Supplemental Digital Content is Available in the Text.
Neuropathic pain in leprosy may respond to drugs usually used to control pain of neuropathic profile and amitriptiline may constitute a candidate drug for future formal clinical trials.
Keywords: Neuropathic pain, Leprosy, Candidate drug, Symptom profile, Neuropathy, Symptom
Abstract
Introduction:
Previous studies reported a high prevalence of neuropathic pain in leprosy, being especially present in “pharmacologically cured” patients. The presence of neuropathic pain in leprosy poses a supplementary burden in patient's quality of life, daily activities, and mood.
Objectives:
The aim of this study was to assess whether neuropathic pain in leprosy has similar symptom profile as neuropathic pain of other etiologies and to retrospectively assess the efficacy of neuropathic pain medications regularly prescribed to leprosy.
Methods:
Leprosy and nonleprosy patients had their neuropathic pain characterized by the neuropathic pain symptom inventory (NPSI, ranges from 0 to 100, with 100 being the maximal neuropathic pain intensity) in a first visit. In a second visit, leprosy patients who had significant pain and received pharmacological treatment in the first evaluation were reassessed (NPSI) and had their pain profile and treatment response further characterized, including information on drugs prescribed for neuropathic pain and their respective pain relief.
Results:
The pain characteristics based on NPSI did not significantly differ between leprosy and nonleprosy neuropathic pain patients in visit 1 after correction for multiple analyses, and cluster analyses confirmed these findings (ie, no discrimination between leprosy and nonleprosy groups; Pearson χ2 = 0.072, P = 0.788). The assessment of pain relief response and the drugs taken by each patient, linear regression analysis showed that amitriptyline, when effective, had the highest percentage of analgesic relief.
Conclusions:
Neuropathic pain in leprosy is as heterogeneous as neuropathic pain of other etiologies, further supporting the concept that neuropathic pain is a transetiological entity. Neuropathic pain in leprosy may respond to drugs usually used to control pain of neuropathic profile in general, and amitriptiline may constitute a potential candidate drug for future formal clinical trials aimed at controlling neuropathic pain in leprosy.
1. Introduction
Leprosy is caused by the infection by Mycobacterium leprae, which currently affects 250,000 new patients annually,37 mainly affecting economically restricted countries where income is unequally distributed across the population.16 Because up to 5% of the general population is susceptible to infection by the Mycobacterium,1,24,28 leprosy cases may occur in nonendemic areas, especially in times of high population geographical dislocation.22 Leprosy is associated with a pleiad of complications, with peripheral neuropathy being one among them. Neuropathic pain occurs in up to 22% of patients and may develop during or after bacteriological cure. In fact, more than 85% of patients with leprosy-related neuropathic pain developed it after the end of the antimicrobial treatment period, constituting a heavy long-term handicap of the disease, and frequently affecting patients who were considered cured and were already discharged from health care.19,34 Although several groups have validated screening tools used to detect neuropathic pain in leprosy patients,30 its symptom profile has not so far been compared with neuropathic pain of other etiologies. Additionally, there is, so far, no evidence-based treatment for neuropathic pain in leprosy, which leads to a vicious cycle where leprosy patients have a low propensity of being diagnosed with neuropathic pain, and, when they receive a diagnosis, disinformation on how to conduct the treatment and drug shortage are the rule, in part due to the lack of published data on this issue.
The different forms of leprosy, its evolving clinical phases, and treatment status may all be associated with the occurrence of chronic pain, and leprosy patients present chronic pain in a higher proportion than the general population.29 Neuropathic pain is also prevalent in leprosy, occurring in 11.2% to 78.9% of patients,7,18,23,34 and varying according to the setting (eg, field vs referral centers), the timing of assessment (before, during, or after the completion of the antimicrobial treatment)7,18,23, and the clinical presentation of the disease (pauci vs multibacillar).26 Similar to other etiologies of peripheral neuropathy, the presence of neuropathic pain in leprosy poses a supplementary burden in patient's quality of life, daily activities, and mood.34 Neuropathic pain in leprosy is also considered a long-term sequel of the disease because it frequently occurs after antimicrobial elimination of the Bacillus and affects patients who were otherwise cured and discharged from care. In the last 10 years, it has been shown that different screening tools used in neuropathic pain of other etiologies could also be used to detect pain of neuropathic characteristics in these patients.30 However, there is still scarce information on the clinical phenotype of neuropathic pain and whether it would differ from the neuropathic pain of other etiologies. Additionally, there is currently no evidence-based treatment for leprosy-associated neuropathic pain, which hampers formal public health policies targeted to pain control in endemic areas.
The objectives of this study are 2-fold. First, we assessed whether neuropathic pain in leprosy had a similar symptom profile as neuropathic pain of other etiologies. It has been repetitively demonstrated that neuropathic pain is a transaetiological entity; therefore, one would expect leprosy patients with neuropathic pain to present similar symptom profiles compared with neuropathic pain of other causes.15 However, this assumption has never been formally assessed or demonstrated, and remained to be confirmed in a controlled basis. A second aim was to assess the efficacy of neuropathic pain medications regularly prescribed to leprosy patients in an off-label basis in an outpatient setting and to evaluate which pharmacological agents would potentially provide a stronger analgesic relief and constitute good candidate drug for a double-blinded randomized clinical trial.
Thus, this study aimed to characterize symptom profiles of neuropathic pain in leprosy and aimed to compare them with those of neuropathic pain due to other etiologies in age- and sex-matched individuals from the same population and to analyze the pattern of analgesic use and their efficacy in this sample of patients to detect “candidate” drugs for future clinical trials.
2. Methods
2.1. Patients
The institutional ethics committee from Instituto Lauro de Souza Lima and Hospital das Clínicas approved this study and informed consent was obtained from all participants. Patients were prospectively screened for participation at the Pain Center of Instituto Lauro De Souza Lima (leprosy center in Bauru, São Paulo, Brazil), which is a regional reference facility for a population of 356,680 inhabitants. Patients were regularly seen in an outpatient setting in the institution and were screened for participation during a regular prescheduled medical consultation. Leprosy was diagnosed when a patient had one of the following findings: skin lesions typical for leprosy; and/or thickened peripheral nerves; and/or acid-fast bacilli on slit skin smears.39 Patients with leprosy were then classified using the 1998 World Health Organization classification in which patients are classified as paucibacillary if they have up to 5 skin lesions and as multibacillary if they have 5 or more skin lesions based on World Health Organization diagnostic recommendations.39 Adult patients with neuropathic pain based on a specialist physical examination and assessment based on proposed criteria36 were screened for publication. Neuropathic pain was defined as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system. Patients were diagnosed with neuropathic pain if the pain distribution was neuroanatomically plausible and if clinical examination confirmed that negative or positive sensory signs (ie, hypo/anesthesia, hypo/analgesia, hyperalgesia, or allodynia) were confined to innervation territory of the affected nervous structure ie, pain of neuropathic characteristics (positive Douleur Neuropathique 4 questionnaire), had leprosy (disease causing neuropathy) and sensory deficits based on a bedside physical examination, which included visual inspection of muscle atrophy and wasting, and cold (metal tuning fork) mechanical detection (Von Frey monofilaments; SORRI, Bauru, Brazil) and pinprick detection (pin).4 Control body areas were warmer skin areas where neuropathy is either absent or nuanced such as inner thighs, axillae, and interdigital area. Only patients who had finished the multidrug treatment for at least 6 months were included. This was a convenience sample of leprosy patients and we intended to include the largest number of patients during the study period (May–July 2015). Patients with major systemic conditions such as diabetes, excess alcohol consumption, human immunodeficiency virus, or any major mental disorder (DSM-IV) were excluded. One hundred four leprosy patients were screened to participate (Fig. 1-STROBE). Leprosy patients who were under reaction or were currently taking drugs used to treat leprosy reactions (thalidomide and prednisone) were not included. Reaction type 1 or reversal reaction was diagnosed when a patient had erythema and edema of skin lesions or neuritis. Reaction type 2 or erythema nodosum was diagnosed when a patient had tender subcutaneous skin lesions, accompanied or not by neuritis, iritis, arthritis, orchitis, dactylitis, lymphadenopathy, edema, or fever.25
Figure 1.
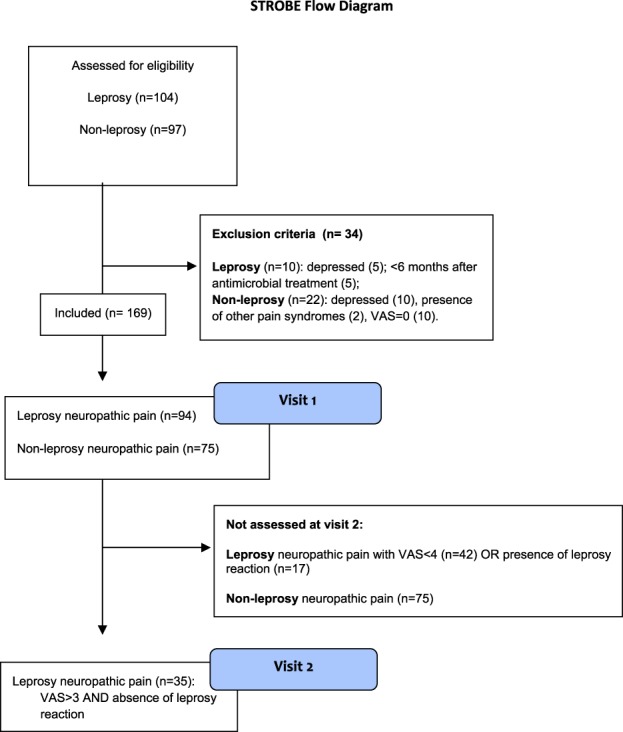
Strobe flow diagram from visit 1 and visit 2.
Ninety-seven age-, sex-, and pain intensity–matched patients with neuropathic pain due to peripheral etiologies other than leprosy were sequentially screened for participation and included (n = 75) in blocks of 10, and assessed at the Hospital das Clínicas, Universidade de São Paulo, São Paulo, Brazil.
2.2. Clinical assessments
Leprosy and nonleprosy neuropathic pain patients were evaluated at a baseline visit (visit 1) for the presence of neuropathic pain based on a clinical assessment. Leprosy patients were invited to return for a second visit from 3 to 5 months after visit 1 if they had moderate/severe neuropathic pain (neuropathic pain symptom inventory [NPSI] > 30).8
2.2.1. Study design
2.2.1.1. Visit 1
A leprosy specialist performed clinical diagnosis of leprosy and classified the participant according to Ridley–Jopling system.31 All patients with clinical diagnosis of neuropathic pain were asked to fill out the NPSI questionnaire assisted by a blinded dermatologist.6,9 Leprosy patients presenting moderate to severe neuropathic pain (NPSI > 30) had analgesic treatment implemented. The choice of drug treatment, dosing, and titration method was at each treating physician's discretion, which had no role in the study. Those with low pain intensity had their treatment maintained as it was, or received minor adjustments and were no longer assessed in the study. Nonleprosy neuropathic pain patients were evaluated by a pain specialist and also filled out the NPSI.
2.2.1.2. Visit 2
Leprosy patients who had significant pain on visit 1 and had analgesic treatment changes or implementation were reassessed and filled in the NPSI and the brief pain inventory.12 The BPI included questions on the intensity of the current pain, pain in the last week at its worst, at its least, and on average using a numeric rating scale (0–10). Also, pain interference in general activity, walking, mood, sleep, work, relationship with others, and enjoyment of life was rated using the same 11-point Likert scale.
2.3. Evaluation of neuropathic pain symptom profile between groups on visit 1
2.3.1. Neuropathic pain symptom inventory analysis of leprosy and nonleprosy patients and neuropathic pain symptom comparisons
Each item of the NPSI questionnaire was compared between leprosy and nonleprosy patients. Cronbach alpha from the NPSI questionnaire responses was used to assess internal consistency and reliability. Then, clusters of NPSI questionnaire symptoms from leprosy patients with neuropathic pain were compared with clusters of NPSI symptoms from patients with defined neuropathic pain of other etiologies.
2.3.2. Burden of neuropathic pain
Leprosy patients who had significant pain and were not clinically experiencing leprosy reactions were invited for a second visit and had their NPSI scores assessed for correlation with pain-related negative impact in daily activities measured by the interference scores of the BPI.
2.4. Evaluation of treatment response
2.4.1. Effect of pharmacological treatment in neuropathic pain in leprosy
Patients with leprosy and pain were offered pharmacological treatment at the discretion of their treating physician. The name of the drugs, its dosage, and the total number of medications were recorded using the BPI. The effect of treatment in neuropathic pain was measured by the percentage of improvement in pain obtained by pharmacological treatment as measured by the BPI (0%–100% improvement, item 8) during visit 2.
2.4.2. Analgesic effect of pharmacological treatment of neuropathic pain in leprosy patients
At visit 2, the percentage of pain improvement from BPI (question 8) was used to build a score (percentage of improvement) from BPI given by the patient. These scores were recorded as the therapeutic successes for neuropathic pain treatment of all possible combinations of drugs. The drug combinations were assessed by their frequency; combinations used by at least 4 patients were included in the analyses (Supplementary material-1, Available at: http://links.lww.com/PR9/A14). The coefficients were obtained by a linear regression with least absolute shrinkage and selection operator (LASSO) methodology to choose explicative variables. This method allows to choose (and then analyze) variables together, and not separately as occurs in traditional regression models. Thus, it works as a model selection method that evaluates all covariates jointly, which is more robust than selecting explanatory variables one by one (because it is possible that one or more variables are significant only in the presence of others). It is also applicable when the number of covariates is equal or greater than the number of observations, which is also not possible with classic linear regression models.35 The results were presented in a descriptive output highlighting drugs with the highest potential to be tested in a future clinical trial (potentially highest analgesic effect).
2.5. Statistical analysis
Data were expressed as mean ± SD for continuous quantitative variables and as percentage for categorical variables. Normality was assessed using the Kolmogorov–Smirnov test (P < 0.05). Nonparametric data were compared using Mann-Whitney U test for paired data and the Kruskall–Wallis test for group comparisons (leprosy vs nonleprosy-related neuropathic pain). Analyses were performed using SPSS 20.20 Cluster analysis was carried out in order to further confirm the findings from nonparametric group comparisons (Supplementary material-1, Available at: http://links.lww.com/PR9/A14) with objective to assign observations from a sample or a population to groups in such a way that observations within each group (called a cluster) was more similar or homogeneous to each other that to those in other groups (clusters) regarding the variables (features) that were measured.17 Hierarchical cluster analysis was performed to identify similarities in the individual response to NPSI on symptoms profiles. The algorithm generated the preclusters which are clusters from the original cases, replacing them with the objective of reducing the number of cases for the next step and decreasing the size of the array that contains the distances between all possible cases matched. This strategy represented an approach to sequential grouping. The algorithm scanned the records one by one and decided whether a certain record should merge with the previously formed clusters or give rise to a new cluster based on the distance criteria. After the preclustering was completed, all cases in the same precluster were treated as a single entity with a technique based on their characteristics and then they are used as new cases. The size of the distance's matrix was then no longer dependent on the number of cases, but on the number of preclusters. The 2-step method scored the quality of clustering with values between 0 and 1: dimensionless value. The closer to 1, the better was the clustering, with fewer cases being left out of the identified clusters.
3. Results
3.1. Baseline characteristics
We evaluated 94 patients with leprosy and neuropathic pain (52.8 ± 11.3 years; 39 female) and 75 nonleprosy patients with neuropathic pain (54.5 ± 15.2 years; 37 female). Pain score at baseline was 4.9 ± 3.1/10 in leprosy patients and 6.7 ± 2.2/10 in nonleprosy patients (P < 0.004). Of the 94 leprosy patients with neuropathic pain included in the study, 84% had finished multidrug treatment more than twelve months previously. According to Ridley and Jopling system, the largest number of patients had the borderline form of the disease [borderline-lepromatous (12.4%), borderline-borderline (29.2%), and borderline-tuberculoid (13.5%)], followed by lepromatous (38.2%) and tuberculoid-tuberculoid (6.7%) forms. Patients had completed the antibiotic regimen in average 95 months before the evaluation. The group of neuropathic pain of “nonleprosy” peripheral etiologies had postherpetic neuralgia (41.3%), traumatic brachial plexus injury (25.3%), other traumatic peripheral neuropathies (15.9%), lumbar radiculopathy (11.1%), and diabetic peripheral neuropathy (6.4%).
3.2. Symptoms profiles comparison at visit 1
Each descriptor of NPSI was compared separately between leprosy neuropathic pain and nonleprosy neuropathic pain groups. We found no statistically significant difference between the groups, except for pain provoked by brushing (2.6 ± 3.8 vs 4.1 ± 3.8, P = 0.009), by pressure (5.9 ± 4.0 vs 4.2 ± 4.1, P = 0.006), and by cold (1.5 ± 3.1 vs 2.7 ± 3.9, P = 0.022). These differences did not remain significant after correction for multiple analyses (Table 1 and Fig. 2).
Table 1.
Comparison between leprosy NeP and nonleprosy NeP symptom profile.
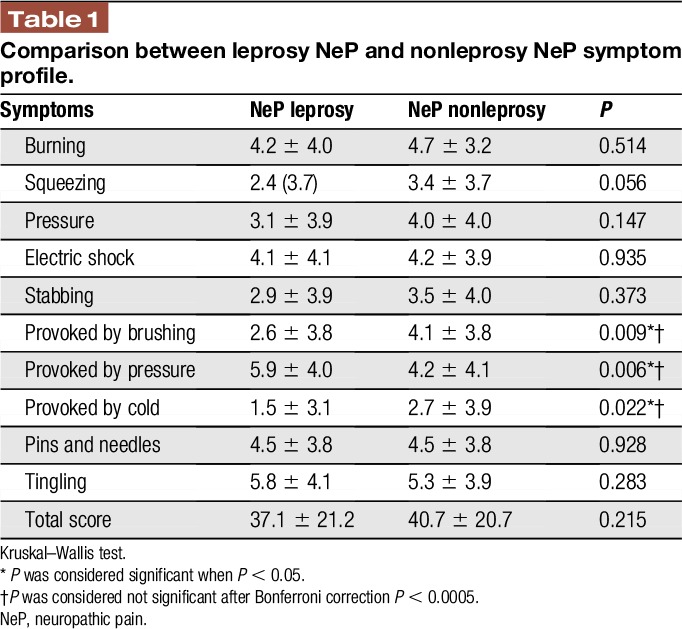
Figure 2.
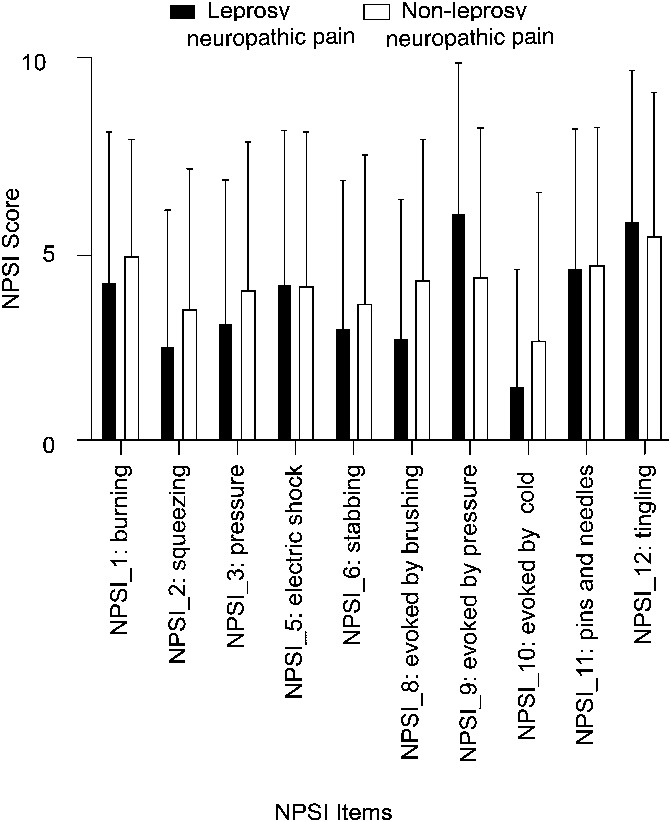
Symptom profiles' comparison between leprosy neuropathic pain and nonleprosy neuropathic pain. NPSI, neuropathic pain symptom inventory.
3.3. Cluster analysis
Cronbach alpha was 0.716, showing a good reliability by responders. Hierarchical cluster analyses of NPSI scores were performed (Supplementary material-1, Available at: http://links.lww.com/PR9/A14).
3.4. Visit 2: effects of treatments on pain relief in leprosy patients
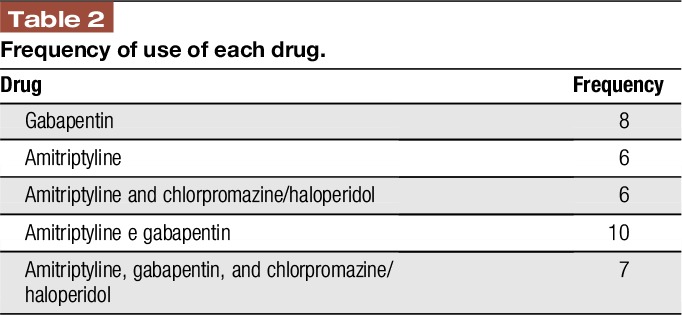
At visit 1, 52 leprosy patients had moderate to severe pain, and among those, 35 were not experiencing a clinically detectable leprosy reaction, and were then invited to attend visit 2, when the medication use profile was then assessed by the BPI, as well as the percentage of pain relief brought about by the treatment regimen. The frequency of use of each drug and the association treatments were described in Table 2.
Table 2.
Frequency of use of each drug.
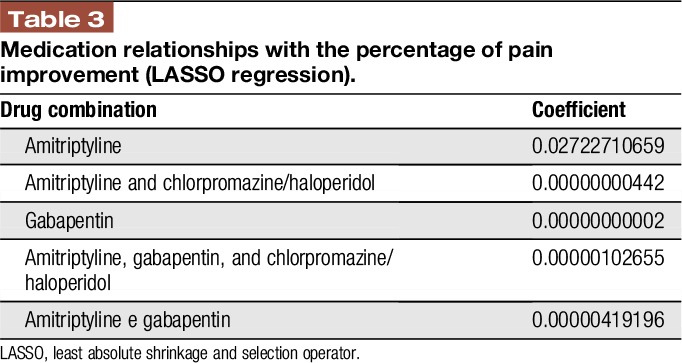
The linear regression with LASSO analyses showed 3 coefficients with positive relationship with the percentage of pain improvement from the BPI: the first drug in Table 3 is the most relevant. Higher coefficients are associated with the importance of each drug combination to explain the percentage of pain improvement.
Table 3.
Medication relationships with the percentage of pain improvement (LASSO regression).
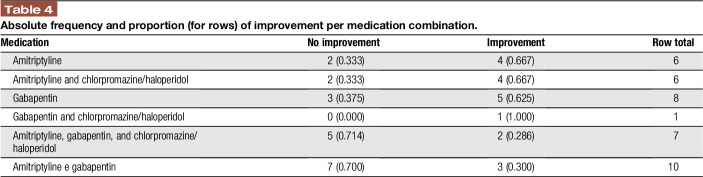
Considering these 3 drugs (amitriptyline, gabapentin, and chlorpromazine/haloperidol), amitriptyline and amitriptyline combined to chlorpromazine/haloperidol were the medicines with higher percentages of pain relief (66.7%). The combination of amitriptyline and gabapentin seemed to offer lower analgesic effects to patients, with greater proportions of patients without improvement (70.0% to amitriptyline + gabapentin), as expressed in Table 4.
Table 4.
Absolute frequency and proportion (for rows) of improvement per medication combination.
The linear regression analysis showed that the use of amitriptyline, when effective, had the highest percentage of analgesic relief.
4. Discussion
Neuropathic pain is prevalent, affecting up to 7% of the general population.3 Also, it is well known that patients with chronic pain with neuropathic components have a higher negative impact in quality of life when compared with those with chronic pain conditions of similar intensity, but which lack neuropathic components.10 Although leprosy is rare in developed countries, it is a common cause of disability and chronic pain in developing countries such as Brazil and India.7,18,23,34 Also, leprosy is associated with neuropathic pain in a significant proportion of cases, which frequently occurs after the end of microbiological treatment of disease. In fact, it has been reported that about one-third of patients treated for leprosy more than 10 years previously had pain of neuropathic characteristics, which was severe in intensity in 40% of the cases.32 For this reason, neuropathic pain in leprosy is also considered as a long-term sequel of this disease, and has been proposed to be included in the care after cure program.19 In the past years, several studies have validated and assessed the sensitivity and specificity of screening tools habitually used to detect neuropathic pain of other etiologies in leprosy patients.30,34 These tools have been shown to be useful when used in this setting, and are currently used by some groups to screen for neuropathic pain in leprosy patients. However, the next step in the care chain in the management of these patients is lacking. Neuropathic pain is known to be responsive to antidepressants and anticonvulsants with antihyperalgesic and antiallodynic properties.13 These drugs have varied efficacy,33 costs, side-effect profile, and mechanisms of action.2,5 To date, none of the drugs known to be effective in neuropathic pain in general have been formally tested in leprosy-related neuropathic pain. In fact, the characteristics of neuropathic pain in leprosy have rarely been described and never compared with neuropathic pain of other etiologies. This is the first step if one wishes to explore the drugs currently used to treat neuropathic pain in general in a leprosy patient. In this study, we have shown that the sensory profile of neuropathic pain related to leprosy had no major differences compared with neuropathic pain caused by other etiologies. Despite the higher baseline pain intensity in the leprosy group, the higher scores found for evoked pain in the leprosy group did not remain significant after correction for multiple comparisons. This suggests that leprosy is capable of causing neuropathic pain with different clinical presentations, similar to what is believed to occur to other frequent and more prevalent etiologies of neuropathic pain such as postherpetic neuralgia and diabetes mellitus, and its treatment should not be different from that of neuropathic pain caused by other etiologies.
The second step in this line of reasoning would be to assess potential drugs to be included in a drug trial. The choice of the best drug to be included in such a trial is not a trivial matter. One can perform bench studies to try to screen for potential candidate drugs,27 one can propose drugs based on putative mechanisms of action derived from experimental works,11 or one can observe what is currently offered to these patients in the clinical practice and explore potential drugs from this “real-life scenario.” We have followed this last choice. In this study, we have assessed the effects of different drugs prescribed to leprosy patients in an uncontrolled, unblinded fashion and, after a cluster assessment of the pain relief provided by the different drug regimens, we have found that amitriptyline would be a potential candidate.
Our study has obvious limitations because the sample size was a convenience sample, and the availability of drugs, the dosages, and the titration methods were not controlled for. Also, it is based on recall of efficacy of a group of drugs, with its inherent potential bias. On the other hand, tricyclic antidepressants have been shown to have the highest efficacy profile and recent meta-analyses13 and are widely available worldwide.21 We have also assessed pain treatment profile and efficacy at visit 2. We have no information on the pharmacological treatment patients were already on during visit 1. Also, despite that exclusion of clinically detectable leprosy reaction at visit 2 (which can co-occur with neuropathic pain and confound the assessment of pain), we only assessed patients with moderate to severe pain at visit 2, which has probably excluded patients with lighter and less severe neuropathic pain symptoms, which may have responded to other pharmacological agents or combinations not assessed here. Another issue that merits discussion is the definition of neuropathic pain in economically restricted areas. This is a challenge not limited to leprosy,38 and it opens important discussions when trying to adapt the current diagnostic grading system of neuropathic pain in regions where confirmatory tests aimed at confirmation of lesion or disease of the somatosensory system are difficult or impossible to perform. The present data collection has been undertaken before the latest diagnostic grading system recommendations.14 However, even if one applied these criteria to our sample of patients, we would be able to consider them as having definite neuropathic pain. The grading system requires a confirmatory test to provide the definite diagnosis of neuropathic pain, but when mentioning the effects of direct surgical lesions to nerves recognizes that: “(…) direct anatomical or surgical evidence (ie, of disease or lesion to the somatosensory system) counts as a confirmatory test.” Because our patients had major limb atrophic changes due to nerve lesions in the area of neuropathic pain, one can use it as surrogate markers of abnormal confirmatory tests.
Despite these limitations, we believe that the controlled comparison of leprosy and nonleprosy neuropathic pain allows us to conclude that leprosy can cause neuropathic pain of different clinical presentations, just as any other etiology of neuropathic pain. Also, it may respond to drugs usually used to control pain of neuropathic profile and amitriptyline may constitute a candidate drug for future formal clinical trials.
Disclosures
D. Ciampi de Andrade and M. J. Teixeira received researcher scholarship form Conselho Nacional de Desenvolvimento Científico e Tecnológico.
The institutional ethics committee at Instituto Lauro de Souza Lima approved the project number 230-A/14. All study participants provided written informed consent.
Acknowledgments
The authors sincerely thank the staff from Instituto Lauro de Souza Lima for contribution with their duties. Authors' contributions: I. Raicher, P. R. N. A. G. Stump, M. J. Teixeira, and D. Ciampi de Andrade designed the study. I. Raicher, P. R. N. A. G. Stump, R. Baccarelli, L. H. S. C. Marciano, S. Ura, and M. C. L. Virmond organized the project and collected the data. I. Raicher, S. B. Harnik, R. A. de Oliveira, D. Ciampi de Andrade, M. J. Teixeira, and M. C. L. Virmond performed data analyses and data interpretation. I. Raicher and D. Ciampi de Andrade drafted the initial manuscript and all authors assisted in the manuscript preparation and approved the manuscript.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A14.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Alter A, Grant A, Abel L, Alcaïs A, Schurr E. Leprosy as a genetic disease. Mamm Genome 2011;22:19–31. [DOI] [PubMed] [Google Scholar]
- [2].Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? PAIN 2015;156(suppl 1):S104–14. [DOI] [PubMed] [Google Scholar]
- [3].Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. PAIN 2011;152:2836–43. [DOI] [PubMed] [Google Scholar]
- [4].Avaliação sensitiva na neuropatia hansenica. In: Duerksen F, Virmond M, editors. Cirurgia Reparadora e Reabilitação em Hanseníase. 1st ed Rio de Janeiro: ALM International, 1997. p. 75–83. [Google Scholar]
- [5].Beal BR, Wallace MS. An overview of pharmacologic management of chronic pain. Med Clin North Am 2016;100:65–79. [DOI] [PubMed] [Google Scholar]
- [6].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the neuropathic pain symptom inventory. PAIN 2004;108:248–57. [DOI] [PubMed] [Google Scholar]
- [7].Chen S, Qu J, Chu T. Prevalence and characteristics of neuropathic pain in the people affected by leprosy in China. Lepr Rev 2012;83:195–201. [PubMed] [Google Scholar]
- [8].Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimeters? PAIN 1997;72:95–7. [DOI] [PubMed] [Google Scholar]
- [9].de Andrade DC, Ferreira KA, Nishimura CM, Yeng LT, Batista AF, de Sá K, Araujo J, Stump PR, Kaziyama HH, Galhardoni R, Fonoff ET, Ballester G, Zakka T, Bouhassira D, Teixeira MJ. Psychometric validation of the Portuguese version of the neuropathic pain symptoms inventory. Health Qual Life Outcomes 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the chikungunya fever: long lasting burden of an acute illness. BMC Infect Dis 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Devor M. Strategies for finding new pharmacological targets for neuropathic pain. Curr Pain Headache Rep 2004;8:187–91. [DOI] [PubMed] [Google Scholar]
- [12].Ferreira KA, Teixeira MJ, Mendonza TR, Cleeland CS. Validation of brief pain inventory to Brazilian patients with pain. Support Care Cancer 2011;19:505–11. [DOI] [PubMed] [Google Scholar]
- [13].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. PAIN 2014;155:367–76. [DOI] [PubMed] [Google Scholar]
- [16].Global leprosy situation, 2012. Wkly Epidemiol Rec 2012;87:317–28. [PubMed] [Google Scholar]
- [17].Hair JF, Jr, Anderson RE, Tatham RL, Black WC. Análise multivariada de dados. Tradução Adonai Schlup Sant'Anna e Anselmo Chaves Neto. 5th ed Porto Alegre: Bookman, 2005. [Google Scholar]
- [18].Haroun OM, Hietaharju A, Bizuneh E, Tesfaye F, Brandsma JW, Haanpää M, Rice AS, Lockwood DN. Investigation of neuropathic pain in treated leprosy patients in Ethiopia: a cross-sectional study. PAIN 2012;153:1620–4. [DOI] [PubMed] [Google Scholar]
- [19].Hietaharju A, Croft R, Alam R, Birch P, Mong A, Haanpää M. Chronic neuropathic pain in treated leprosy. Lancet 2000;356:1080–1. [DOI] [PubMed] [Google Scholar]
- [20].IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk: IBM Corp, 2011. [Google Scholar]
- [21].Kamerman PR, Wadley AL, Davis KD, Hietaharju A, Jain P, Kopf A, Meyer AC, Raja SN, Rice AS, Smith BH, Treede RD, Wiffen PJ. World Health Organization essential medicines lists: where are the drugs to treat neuropathic pain? PAIN 2015;156:793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet 2014;15:379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lasry-Levy E, Hietaharju A, Pai V, Ganapati R, Rice AS, Haanpää M, Lockwood DN. Neuropathic pain and psychological morbidity in patients with treated leprosy: a cross-sectional prevalence study in Mumbai. PLoS Negl Trop Dis 2011;5:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lázaro FP, Werneck RI, Mackert CC, Cobat A, Prevedello FC, Pimentel RP, Macedo GM, Eleutério MA, Vilar G, Abel L, Xavier MB, Alcaïs A, Mira MT. A major gene controls leprosy susceptibility in a hyperendemic isolated population from north of Brazil. J Infect Dis 2010;201:1598–605. [DOI] [PubMed] [Google Scholar]
- [25].Lockwood DN, Nicholls P, Smith WC, Das L, Barkataki P, van Brakel W, Suneetha S. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian patients with multibacillary leprosy. PLoS Negl Trop Dis 2012;6:e1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lockwood DN, Saunderson PR. Nerve damage in leprosy: a continuing challenge to scientists, clinicians and service providers. Int Health 2012;4:77–85. [DOI] [PubMed] [Google Scholar]
- [27].Maia RD. Recent trends in neuropathic pain patents. Expert Opin Ther Pat 2017;27:539–46. [DOI] [PubMed] [Google Scholar]
- [28].Mira MT, Alcaïs A, Nguyen VT, Moraes MO, Di Flumeri C, Vu HT, Mai CP, Nguyen TH, Nguyen NB, Pham XK, Sarno EN, Alter A, Montpetit A, Moraes ME, Moraes JR, Doré C, Gallant CJ, Lepage P, Verner A, Van De Vosse E, Hudson TJ, Abel L, Schurr E. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 2004;427:636–40. [DOI] [PubMed] [Google Scholar]
- [29].Passo IP, Palmeira CCA, Vieira ÉBM. Epidemiology of neuropathic pain. Rev Dor 2016;17(suppl 1):S11–4. [Google Scholar]
- [30].Raicher I, Stump PR, Baccarelli R, Marciano LH, Ura S, Virmond MC, Teixeira MJ, Ciampi de Andrade D. Neuropathic pain in leprosy. Clin Dermatol 2016;34:59–65. [DOI] [PubMed] [Google Scholar]
- [31].Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev 1962;33:119–28. [DOI] [PubMed] [Google Scholar]
- [32].Saunderson P, Bizuneh E, Leekassa R. Neuropathic pain in people treated for multibacillary leprosy more than ten years previously. Lepr Rev 2008;79:270–6. [PubMed] [Google Scholar]
- [33].Sidhu HS, Sadhotra A. Current status of the new antiepileptic drugs in chronic pain. Front Pharmacol 2016;7:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stump PRNAG, Baccarelli R, Marciano LHSC, Lauris JR, Teixeira MJ, Ura S, Virmond MC. Neuropathic pain in leprosy patients. Int J Lepr 2004;72:134–8. [DOI] [PubMed] [Google Scholar]
- [35].Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodological) 1996;58:267–88. [Google Scholar]
- [36].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5. [DOI] [PubMed] [Google Scholar]
- [37].WHO Expert Committee on leprosy. World Health Organ Tech Rep Ser 1998;874:1–43. [PubMed] [Google Scholar]
- [38].Woldeamanuel YW, Kamerman PR, Veliotes DG, Phillips TJ, Asboe D, Boffito M, Rice AS. Development, validation, and field-testing of an instrument for clinical assessment of HIV-associated neuropathy and neuropathic pain in resource-restricted and large population study settings. PLoS One 2016;11:e0164994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].World Health Organization Expert Committee on Leprosy. Seventh report. Technical report series, Geneva, Switzerland, 1998. p. 874. [PubMed] [Google Scholar]