ABSTRACT
Isocitrate dehydrogenase 1 (IDH1) is a metabolic enzyme implicated in cancer cell metabolic reprogramming. This is underscored by the detection of functional, somatic IDH1 mutations frequently found in secondary glioblastoma. To our knowledge, there has never been a reported, validated case of an IDH1 mutation in a pancreatic ductal adenocarcinoma (PDA). Herein, we present a case of a patient with metastatic PDA that harbored a potentially actionable, albeit rare, IDH1 mutation. As part of the Know Your Tumor project (Pancreatic Cancer Action Network), a 48-year-old female was diagnosed with metastatic PDA and subsequently started on standard of care chemotherapy, during which her hepatic lesions progressed. Detailed molecular profiling was performed on a biopsy from a liver lesion that demonstrated an IDH1 mutation, R132H. This mutation was confirmed by an independent sequencing reaction from the tumor sample, and by immunohistochemistry using an antibody specific for the IDH1 R132H mutation. The patient subsequently received a mutant IDH1 inhibitor (AG-120, Agios Pharmaceuticals, Cambridge, MA), but with no response. IDH1 mutations are common in certain cancer types, but have not been reported in PDA. We report the first case of an IDH1 mutation in this tumor type, perhaps providing a rare opportunity for a targeted therapy as a treatment option for PDA.
KEYWORDS: IDH1, IDH1 mutation, multimodal treatment, pancreatic cancer, pancreatic ductal adenocarcinoma, personalized medicine, targeted therapy
Introduction
Isocitrate dehydrogenase 1 (IDH1) is the most commonly mutated metabolic enzyme in human cancer.1,2 The recent discovery of somatic IDH1 mutations in diverse cancer types emphasizes the importance of metabolic pathways in cancer biology. Moreover, the discovery of small compounds that selectively target this enzyme (but spare the wild type isoenzyme) has ushered in a wave of optimism for the treatment of IDH1 mutant tumors.3 Despite widespread molecular profiling for the purposes of targeted therapy, the FDA has approved therapies against just 12 genetic mutations,4-18 underscoring the infrequency of this type of discovery. Overall 25,000 new cancer diagnoses per year are associated with an IDH1 mutation, including: secondary glioblastoma, low grade gliomas, anaplastic gliomas, central chondrosarcomas, intrahepatic cholangiocarcinomas, melanoma, and anaplastic thyroid cancer. To our knowledge, there has not been a reported IDH1 mutation in pancreatic ductal adenocarcinoma (PDA).19-21
Functionally, IDH1 is a cytoplasmic enzyme that catalyzes the reversible interconversion of isocitrate and α-ketoglutarate. NADP is a cofactor for this reaction, and is either oxidized (with isocitrate formation) or reduced (with α-ketoglutarate formation).22 The mutant isoenzyme typically contains a heterozygous missense substitution at arginine 132. The wild type amino acid is most commonly replaced by a histidine, but may also be replaced by a serine, cysteine, or glycine.23,24 The mutated enzyme diverts α-ketoglutarate into an alternative reductive pathway that produces an oncometabolite, 2-hydroxyglutarate (D-2HG).25 The accumulation of D-2HG leads to increased DNA and histone methylation, which in turn drives cancer cell dedifferentiation.26-28 Previous studies suggest that IDH1 mutant tumors have a more favorable prognosis compared to IDH1 wild type tumors, and they are more vascularized. In addition, IDH1 mutant tumors are more sensitive to both harsh metabolic conditions and chemotherapy than their wild type counterparts.29,30 Since PDA is characterized by a harsh and nutrient deprived microenvironment (similar to primary glioblastomas which contain wild type IDH1),31-33 it stands to reason that IDH1 mutations may in fact be deleterious for PDA and selected against, which could account for the absence of any reported IDH1 mutations to date. Herein, we report for the first time, a case of PDA with a bona fide and validated IDH1 R132H mutation.
Clinical case report
A 48-year-old woman presented with stage IV PDA, and was noted to have a 7 cm liver mass, enlarged retroperitoneal lymphadenopathy, a 1.8 × 1.4 cm lesion in the uncinate process of the pancreas (likely the primary), and a 5 × 4 mm right middle lobe lung lesion. Notably, the details of her clinical presentation were not known to the study authors, since her care was performed at another institution and we were privy to just clinical information provided with biopsy material obtained for molecular profiling. The patient had a family history of prostate cancer in her father, and breast cancer in her maternal grandmother. A percutaneous biopsy of the liver lesion was presumed to be metastatic adenocarcinoma of pancreatic origin by histology.
As first line therapy, the patient received a standard combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX).34 This regimen temporarily controlled the disease until progression was observed after 9 months. As second line therapy, a course of gemcitabine, nab-paclitaxel, and pembrolizumab (a PD-1 inhibitor) was given, as part of a clinical trial.35 The patient had stable disease for an additional 5 months on this regimen. Upon progression, a second biopsy of the liver lesion was performed with repeat molecular profiling. Histologic evaluation revealed foci of both well and poorly differentiated tumor, including features consistent with a high grade histologic phenotype, as shown in Figs. 1A and 1C.
Figure 1.
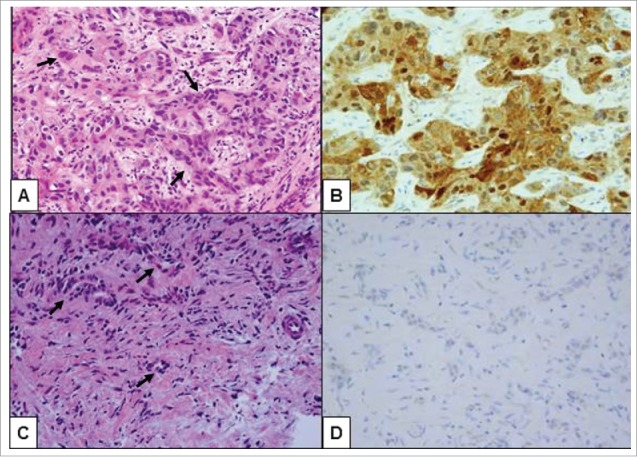
Histologic review of the second biopsy showed high grade pancreatic ductal adenocarcinoma (PDA), metastatic to the liver throughout the tissue cores (original magnification 400X). (A) Areas of undifferentiated tumor cells containing markedly pleomorphic nuclei were arranged in highly cellular nests and clusters of malignant cells (arrows) within a relatively scarce fibrotic stroma. (B) These undifferentiated tumor cells were positive by immunohistochemistry for IDH1 (R132H) mutation. (C) Distinct areas of the tumor biopsy demonstrated less tumor pleomorphism that was more characteristic of conventionally tubular morphology (arrows). (D) In contrast to high grade foci, the well-differentiated foci were negative by immunohistochemical stain for IDH1 (R132H) mutation, consistent with IDH1 wild type status of tumor cells in these areas.
The biopsy specimen was sent for molecular profiling (Foundation Medicine, Cambridge, MA), which identified mutations in KRAS, CDKN2A, CDKN2B, PLCG2, and IDH1. Sanger sequencing and next-generation sequencing (NGS) were used to confirm the IDH1 mutation (R132H) (Figs. 2A and 2B) at Thomas Jefferson University (Department of Pathology). Immunohistochemistry with a mutant-IDH1 specific antibody (Dianova, Hamburg, Germany) provided additional confirmation36 (Fig. 1B and 1D). A Catalog of Somatic Mutations in Cancer (COSMIC, http://www.sanger.ac.uk/genetics/CGP/cosmic/) database search of IDH1 mutations demonstrates the high prevalence of this mutation in central nervous system malignancies, as opposed to the rare occurrence in pancreatic cancers (Table 1).
Figure 2.
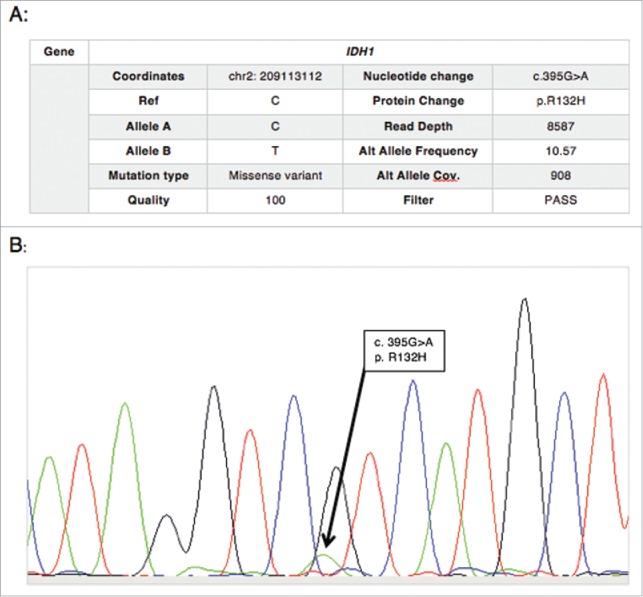
(A) NGS results, and (B) chromatogram from Sanger sequencing confirming the IDH1 mutation (R132H) in the pancreatic tumor sample.
Table 1.
COSMIC database search of IDH1 mutation frequencies in various malignancies.
| Tissue | Overall IDH1 Mutation Frequency | Frequency of IDH1 R132 Mutations |
|---|---|---|
| Central nervous system | 6,975/20,299 (34.4%) | 6,956/6,975 (99.7%) |
| Bone | 357/1,775 (20.1%) | 340/357 (95.2%) |
| Biliary tract | 93/1,097 (8.48%) | 91/93 (97.8%) |
| Pancreas | 4/1,712 (0.23%) | 2/4* (50%) |
The 2 R132 mutations were in intraductal papillary neoplasms, while the 2 non-R132 mutations were synonymous variants.
The patient enrolled in a Phase I trial of an inhibitor targeted to mutant IDH1 (AG-120, Agios Pharmaceuticals, Cambridge, MA) for advanced solid tumors,37 but progressed after just 1 cycle of therapy. The patient's current clinical status is unknown.
Discussion
Somatic mutations in IDH1 (R132H, R132G) have been reported in intraductal papillary mucinous neoplasms of the pancreas.38 However, this report provides the first evidence of an intragenic IDH1 R132H mutation in PDA.20,21 A close examination of the mutant and wild type IDH1 literature hints at why IDH1 mutations may be a rare event in PDA. While IDH1 mutations are considered a gain of function mutation that leads to the generation of 2-hydroxyglutarate, there is also a clear element of functional loss, due to haploinsufficiency of the wild type allele. For instance, mutant IDH1 isoenzymes are less efficient at catalyzing the reductive carboxylation of α-ketoglutarate (into isocitrate), they are more sensitive to chemotherapy, and they are more susceptible to the ill effects of hypoxia.1,2,29,30 Moreover, in glioblastoma multiforme, tumors with biallelic wild type IDH1 are more aggressive and associated with worse outcomes than their mutant counterparts.1 Thus, PDA may in fact be maladapted for IDH1 mutations due to the harsh tumor microenvironment that is a hallmark of the disease.31
This particular example of PDA reported herein was notable for having a mixed histology of both differentiated and undifferentiated components (Fig. 1). A prior study of noncohesive PDA (defined as tumors having any foci lacking infiltrating glands, including anaplastic and undifferentiated PDA) revealed that this phenotype is a common finding at autopsy, but rare in resected specimens. This finding suggests that an epithelial-to-mesenchymal transition (EMT) occurs at a very late stage in many PDAs.39 The authors noted a hypermethylation phenotype in some tumors with undifferentiated foci, and frequent loss of E-cadherin associated with high grade morphology. It is notable that IDH1 mutations result in a hypermethylation phenotype due to enhanced 2-HG, and are believed to drive neomorphic dedifferentiation in affected tumors.40,41 Based on this connection, we tested the index tumor for E-cadherin expression and observed that the differentiated foci lacked expression, while high grade foci paradoxically had intact expression (data not shown). Thus, E-cadherin loss cannot explain the dedifferentiated phenotype in this sample. Still, the labeling pattern observed in this metastatic lesion leaves open the possibility that a late IDH1 mutation is responsible for the observed morphologic findings and a driver of EMT in at least this case of advanced PDA.
In conclusion, we describe the first validated case of an IDH1-mutant PDA. The mutant IDH1 inhibitor proved to be ineffective in this patient. It is not known if the lack of inactivity was due to polyclonality and heterogeneity of the tumor (e.g., the IDH1 mutation was likely a late event), the advanced stage of the cancer, poor performance status of the patient, or some other reason. As noted, at the time of submission the KYT program identified the same IDH1 mutation in a PDA patient (data not shown), we are hopeful this patient identified earlier in the disease process may have a better chance to respond to targeted therapy. These case studies were identified under the auspices of the “Know Your Tumor” program led by the Pancreatic Cancer Action Network (PanCAN) wherein multi-omic broad-scale molecular profiling is performed for all eligible pancreatic cancer patients. To date, 285 patients in this program have undergone NGS analysis. Such systematic molecular screening initiatives are extremely useful to identify patients with rare or infrequent molecular alterations that would be eligible for targeted therapy-based clinical trials and/or off-label therapy for existing FDA approved therapies. Future studies are required to better understand the role of IDH1 mutations in this small subset of PDAs, and the susceptibility of these tumors to “metabolic” targeted therapy.
Funding Statement
Pancreatic Cancer Action Network (PanCAN) (15-90-25-BROD); American Cancer Society (ACS) (MRSG-14-019-01-CDD).
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
American Cancer Society, MSG-Winter, Brody; Know Your Tumor; Pancreatic Cancer Action Network; and Perthera
References
- 1.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al.. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321:1807-12; PMID:18772396; https://doi.org/ 10.1126/science.1164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013; 19:764-72; PMID:23209033; https://doi.org/ 10.1158/1078-0432.CCR-12-3002 [DOI] [PubMed] [Google Scholar]
- 3.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al.. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013; 340:626-30; PMID:23558169; https://doi.org/ 10.1126/science.1236062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Kohne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012; 48:1466-75; PMID:22446022; https://doi.org/ 10.1016/j.ejca.2012.02.057 [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370:2537-9; PMID:24963575; https://doi.org/ 10.1056/NEJMoa1311107 [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, et al.. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371:1963-71; PMID:25264305; https://doi.org/ 10.1056/NEJMoa1406766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al.. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347:472-80; PMID:12181401; https://doi.org/ 10.1056/NEJMoa020461 [DOI] [PubMed] [Google Scholar]
- 8.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al.. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366:2171-9; PMID:22670903; https://doi.org/ 10.1056/NEJMoa1113713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei G, Rafiyath S, Liu D. First-line treatment for chronic myeloid leukemia: dasatinib, nilotinib, or imatinib. J Hematol Oncol 2010; 3:47; PMID:21108851; https://doi.org/ 10.1186/1756-8722-3-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al.. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30-9; PMID:25399551; https://doi.org/ 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al.. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; https://doi.org/ 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, et al.. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366:1382-92; PMID:22452356; https://doi.org/ 10.1056/NEJMoa1105535 [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, et al.. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013; 14:461-71; PMID:23602601; https://doi.org/ 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrill B, Amonkar MM, Sherif B, Maltzman J, O'Rourke L, Johnston S. Quality of life in hormone receptor-positive HER-2+ metastatic breast cancer patients during treatment with letrozole alone or in combination with lapatinib. Oncologist 2010; 15:944-53; PMID:20798196; https://doi.org/ 10.1634/theoncologist.2010-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med 2005; 353:1734-6; PMID:16236745; https://doi.org/ 10.1056/NEJMe058196 [DOI] [PubMed] [Google Scholar]
- 16.Cataldo VD, Gibbons DL, Perez-Soler R, Quintas-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med 2011; 364:947-55; PMID:21388312; https://doi.org/ 10.1056/NEJMct0807960 [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration Hematology/Oncology (Cancer) Approvals & Safety Notifications. Silver Spring, MD: US. Food and Drug Administration, 2016. [Google Scholar]
- 18.Mullard A. Pioneering apoptosis-targeted cancer drug poised for FDA approval. Nat Rev Drug Discov 2016; 15:147-9; PMID:26931080; https://doi.org/ 10.1038/nrd.2016.23 [DOI] [PubMed] [Google Scholar]
- 19.Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta 2014; 1846:326-41; PMID:24880135; https://doi.org/10.1016/j.bbcan.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al.. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491:399-405; PMID:23103869; https://doi.org/ 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al.. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321:1801-6; PMID:18772397; https://doi.org/ 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margittai E, Banhegyi G. Isocitrate dehydrogenase: A NADPH-generating enzyme in the lumen of the endoplasmic reticulum. Arch Biochem Biophys 2008; 471:184-90; PMID:18201546; https://doi.org/ 10.1016/j.abb.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al.. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360:765-73; PMID:19228619; https://doi.org/ 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R, Flanagan S, Li CC, Lee M, Shivalingham B, Maleki S, Wheeler HR, Buckland ME. Expanding the spectrum of IDH1 mutations in gliomas. Mod Pathol 2013; 26:619-25; PMID:23307057; https://doi.org/ 10.1038/modpathol.2012.210 [DOI] [PubMed] [Google Scholar]
- 25.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al.. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009; 462:739-44; PMID:19935646; https://doi.org/ 10.1038/nature08617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, et al.. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 2012; 483:484-8; PMID:22343896; https://doi.org/ 10.1038/nature10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, Cowley GS, Root DE, Ebert BL, Kaelin WG Jr. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013; 339:1621-5; PMID:23393090; https://doi.org/ 10.1126/science.1231677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al.. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012; 483:474-8; PMID:22343901; https://doi.org/ 10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonardi R, Subramanian C, Jackowski S, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem 2012; 287:14615-20; PMID:22442146; https://doi.org/ 10.1074/jbc.C112.353946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JB, Dong DF, Wang MD, Gao K. IDH1 overexpression induced chemotherapy resistance and IDH1 mutation enhanced chemotherapy sensitivity in Glioma cells in vitro and in vivo. Asian Pac J Cancer Prev 2014; 15:427-32; PMID:24528069; https://doi.org/ 10.7314/APJCP.2014.15.1.427 [DOI] [PubMed] [Google Scholar]
- 31.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012; 18:4266-76; PMID:22896693; https://doi.org/ 10.1158/1078-0432.CCR-11-3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 2015; 129:829-48; PMID:25943888; https://doi.org/ 10.1007/s00401-015-1432-1 [DOI] [PubMed] [Google Scholar]
- 33.Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res 2009; 69:9157-9; PMID:19996293; https://doi.org/ 10.1158/0008-5472.CAN-09-2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al.. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817-25; PMID:21561347; https://doi.org/ 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 35.Western Regional Medical Center A Phase Ib/II study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus) Clinicaltrials.gov [NCT02331251]. Bethesda, MD: National Library of Medicine (US), 2015. [Google Scholar]
- 36.Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DTW. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathologica 2015; 129:133-46; PMID:25427834; https://doi.org/ 10.1007/s00401-014-1370-3 [DOI] [PubMed] [Google Scholar]
- 37.Agios Pharmaceuticals Inc A phase 1, multicenter, open-label, dose-escalation and expansion, safety, pharmacokinetic, pharmacodynamic, and clinical activity study of orally administered AG-120 in subjects with advanced solid tumors, including glioma, with an IDH1 mutation ClinicalTrialsgov [Internet]. Bethesda, MD: National Library of Medicine (US), 2015. [Google Scholar]
- 38.Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, et al.. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014; 233:217-27; PMID:24604757; https://doi.org/ 10.1002/path.4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winter JM, Ting AH, Vilardell F, Gallmeier E, Baylin SB, Hruban RH, Kern SE, Iacobuzio-Donahue CA. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res 2008; 14:412-8; PMID:18223216; https://doi.org/ 10.1158/1078-0432.CCR-07-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, Viale A, Riggins GJ, Chan TA. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget 2013; 4:1729-36; PMID:24077826; https://doi.org/ 10.18632/oncotarget.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grassian AR, Lin F, Barrett R, Liu Y, Jiang W, Korpal M, Astley H, Gitterman D, Henley T, Howes R, et al.. Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/microRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT). J Biol Chem 2012; 287:42180-94; PMID:23038259; https://doi.org/ 10.1074/jbc.M112.417832 [DOI] [PMC free article] [PubMed] [Google Scholar]


