ABSTRACT
In clinical practice, one subgroup patients of breast cancer might have developed resistance to multi-anti-HER2 targeted drugs(trastuzumab, lapatinib and/or T-DM1) and can not benefit from the anti-HER2 targeted therapy continuously. We attempt to change the next therapic way for these patients. Two patients with metastatic breast cancer who have failed to multi-anti-HER2 targeted therapy were treated with pembrolizumab (2 mg/Kg, day1) plus albumin-bound paclitaxel (125 mg/m2, day1,8) every 3 weeks. CT evaluation and HER2 ECD test were performed every 2 cycles. Both of the two patients achieved remarkable response with Partial Remission (PR), meanwhile serum HER2 ECD levels (the upper normal limit is 15 ng/ml) showed a remarkable decreases(compared to the base line decreases 75% and 60% respectively). The results indicate that regimen of pembrolizumab combination with albumin-bound paclitaxel might produce response in patients with HER2-positive metastatic breast cancer who have failed to multi-anti-HER2 targeted therapy.
KEYWORDS: Albumin-bound paclitaxel, Pembrolizumab, metastatic breast cancer, multi-anti-HER2 targeted therapy
Introduction
Breast cancer is the most common female cancer diagnosis in China, with nearly 250,000 new cases occurring each year. Moreover, 60,000 death cases occurring every year are due to the metastatic disease.1 Approximately 25% of invasive breast cancers overexpress the human epidermal growth factor receptor-2 (HER2), which is associated with the poor prognosis.2 NCCN guidelines recommend combination chemotherapy with anti-HER2 monoclonal antibody (trastuzumab) as the first line therapy for HER2 positive metastatic breast cancer. When the patients failed to trastuzumab, they can consider accepting the trastuzumab emtansine (T-DM1) or the tyrosine kinase inhibitor targeting both HER1 and HER2 (lapatinib) plus capecitabine.3 Unfortunately, a lot of patients developed resistance to trastuzumab, lapatinib, and T-DM1 ultimately during the treatment.
Some studies have concluded that continuously administering anti-HER2 targeted therapy after failure of the first line trastuzumab treatment is superior to pause in metastatic breast cancer (MBC),4-5 so the conception that the patients with HER2 positive should recept the “persistent anti-HER2 targeted treatment” was approved widely. But it is still challenging for physicians to determine the salvage therapeutic strategy for these patients who have failed to multi-anti-HER2 targeted therapy (trastuzumab, lapatinib and/or T-DM1) in clinical practice. The subgroup patients might have developed resistance to anti-HER2 targeted drugs and can not benefit from the anti-HER2 targeted therapy continuously. Should we change the next therapic way for these patients?
The checkpoint inhibition of the programmed death-ligand 1/programmed cell death-1 (PD-L1/PD-1) have been used in the clinical treatment.6 Muenst S7 reported that PD-1+ tumor infiltrating lymphocytes (TIL) is associated with poor outcome in HER2 positive breast cancer patients. The subgroup could possibly indicate a higher potential benefit from anti-PD-1 therapy. Here, we report the therapy course of two HER2 positive MBC patients with resistance to multi-anti-HER2 targeted terapy who displayed a remarkable response to the inhibitory antibody against PD-1 (pembrolizumab) plus albumin-bound paclitaxel (nab-paclitaxel, Abraxane).
Case presentation
Case 1
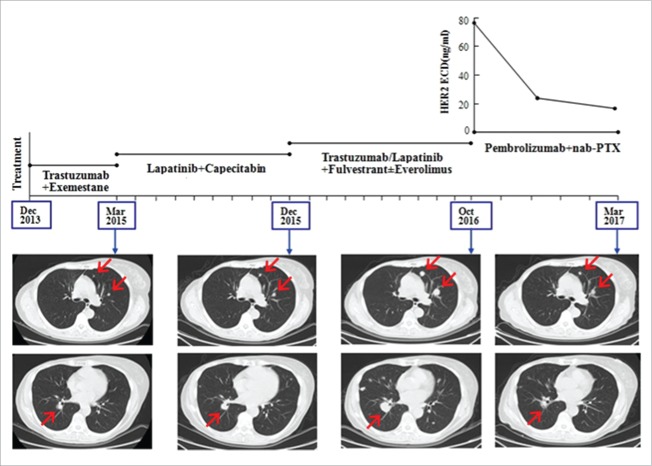
In May 2011, the 57-year-old female presented with a 3.5 cm mass in the right breast. She had a core needle biopsy and the pathology showed it to be infitrating ductal carcinoma of the right breast, and immunohistochemistry showed ER(+, 25–50%), PR(+, 10%), and HER2 (+++), Ki-67 10%. Neoadjuvant therapy was used 6 cycles and the protocol was paclitaxol (300 mg,175 mg/m2, d1) and carboplatin (650 mg, AUC = 5, d2) in combination with trastuzumab (260 mg, week 1; then 130 mg weekly). The patient achieved PR according RECIST v1.1. She then had a surgery “right breast simple mastectomy + right axillary sentinel lymph node biopsy” and the postoperative pathology showed it to be moderate treatment response. She received letrozole and trastuzumab after surgery until November 2012. In August 2013, recurrences with right axillary lymph nodes occurred, then she had the surgery “right axillary lymph node dissection” and the pathology showed 5 metastasis in the 30 lymph node. She received radiation therapy in the regiones of right chest wall, axilla and supraclavicular followed by exemestane plus trastuzumab. In March 2015, PET/CT indicated metastases in both lungs and lymph nodes. She had the therapy of lapatinib plus capecitabine and achieved PR, the PFS was 9 months. Then she had the regimens including fulvestrant+trastuzumab, fulvestrant+lapatinib, fulvestrant + everolimus +trastuzumab in turn, but the metastases gradually enlarged and came to disease progression. From October 27th 2016 to March 27th 2017, she received the regimen of pembrolizumab 150 mg (2.5 mg/kg, d1) and albumin-bound paclitaxel 200 mg (118 mg/m2 d1, d8) every 3 weeks. After 6 weeks CT evaluation revealed a remarkable reduction of the lung metastases that reached PR. After 12 weeks CT showed a further reduction of the disease that confirmed PR, meanwhile serum HER2 ECD levels (the upper normal limit is 15 ng/ml) showed a remarkable decreases of 75% compared to the base line (base line 77.3 ng/ml, 6 weeks 25.9 ng/ml, 15 weeks 19.5 ng/ml) (Fig. 1).
Figure 1.
In case 1, CT images showed a continuously increase of the lung metastases during multi-anti-HER2 targeted therapy and a remarkable decrease after treatment of pembrolizumab plus albumin-bound paclitaxel with the reduction of serum HER2 ECD levels.
We detected the expressions of PD-L1 with a proportion score of less than 1% in the tumor cells and PD-1 with a proportion score of less than 1% in the immune cells that had infiltrated in the tumors using the tumor sample (formalin-fixed paraffin-embedded specimens) from the surgery “right axillary lymph node dissection” by immunohistochemistry (Fig. 3A/B).
Figure 3.
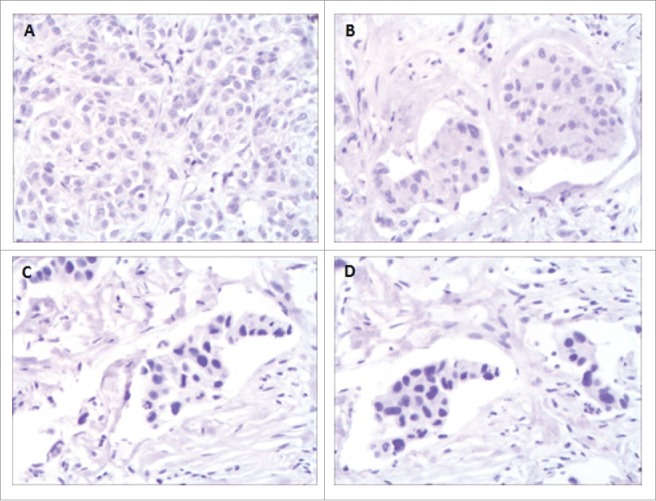
The immunohistochemistry results showed PD-Ll and PD-1 expression of the tumor sample (formalin-fixed paraffm-embedded specimens) obtained from Case 1 patient with a proportion score of less than 1% (A and B), from Case 2 patient also with a proportion score of less than 1% (C and D) at a magnification of 200.
Case 2
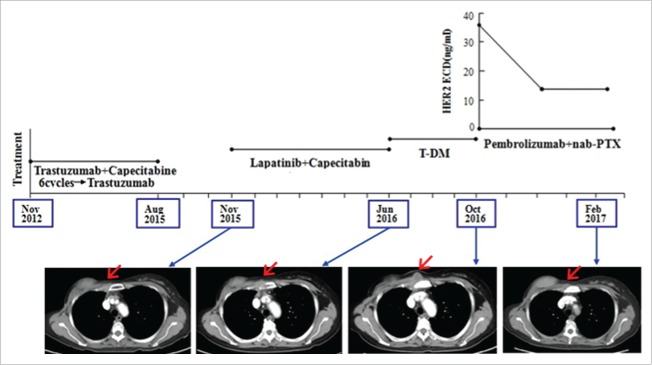
In May 2011, B ultrasound determined a malignant mass was located in the right breast of the 49-year-old female. Then on May 14th 2011, she had a modified radical mastectomy and postoperative pathology indicated it was a right infitrating ductal carcinoma and 5 metastasis in the 15 right axillary lymph node. Immunohistochemical analysis showed ER(−), PR(−), and HER2 (+++), Ki-67 40%. After surgery she received chemotherapy of EC × 4 cycles follow by TH × 4 cycles and trastuzumab until half a year. The disease free survival of the patient is one year and a half. In November 2012, chest CT showed many nodule metastases in both lungs and substernal lymph node metastasis. She had the therapy of trastuzumab plus capecitabine and achieved PR until April 2013, then the chemotherapy was stopped. She continued trastuzumab alone and PET/CT revealed CR until August 2015. In November 2015, CT indicated 3.3 × 1.9 cm metastatic lesion in right chest wall (the second intercostal space beside sternum). She had the therapy of lapatinib plus capecitabine and the metastatic lesion reduced, the PFS was 7 months. In June 2016, she received treatment of trastuzumab emtansine (T-DM1) and the metastatic lesion reduced again, the PFS was 4 months. From October 27th 2016 to February 9th 2017, she received the regimen of pembrolizumab 100 mg (2 mg/kg, d1) and albumin-bound paclitaxel 200 mg (140 mg/m2 d1, d8) every 3 weeks. After 6 weeks, the CT evaluation was PR, and after 12 weeks, the CT confirmed PR, meanwhile serum HER2 ECD levels showed a remarkable decreases of 60% compared to the base line (base line 36.3 ng/ml, 6 weeks 14 ng/ml, 12 weeks 14.3 ng/ml) (Fig. 2). We detected the expressions of PD-L1 and PD-1 also with a proportion score of less than 1% using the tumor sample from the biopsy of metastatic lesion in right chest wall by immunohistochemistry (Fig. 3C/D).
Figure 2.
In case 2, CT images showed a continuously increase of the right chest wall metastasis during multi-anti-HER2 targeted therapy and a remarkable decrease after treatment of pembrolizumab plus albumin-bound paclitaxel with the reduction of serum HER2 ECD levels.
Discussion
Pembrolizumab is a regulator of antitumor immunity as an inhibitory antibody against PD-1. It is approved for treatment of unresectable or metastatic melanoma and for metastatic non-small cell lung cancer following PD during or after platinum-based chemotherapy. Pembrolizumab is also being investigated in breast cancer. However, the objective response rate (ORR) of anti-PD-1 antibody alone to advanced breast cancer is less than 20%, so several studies are focusing on how to increase the ORR by combination with other drugs. Among these combination strategis, the chemotherapy plus is an important regimen. The major molecular mechanisms that chemotherapy promotes tumor immunity are by inducing immunogenic cell death and by disrupting strategies that tumors use to evade immune recognition.8
Pembrolizumab plus chemotherapy (carboplatin and pemetrexed) increase the efficacy remarkably in patients with advanced non small cell lung cancer (NSCLC) has been confirmed in KEYNOTE-021 study. The ORR was 55% in the pembrolizumab plus chemotherapy group compared with 29% in the chemotherapy alone group (p = 0.0016).9 The anti-PD-1 antibody combination with chemotherapy was explored in advanced breast cancer as well as advanced NSCLC. It was reported in 2015 san antonio breast cancer symposium that the phase Ιclinical trail about nivolumab plus nab-paclitaxel in HER2 negative MBC (abstract No. OT1-01-07). Patients were treated until disease progression or allowed to continue treatment beyond RECIST v1.1-defined PD if they continue to meet study eligibility; do not have rapid PD or clinical deterioration or unacceptable toxicities; and can benefit from continuation of study treatment in the treating physician's opinion. Exploratory biomarkers included tumor-associated PD-L1 expression and nivolumab serum level. Currently the KEYNOTE-355 phase ΙΙΙ trail is ongoing which explore the efficacy of pembrolizumab plus chemotherapy (albumin-bound paclitaxel or paclitaxel or gemcitabine/carboplatin) for the first line treatment in MBC patients.
Here we reported two metastatic breast cancer patients who have failed to multi-anti-HER2 targeted therapy was treated with pembrolizumab plus albumin-bound paclitaxel. Both of the two patients achieved remarkable response with Partial Remission (PR), and meanwhile serum HER2 ECD levels showed continually decreases (compared to the base line decreases 75% and 60% respectively). Our previous study suggests that a decrease in serum ECD≥20% after treatment in HER2-positive patients indicates therapeutic success, regardless of whether or not therapy is specifically anti-HER2 directed.10
Conclusion
Combination of pembrolizumab with albumin-bound paclitaxel could be an effective therapy option for patients with HER2-positive metastatic breast cancer who have failed to multi-anti-HER2 targeted terapy. Further prospective randomized trials are needed to confirm the finding in this particular patients.
Funding Statement
The National Natural Science Foundation of China [Grant number 81602314].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Our sincere gratitude to the patients for their permission to use the data and pictures for this paper, and to the colleagues of the Pathology department and the Laboratory of Oncology in Chinese PLA 307 Hospital.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer:correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, et al.. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al.. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J, et al.. Trastuzumab beyond progression:overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47(15):2273–81. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8(2):2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer.Breast Cancer Res Treat. 2013;139(3):667–76. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–43. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al.. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Zhou J, Zhang S, Bian L, Hu H, Xu C, Hao X, Liu B, Ye Q, Liu Y, et al.. Meaningful interpretation of serum HER2 ECD levels requires clear patient clinical background, and serves several functions in the efficient management of breast cancer patients.Clin Chim Acta. 2016;458:23–9. doi: 10.1016/j.cca.2016.04.025. [DOI] [PubMed] [Google Scholar]