128/JVI.02605-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fraile-Ramos A, Cepeda V, Elstak E, van der Sluijs P. Rab27a is required for human cytomegalovirus assembly. PLoS One 2010; 5:e15318; PMID:21170347; https://doi.org/ 10.1371/journal.pone.0015318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Krzyzaniak MA, Mach M, Britt WJ. HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic 2009; 10:1439-57; PMID:19761540; https://doi.org/ 10.1111/j.1600-0854.2009.00967.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murray JL, Mavrakis M, McDonald NJ, Yilla M, Sheng J, Bellini WJ, Zhao L, Le Doux JM, Shaw MW, Luo CC, et al. . Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J Virol 2005; 79:11742-51; PMID:16140752; https://doi.org/ 10.1128/JVI.79.18.11742-11751.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ploen D, Hafirassou ML, Himmelsbach K, Schille SA, Biniossek ML, Baumert TF, Schuster C, Hildt E. TIP47 is associated with the hepatitis C virus and its interaction with Rab9 is required for release of viral particles. Eur J Cell Biol 2013; 92:374-82; PMID:24480419; https://doi.org/ 10.1016/j.ejcb.2013.12.003 [DOI] [PubMed] [Google Scholar]
- [39].Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 2007; 9:1089-97; PMID:17721513; https://doi.org/ 10.1038/ncb1631 [DOI] [PubMed] [Google Scholar]
- [40].Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Höning S. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol 2009; 185:641-55; PMID:19451273; https://doi.org/ 10.1083/jcb.200812042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gerber PP, Cabrini M, Jancic C, Paoletti L, Banchio C, von Bilderling C, Sigaut L, Pietrasanta LI, Duette G, Freed EO, et al. . Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. J Cell Biol 2015; 209:435-52; PMID:25940347; https://doi.org/ 10.1083/jcb.201409082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A 2006; 103:11364-9; PMID:16840558; https://doi.org/ 10.1073/pnas.0602818103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol 2003; 162:443-55; PMID:12885763; https://doi.org/ 10.1083/jcb.200304008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tan J, Sattentau QJ. The HIV-1-containing macrophage compartment: a perfect cellular niche? Trends Microbiol 2013; 21:405-12; PMID:23735804; https://doi.org/ 10.1016/j.tim.2013.05.001 [DOI] [PubMed] [Google Scholar]
- [45].Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol 2007; 177:329-41; PMID:17438075; https://doi.org/ 10.1083/jcb.200609050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hammonds JE, Beeman N, Ding L, Takushi S, Francis AC, Wang JJ, Melikyan GB, Spearman P. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog 2017; 13:e1006181; PMID:28129379; https://doi.org/ 10.1371/journal.ppat.1006181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gaudin R, Berre S, Cunha de Alencar B, Decalf J, Schindler M, Gobert FX, Jouve M, Benaroch P. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS One 2013; 8:e69450; PMID:23922713; https://doi.org/ 10.1371/journal.pone.0069450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nkwe DO, Pelchen-Matthews A, Burden JJ, Collinson LM, Marsh M. The intracellular plasma membrane-connected compartment in the assembly of HIV-1 in human macrophages. BMC Biol 2016; 14:50; PMID:27338237; https://doi.org/ 10.1186/s12915-016-0272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Qi M, Williams JA, Chu H, Chen X, Wang JJ, Ding L, Akhirome E, Wen X, Lapierre LA, Goldenring JR, et al. . Rab11-FIP1C and Rab14 direct plasma membrane sorting and particle incorporation of the HIV-1 envelope glycoprotein complex. PLoS Pathog 2013; 9:e1003278; PMID:23592992; https://doi.org/ 10.1371/journal.ppat.1003278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Caillet M, Janvier K, Pelchen-Matthews A, Delcroix-Genete D, Camus G, Marsh M, Berlioz-Torrent C. Rab7A is required for efficient production of infectious HIV-1. PLoS Pathog 2011; 7:e1002347; PMID:22072966; https://doi.org/ 10.1371/journal.ppat.1002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol 2007; 55:347-87; PMID:17586320 [DOI] [PubMed] [Google Scholar]
- [52].Freed EO. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 1998; 251:1-15; PMID:9813197; https://doi.org/ 10.1006/viro.1998.9398 [DOI] [PubMed] [Google Scholar]
- [53].Ono A, Orenstein JM, Freed EO. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J Virol 2000; 74:2855-66; PMID:10684302; https://doi.org/ 10.1128/JVI.74.6.2855-2866.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci U S A 2004; 101:14889-94; PMID:15465916; https://doi.org/ 10.1073/pnas.0405596101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem 2001; 276:39067-75; PMID:11495908; https://doi.org/ 10.1074/jbc.M104831200 [DOI] [PubMed] [Google Scholar]
- [56].Lindsay AJ, Hendrick AG, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey MW. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem 2002; 277:12190-9; PMID:11786538; https://doi.org/ 10.1074/jbc.M108665200 [DOI] [PubMed] [Google Scholar]
- [57].Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans 2009; 37:1032-6; PMID:19754446; https://doi.org/ 10.1042/BST0371032 [DOI] [PubMed] [Google Scholar]
- [58].Prekeris R. Rabs, Rips, FIPs, and endocytic membrane traffic. ScientificWorldJournal 2003; 3:870-80; PMID:14532427; https://doi.org/ 10.1100/tsw.2003.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 2008; 183:143-55; PMID:18838556; https://doi.org/ 10.1083/jcb.200804140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jing J, Junutula JR, Wu C, Burden J, Matern H, Peden AA, Prekeris R. FIP1/RCP binding to Golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network. Mol Biol Cell 2010; 21:3041-53; PMID:20610657; https://doi.org/ 10.1091/mbc.E10-04-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R. The RCP-Rab11 complex regulates endocytic protein sorting. Mol Biol Cell 2004; 15:3530-41; PMID:15181150; https://doi.org/ 10.1091/mbc.E03-12-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kelly EE, Horgan CP, Adams C, Patzer TM, Ni Shuilleabhain DM, Norman JC, McCaffrey MW. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell 2009; 102:51-62; PMID:19702578; https://doi.org/ 10.1042/BC20090068 [DOI] [PubMed] [Google Scholar]
- [63].Checkley MA, Luttge BG, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol 2011; 410:582-608; PMID:21762802; https://doi.org/ 10.1016/j.jmb.2011.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Murakami T, Freed EO. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci U S A 2000; 97:343-8; PMID:10618420; https://doi.org/ 10.1073/pnas.97.1.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Qi M, Chu H, Chen X, Choi J, Wen X, Hammonds J, Ding L, Hunter E, Spearman P. A tyrosine-based motif in the HIV-1 envelope glycoprotein tail mediates cell-type- and Rab11-FIP1C-dependent incorporation into virions. Proc Natl Acad Sci U S A 2015; 112:7575-80; PMID:26034275; https://doi.org/ 10.1073/pnas.1504174112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brock SC, Goldenring JR, Crowe JE Jr. Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc Natl Acad Sci U S A 2003; 100:15143-8; PMID:14630951; https://doi.org/ 10.1073/pnas.2434327100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Utley TJ, Ducharme NA, Varthakavi V, Shepherd BE, Santangelo PJ, Lindquist ME, Goldenring JR, Crowe JE Jr. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc Natl Acad Sci U S A 2008; 105:10209-14; PMID:18621683; https://doi.org/ 10.1073/pnas.0712144105 [DOI] [PMC free article] [PubMed] [Google Scholar]


