The capsaicin 8% patch is a treatment option in patients with localized peripheral neuropathic pain. We provide first data on the effect of capsaicin on the electrophysiological properties of A-delta fibers.
Keywords: Capsaicin 8% patch, Pain-related evoked potentials, Neuropathic pain, Quantitative sensory testing
Abstract
Introduction:
The capsaicin 8% patch is a treatment option in patients with localized peripheral neuropathic pain. Better understanding of its mechanisms of action and knowledge on predictive biomarkers for a treatment response is warranted.
Objectives:
To use electrically evoked pain-related potentials for investigation of A-delta fiber conduction after capsaicin 8% patch treatment.
Methods:
We studied 11 healthy controls at the dorsal hand and the foot and 12 patients with neuropathic pain at the area affected by neuropathic pain before and 2 hours after application of a capsaicin 8% patch (Qutenza). Patients were additionally phenotyped using quantitative sensory testing and skin biopsy.
Results:
Peak-to-peak N1-P1 amplitudes (PPA) were reduced after Qutenza application by a median of 60% in 6/11 controls and by 33% in patients with neuropathic pain compared with baseline; they were increased in 3 controls that did not develop capsaicin-induced pain. Patients with elevated cold detection thresholds more often had reduced PPA after Qutenza than those with normal cold detection threshold. Patients with reduced PPA after capsaicin application and with capsaicin-induced pain were more likely to achieve pain reduction on Qutenza.
Conclusion:
The capsaicin 8% patch induces a reduction in A-delta PPA in healthy persons and in patients with neuropathic pain adding to the mechanistic understanding of its effect.
1. Introduction
The transdermal capsaicin 8% patch (Qutenza) containing 179 mg capsaicin was launched in 2009 for focal peripheral neuropathic pain syndromes.31 Capsaicin is a natural ligand of the transient receptor potential vanilloid 1 (TRPV1) channel, a nonselective cation channel of major importance in pain perception.2 TRPV1 is expressed on A-delta and C-nerve fibers, which can be activated in the skin by transdermal capsaicin. After an initial excitation of the cutaneous nociceptors with additional burning pain reported by a subgroup of treated patients, intraepidermal axons retract and become “de-sensitized.”29 Topically applied capsaicin increases thermal perception thresholds as measured with quantitative sensory testing (QST) in parallel to nerve fiber degeneration, sparing A-beta fiber function.13,19,23 Although in healthy controls cutaneous nociceptors regenerate within 24 weeks after treatment with the capsaicin 8% patch,12 nerve fiber regeneration kinetics are unknown in patients with neuropathic pain.
As with other costly drugs, it would be useful to know predictive factors for the response to Qutenza. In a post hoc analysis of clinical trial data, the efficacy of lidocaine pretreatment and high pretreatment variability of pain intensity were predictive of a positive capsaicin response.20 A meta-analysis of 6 Qutenza trials identified a lower pain score as response predictor in patients with postherpetic neuralgia and HIV-neuropathy, and additionally female sex, absence of allodynia, and the presence of hypoesthesia as predictors in postherpetic neuralgia.10 In a prospective study, a shorter pretreatment pain duration was suggested a response predictor.17 Using QST, the presence of mechanical and cold hyperalgesia was predictive for capsaicin 8% patch responders.18
Given these heterogeneous reports, we hypothesized that knowledge on the electrophysiological properties of nociceptors exposed to capsaicin 8% might help to better understand the mechanisms of capsaicin analgesia and potentially identify objective predictors. To achieve this, we assessed A-delta nerve fibers and their ascending tracts using electrically evoked pain-related potentials (PREPs) before and after topical capsaicin 8% patch application in control subjects and patients with neuropathic pain. We hypothesized that either baseline PREP parameters or the change of PREP after treatment would differentiate responders from nonresponders.
2. Patients and methods
2.1. Healthy controls and patients
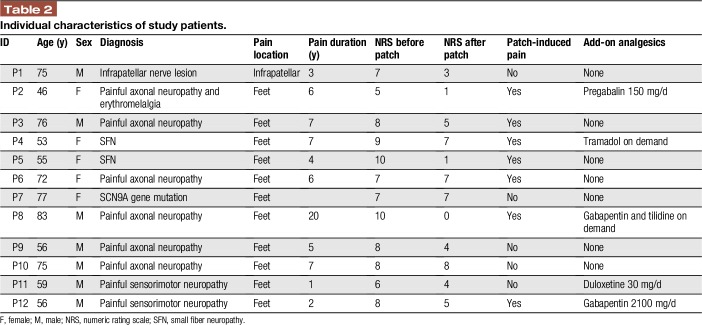
In this case series, we investigated 11 healthy volunteers and 12 patients with focal neuropathic pain at the lower extremities who received capsaicin 8% patch treatment at our Department of Neurology, University of Würzburg, Germany, between 2013 and 2017. The control group consisted of 8 women and 3 men with a median age of 49 years (23–70) reporting no neurological or other diseases and no pain. The patient group comprised 4 women and 8 men with a median age of 66 years (46–83) suffering from neuropathic pain of different etiologies at the lower extremities. Patients were investigated during a routine ambulatory appointment at our department where they receive a 3-monthly capsaicin 8% patch application. Treatment effect was assessed during an interview at the following regular appointment 3 months later. A positive treatment response was recorded if the patient's pain on a numeric rating scale (NRS, 0–10) was reduced by ≥2 points. This definition was chosen according to literature5,27 and the clinical effect by pain reduction in everyday life reported as “relevant” by our patients. Our study was approved by the Würzburg Medical Faculty Ethics Committee and subjects gave written informed consent before inclusion.
2.2. Quantitative sensory testing
Quantitative sensory testing (Somedic, Hörby, Sweden) was performed as described earlier35 at the dorsal foot of 8/11 (73%) healthy controls and in 8/12 (67%) patients as part of diagnostic work-up. Cold detection threshold (CDT) and heat detection threshold, the ability to detect temperature changes (thermal sensory limen), mechanical detection and pain thresholds, mechanical pain sensitivity, pressure pain threshold, paradoxical heat sensation, and vibration detection threshold were determined following the standardized procedure of the German Research Network of Neuropathic Pain (Deutscher Forschungsverbund Neuropathischer Schmerz, DFNS).26
2.3. Pain-related evoked potentials
Pain-related evoked potentials were recorded as previously described.35 Potentials were elicited by consecutive stimulation at the area of interest with superficial concentric planar electrodes (Inomed Medizintechnik GmbH, Lübeck, Germany) and using a stimulator (Digitimer DS7A, Welwyn Garden City, United Kingdom). Potentials were recorded from Cz using a subcutaneously placed needle electrode referred to linked earlobes (A1–A2) of the international 10 to 20 system. Signal Software (Version 2-16; Cambridge Electronic Design, Ltd, United Kingdom) was used for data acquisition. Twenty triple pulses with twice the intensity of the individual pain threshold, a duration of 0.5 milliseconds, and a random interstimulus interval of 15 to 17 seconds were applied to avoid habituation. To achieve similar attention levels, we asked all subjects to lightly close their eyes during the recordings, count the stimulations applied, and rate the painfulness of the pin-prick sensation.
Pain-related evoked potentials were recorded using the following setting: gain: ×5000, bandwidth: 1 Hz to 1 kHz, sweep length: 400 milliseconds, and digitalization sampling rate: 2.5 kHz. Individual pain thresholds were determined by triple stimulation of the area of interest twice with increasing and decreasing current intensities until the subject reported a pin-prick sensation. The average value was determined as the individual pain threshold. The upper limit for PREP stimulation was set at 2.4 mA to avoid A-beta fiber stimulation. Two sets of averaged curves (from n = 10 single sweeps each) were investigated for reproducible N1- (ie, first negative peak), P1- (ie, subsequent positive peak) latencies, and N1-P1 peak-to-peak amplitudes (PPA) using MATLAB software (Version 7.7.0.471; The MathWorks, Ismaning, Germany). The early N1-P1 complex of the PREP potential obtained from above Cz was used instead of the later N2-P2 complex in accordance with PREP literature.22,24,25 All PREP records were individually and manually evaluated by an investigator blinded to subjects' identity; data were assessed off-line using coded files.
2.4. Skin punch biopsy
Intraepidermal nerve fiber density (IENFD) was determined on 5-mm skin punch biopsies obtained from the lateral lower leg and the lateral upper thigh of 9/12 (75%) patients as part of diagnostic work-up. Skin samples were processed as reported previously.33 Fifty-micrometer skin sections were immunostained with antibodies against the pan-axonal marker protein-gene product 9.5 (1:1000; Ultra-clone, Isle of Wight, United Kingdom); an appropriate fluorescent secondary antibody was applied (Cy3, 1:100; Dianova, Hamburg, Germany). Intraepidermal nerve fiber density was determined using a fluorescence microscope (Axiophot 2; Zeiss, Oberkochen, Germany) with an Axiocam MRm camera (Zeiss) and SPOT software (Diagnostic Instruments, Inc, Sterming Heights, MI) following published counting rules.14
2.5. Investigation of control subjects
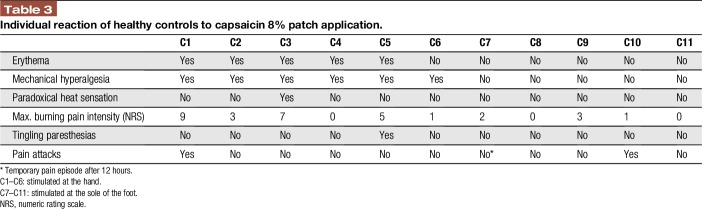
The study protocol of control subjects followed the algorithm illustrated in Figure 1. Pain-related evoked potential stimulation was performed at the dorsal second phalanx of the index finger of the nondominant hand or the sole of the right foot before and after capsaicin 8% patch (Qutenza; Astellas, Munich, Germany) application. Six controls were treated at the hand; 5 controls agreed to treatment at the foot. The experiment started with a baseline PREP recording. In addition, baseline mechanical sensitivity of the test area was assessed using a von Frey filament (0.25 g). The test area was then pretreated with lidocaine–prilocaine cream 2.5%/2.5% (Emla; AstraZeneca GmbH, Wedel, Germany) for 1 hour. After washing off the cream, a 1 × 2 cm stripe of capsaicin 8% patch was applied for 1 hour following the manufacturer's instructions. After another wash off and 2 further hours, a second PREP recording was performed and mechanical sensitivity was tested with the von Frey filament (0.25 g). Measurements (PREP, von Frey) were repeated at days 3 and 7 after capsaicin 8% patch application in controls treated at the hand. At all test time points, subjects were also asked to report patch effects.
Figure 1.
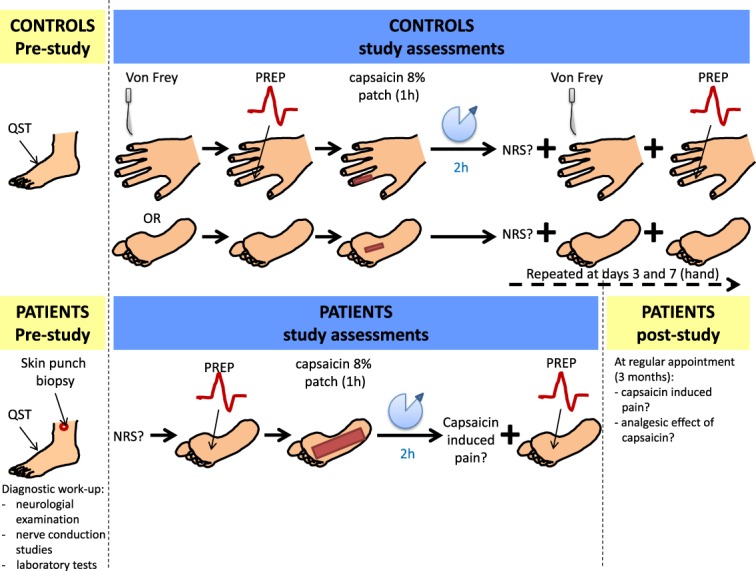
Schematic illustration of the study design for assessment of healthy controls and patients with neuropathic pain. Please note that all patients were stimulated at the plantar surface of the foot except for 1 patient who had pain in the infrapatellar region. NRS, numeric rating scale; PREP, pain-related evoked potentials; QST, quantitative sensory testing.
2.6. Investigation of patients with neuropathic pain
Patients were investigated after the protocol illustrated in Figure 1. Pain-related evoked potential stimulation was performed at the individual painful body area before and after capsaicin 8% patch (Qutenza; Astellas) application. This was the plantar surface of the foot in all cases but one; 1 patient had pain at the infrapatellar region and received capsaicin treatment and PREP stimulation there. The experiment started with a baseline PREP recording. Afterwards, the test area was pretreated with lidocaine–prilocaine cream 2.5%/2.5% (Emla; AstraZeneca GmbH) for 1 hour. After washing off the cream, the respective body area was covered with a capsaicin 8% patch for another hour according to the manufacturer's instructions. After wash off and 2 further hours, a second PREP recording was performed. Patients were interviewed about the individual treatment response at their regular visit at our department 3 months later. A responder was defined as a person who reported a reduction of ≥2 on the NRS or, if the NRS score was not available, at least “moderate” pain relief.
2.7. Statistical analysis
We used IBM SPSS Statistics Version 24.0 (IBM, Ehningen, Germany) for statistical analysis; for graph design, additionally GraphPad Prism 3.0 (San Diego, CA) was used. For group-wise comparisons, the nonparametric Mann–Whitney test was used and for correlation analysis, the Spearman correlation coefficient was applied. To detect potential dependency between outcome parameters, the χ2 test was used. P values < 0.05 were considered significant.
3. Results
3.1. Capsaicin 8% patch tolerability and effect
Table 1 summarizes the characteristics of the study cohort; Table 2 gives patients' individual data. Healthy controls tolerated capsaicin 8% patch application well; however, the majority reported mechanical hyperalgesia and/or local erythema (6/11, 55%) accompanied by local burning pain for several days (8/11, 73%; Table 3). Patients reported capsaicin-induced burning pain in 7/12 (58%) cases; 9/12 (75%) patients experienced a reduction of initial pain intensity in the days and weeks after treatment (Table 2).
Table 1.
Demographic and clinical characteristics of study cohort.
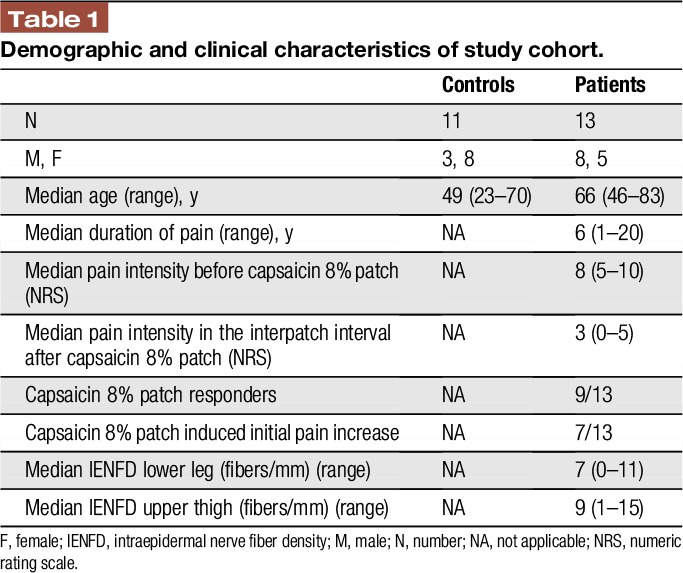
Table 2.
Individual characteristics of study patients.
Table 3.
Individual reaction of healthy controls to capsaicin 8% patch application.
3.2. Baseline pain-related evoked potential data
Pain-related evoked potentials were obtained in all controls stimulated at the hand and at the foot, whereas in patients, who were stimulated at the lower extremities only, PREPs were available in 9/12 cases (75%). Group median at baseline in control subjects stimulated at the hand for N1 latencies was 148.85 milliseconds (85.25–161.75 ms), for P1 latencies 195.40 ms (152.53–252.07 ms), and for PPA 0.05 mV (0.03–0.11 mV). In controls stimulated at the sole median, N1-latency was 213.4 ms (171.9–263.1 ms), P1-latency 233.6 ms (207.8–294.5 ms), and PPA was 0.01 mV (0.001–0.1 mV). Group median for the n = 6 of 9 patients stimulated at the sole of the foot with reproducible responses was 203.37 milliseconds (135.90–261.79) for N1 latencies, 256.68 milliseconds (187.60–305.50) for P1 latencies, and 0.04 mV (0.03–0.06 mV) for PPA.
3.3. Capsaicin 8% patch reduces pain-related evoked potential peak-to-peak amplitude in healthy controls
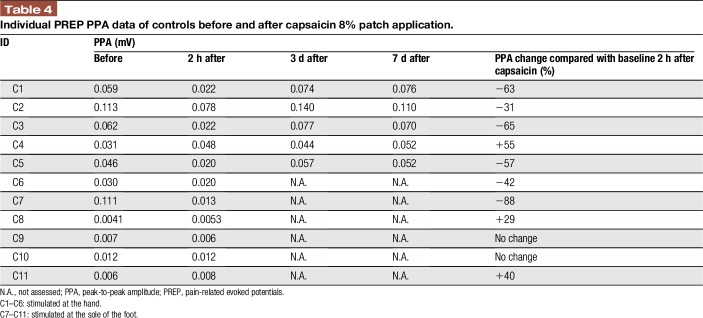
Pain-related evoked potential recordings after capsaicin 8% application at the hand and sole gave reproducible results in all control subjects and at all measurement time points. Although N1 and P1 latencies and PPA of the treated extremity did not differ before and after capsaicin application (data not shown), PPA was decreased at 2 hours after capsaicin 8% patch application in 6/11 (55%) controls (Figure 2 and Table 4). Peak-to-peak amplitude was reduced from baseline by a median of 60% (31–88). At day 3, PPA values had returned to baseline in all cases treated at the hand; no measurements at later time points were available from controls treated at the soles.
Figure 2.
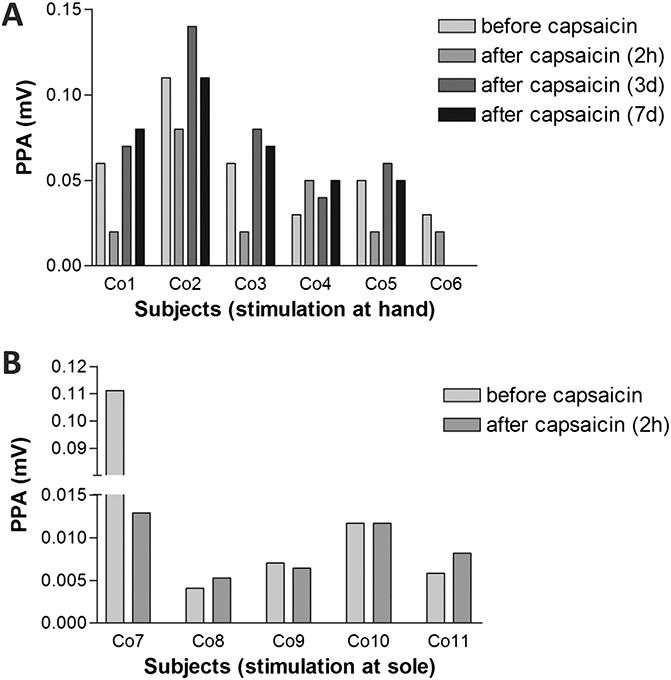
Pain-related evoked potential peak-to-peak amplitudes (PPAs) in healthy controls before and after capsaicin 8% patch treatment. Peak-to-peak amplitude (N1-P1) decreased within 2 hours after capsaicin 8% patch application in 5/6 (83%) control subjects (co) treated at the hand (A) and 2/5 controls treated at the foot (B).
Table 4.
Individual PREP PPA data of controls before and after capsaicin 8% patch application.
Interestingly, 1 healthy male (stimulated at the hand) and 2 female controls (stimulated at the sole) did not report capsaicin-induced pain, and in these cases, PPA after treatment increased from 0.031, 0.0041, and 0.0058 mV at baseline to 0.048, 0.0053, and 0.0082 mV, respectively, 2 hours after capsaicin 8% patch application. Peak-to-peak amplitude was assessed up to day 7 after capsaicin application at the hand and was still elevated in the male subject (0.052 mV). In 2 controls treated at the soles, capsaicin application did not change PPA at 2 hours (Figure 2).
3.4. Capsaicin 8% patch reduces pain-related evoked potential peak-to-peak amplitude in patients with neuropathic pain
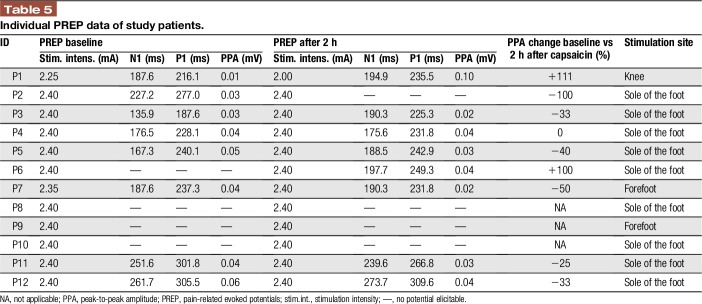
Pain-related evoked potential recordings gave reproducible results at baseline in 8/12 (67%) patients and also at 2 hours after capsaicin 8% application. In 3 patients, no PREP potentials could be elicited at baseline and also not after treatment; in 1 patient, PREPs were lost after capsaicin 8%; and in 1 further patient, PREP could not be elicited before treatment, but afterwards. Although N1 and P1 latencies did not differ before and after capsaicin 8% application (Table 5), PPA decreased in 6/9 (67%) patients within 2 hours after treatment (Figure 3). The median PPA reduction in those 5/7 (71%) patients, in whom precapsaicin and postcapsaicin potentials were available, was 33% (25–50). Pain ratings for the applied electrical stimulus did not change after capsaicin 8% patch application (data not shown).
Table 5.
Individual PREP data of study patients.
Figure 3.
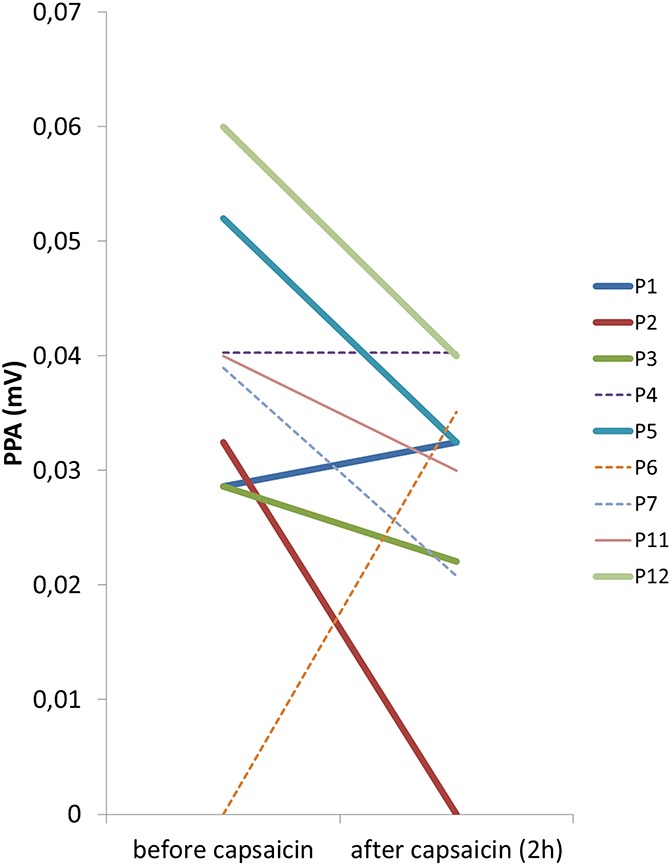
Pain-related evoked potential peak-to-peak amplitudes (PPAs) in patients with neuropathic pain capsaicin 8% patch treatment. Peak-to-peak amplitude (N1-P1) decreased in 7/9 patients within 2 hours after capsaicin 8% patch application. Responders are indicated by thick lines and nonresponders by dotted lines.
3.5. Patient phenotyping and pain-related evoked potential parameters do not provide predictors for treatment response
When assessing responders (n = 9) and nonresponders (n = 3) to capsaicin 8% patch treatment, 6/9 (67%) patients in the responder group reported patch-induced pain in contrast to only 1 patient (33%) in the nonresponder group. No differences were found for age and sex, duration or cause of pain, pain characteristics, PREP parameters (recordable in 1/3 nonresponders), QST profiles, and IENFD (data not shown). When regarding CDT, PPA reduction after treatment, distal IENFD, and pain induced by capsaicin individually, there was a nonsignificant trend towards more patients with the combination of capsaicin-induced pain and reduced distal IENFD in the responder group (4/7 vs 0/2; Table 6, χ2 test, P = 0.167).
Table 6.
Characterization of responders and nonresponders (percentage of assessable data).
Two patients from the responder group reported complete pain relief while having a maximum of 10/10 NRS pain before capsaicin (Table 5: #5, #8). In these patients, CDT was normal at baseline and both reported severe capsaicin-induced pain on the first day after treatment.
3.6. Thermal perception and A-delta nerve fiber excitability
Baseline analysis of A-delta fiber properties revealed patients with normal (n = 5) and elevated (n = 4) CDT (ie, reduced sensitivity for cold stimuli) at baseline. Although PREP parameters did not differ between these 2 groups (data not shown), N1 positively correlated with CDT (correlation coefficient 0.778; P < 0.05; Figure 4). Patients with normal CDT had higher pain ratings before capsaicin treatment than patients with elevated CDT (P < 0.05; Figure 5A). These patients with normal CDT and higher baseline NRS also more frequently developed capsaicin-induced additional pain after treatment (P < 0.05; Figure 5B).
Figure 4.
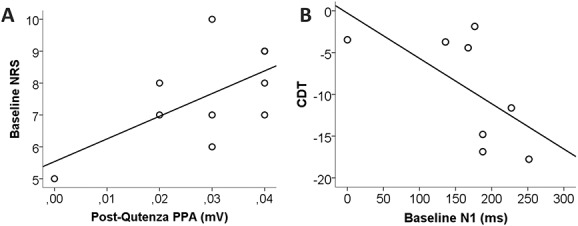
Pain-related evoked potential correlations. A) Baseline pain intensity on an 11-point numeric rating scale (NRS) positively correlated with peak-to-peakamplitude (PPA) after capsaicin application (P, 0.05) and B) baseline N1 negatively correlated with cold perception (P, 0.05). CDT, cold detection threshold.
Figure 5.
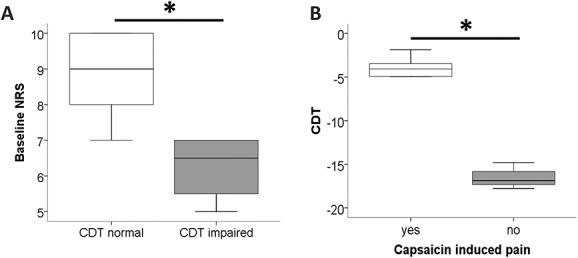
Subgroup analysis. (A) Patients with normal cold detection thresholds (CDTs) had higher pain ratings on an 11-point numeric rating scale (NRS) before capsaicin treatment than patients with increased CDT (P < 0.05). (B) Patients with normal CDT and higher baseline NRS more frequently developed capsaicin-induced additional pain after treatment (P < 0.05).*P<0.05.
4. Discussion
In this case series, we investigated the effect of capsaicin 8% patch treatment on A-delta fiber properties in healthy controls and patients with focal neuropathic pain using PREP recordings. We did not find direct predictors for capsaicin 8% patch treatment response; however, we report on an effect of the capsaicin 8% patch on A-delta fiber evoked potentials, ie, a reduction of PREP PPA amplitudes. We further provide data on potential patient profiles that may have a higher chance to benefit from Qutenza. Thus, patients experiencing capsaicin-induced burning pain after patch application and with reduced distal IENFD were more frequently found in our responder group.
Using PREP, we show that N1 latencies, ie, the duration until a potential can be recorded from Cz after peripheral stimulation, correlate with CDT. Thus, impaired cold detection through A-delta fibers is associated with prolonged PREP N1 latencies. This finding adds to the evidence that PREPs are based on A-delta conduction,9,11,15 provided methodological caveats such as thresholds for stimulation intensities are observed and measures assuring data reproducibility are taken.
Comparable with numbers reported in previous studies,35 PREP could be elicited after stimulation at the hand and the sole in all controls, whereas no potentials could be recorded in 4 patients after stimulation at the foot. Similar to nerve conduction studies, this may be due to the underlying neuropathy; however, it needs to be interpreted with caution because other potential confounding factors such as the age difference between our control and patient group or the presence of pain cannot be excluded.
Besides the fact that pretreatment A-delta fiber impairment was present only in the patient group, the different PREP stimulation sites may have had an impact on our results. The younger median age of our control group may have been another influential factor changing A-delta fiber excitability. Here, also the long duration of the refractory phases after TRPV1 channel activation by capsaicin may be of importance.28 During this period of “desensitization,” TRPV1 carrying nociceptors cannot be excited by a wide range of stimuli, potentially including electrical current30 because the calcium-dependent conformational changes in the TRPV1 protein close the channel pore.16 It is possible that these mechanisms are altered in patients with neuropathic pain. Furthermore, it has to be taken into account that TRPV1 channels are mostly located on C-fibers, thus our results may not reflect the entire spectrum.
In our control group, PREP PPA, which was reduced at 2 hours after capsaicin 8% patch application, returned to baseline values within few days. Because intraepidermal innervation needs several weeks to recover after high-concentration capsaicin exposure,12 PREP parameters seem to be independent of the number of fibers present in the epidermis. This is in accordance with our previous reports where no correlations could be found between PREP parameters and skin nerve fiber counts.8,34,35 Rather than the number of fibers present, nerve fiber excitability based on ion channel presence and activity seems to be crucial for PREP conductance.
It is an intriguing observation that 1 male and 2 female healthy controls did not develop any pain or hypersensitivity on capsaicin 8% patch application at all and also did not show a reduction, but rather a sustained increase in PPA after treatment. These cases may help to better understand the physiological basis of Qutenza responsiveness and A-delta properties. We speculate on physiologically diverse capsaicin susceptibility of TRPV1 receptors. If the receptors can be activated by capsaicin, this leads to pain. Additional electrical stimulation of these already maximally activated nociceptors then can only elicit lower PPA than stimulation of naive nociceptors. By contrast, if the individual TRPV1 receptors are not susceptible to capsaicin, then no pain occurs, and the nociceptors can still be stimulated by electrical current, resulting in normal PPA. Contrary to this speculation, the patient in the nonresponder group with recordable PREP also reported no capsaicin-induced pain but showed PPA reduction within 2 hours after treatment. This, in turn, may be due to the pathologically altered TRPV1 channels in diseased nociceptors. Another possibility are genetic alterations changing TRPV1 activation properties and somatosensorics also in healthy controls.1,6
There are further open questions that need to be considered when interpreting our data. Although it was shown that capsaicin reaches the dermal layers of the skin within 30 minutes of application,36 we did not measure the cutaneous penetration depth of capsaicin in our subjects. Thus, dermal concentrations may have been different between the study participants. Capsaicin may exert its effects depending on the underlying diagnosis10 and our patient cohort consisted of different diagnoses. Effects may change in homogenous large diagnostic groups. The question, why some patients develop capsaicin-induced pain and others do not is still open. Here, the receptor repertoire on the intraepidermal nerve fibers is a crucial factor that is, however, not easy to assess. It is plausible that pain syndromes based on the overexpression or overexcitation of ion channels other than TRPV1 may not respond to capsaicin treatment. Also, the effects of the surrounding keratinocytes and fibroblasts that both express TRPV1 channels have not been taken into account in the studies conducted so far.
One limitation of our study is the low number of subjects investigated, which has also hampered our analysis for predictive markers. However, in this small study group, we applied many tests allowing insights into small nerve fibers from functional, electrical, and histological perspectives. Another limitation is that patients were stimulated at different anatomical sites, depending on their painful area, whereas electrical stimulation was standardized to the hand and sole in controls. This restricts cross-comparability of our results, but is of minor relevance when regarding PREP parameter changes before and after treatment, which was the main goal of our study. Also, we cannot exclude that some of the patients might have had neuropathy of A-delta fibers, which may have affected the PREP stimulation threshold.
The A-delta selectivity of PREP is still debated.3,4,7 Using low currents with high current density, the activation of superficial skin layers is achieved sparing deep skin containing A-beta fibers. This is based on the small anode-to-cathode distance of the concentric electrodes. Pain-related evoked potential stimulation causes a pin-prick sensation that is typically conducted by A-delta fibers. Also, the nerve conduction velocity of the investigated fibers that are stimulated by the concentric electrodes is typical to those of A-delta fibers (unpublished own data9,15,21), both when recorded from above Cz and when recorded contralaterally from above C3 or C4 after stimulation in the innervation territory of a peripheral nerve (unpublished own data). There is also increasing evidence for the high test–retest reliability of PREP recordings.25 Thus, although PREP is prone to artifacts, and A-beta fiber activation may occur when not observing caveat such as low-current intensity, a predominant A-delta fiber stimulation can be assumed.
Pain-related evoked potential amplitude reduction was most prominent at 2 hours after Qutenza which theoretically may have been due to pain caused by capsaicin; however, only a subgroup of subjects developed Qutenza-induced pain. We can only speculate at this point, but assume a relevant influence of the individual genetic background determining ion channel activity and excitability as has been shown for capsaicin-induced changes in thermal perception thresholds of healthy controls carrying different allele variants of a TRPV1 polymorphism.6 Although we cannot completely exclude the influence of attention, we tried to control this confounding factor by asking the patient to lightly close their eyes during the recordings, count the stimulations applied, and rate the painfulness of the pin-prick sensation; this should lead to comparable attention levels in all subjects. To avoid habituation, PREP stimulations were applied with a random and various interstimulus interval of 15 to 17 seconds.
Pain-related evoked potential proves to be a reliable A-delta test that shows capsaicin-induced reduction in PPA in healthy controls and patients with neuropathic pain. To predict a capsaicin response, a set of A-delta properties may be more promising than the search for single predictors, which we also could not detect here. Our data parallel a previous study using laser-evoked potentials (LEPs) to stimulate A-delta nerve fibers and assessing the effect of tramadol on LEP parameters.32 Interestingly, tramadol also led to a reduction in LEP amplitudes in healthy controls, which was partially reversed by naloxone. Thus, assessment of PREPs may become a valuable tool to decipher the underlying mechanisms of topically applicable drugs.
Disclosures
A. Papagianni and G. Siedler: report no conflicts of interest. C. Sommer: received honoraria, travel grants, and research grants from Astellas, Baxalta, Genzyme, Air Liquide, CSL-Bering, Pfizer, UCB, LFB, Grifols, and Kedrion. N. Üçeyler: received honoraria, travel grants, and research grants from Sanofi Genzyme, Shire, Daiichi Sankyo, and Baxalta.
The study was supported by intramural funds of the Department of Neurology, University of Würzburg, Germany.
Acknowledgements
Expert technical help by Judith Sauer and Daniela Urlaub is gratefully acknowledged. We also thank Dr. Daniel Zeller for providing hard- and software equipment for PREP analysis.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Binder A, May D, Baron R, Maier C, Tolle TR, Treede RD, Berthele A, Faltraco F, Flor H, Gierthmuhlen J, Haenisch S, Huge V, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Uceyler N, Ufer M, Wasner G, Zhu J, Cascorbi I. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 2011;6:e17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. [DOI] [PubMed] [Google Scholar]
- [3].de Tommaso M. Reply to “Activating selectively and reliably nociceptive afferents with concentric electrode stimulation: yes we can! provided that low stimulus intensities are used!”. Clin Neurophysiol 2013;124:424–5. [DOI] [PubMed] [Google Scholar]
- [4].de Tommaso M, Santostasi R, Devitofrancesco V, Franco G, Vecchio E, Delussi M, Livrea P, Katzarava Z. A comparative study of cortical responses evoked by transcutaneous electrical vs CO(2) laser stimulation. Clin Neurophysiol 2011;122:2482–7. [DOI] [PubMed] [Google Scholar]
- [5].Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [6].Forstenpointner J, Forster M, May D, Hofschulte F, Cascorbi I, Wasner G, Gierthmuhlen J, Baron R. Short report: TRPV1-polymorphism 1911 A>G alters capsaicin-induced sensory changes in healthy subjects. PLoS One 2017;12:e0183322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garcia-Larrea L. Somatosensory volleys and cortical evoked potentials: “first come, first served”? PAIN 2004;112:5–7. [DOI] [PubMed] [Google Scholar]
- [8].Hansen N, Kahn AK, Zeller D, Katsarava Z, Sommer C, Üçeyler N. Amplitudes of pain-related evoked potentials are useful to detect small fiber involvement in painful mixed fiber neuropathies in addition to quantitative sensory testing - an electrophysiological study. Front Neurol 2015;6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kakigi R, Endo C, Neshige R, Kuroda Y, Shibasaki H. Estimation of conduction velocity of A delta fibers in humans. Muscle Nerve 1991;14:1193–6. [DOI] [PubMed] [Google Scholar]
- [10].Katz NP, Mou J, Paillard FC, Turnbull B, Trudeau J, Stoker M. Predictors of response in patients with postherpetic neuralgia and HIV-associated neuropathy treated with the 8% capsaicin patch (Qutenza). Clin J Pain 2015;31:859–66. [DOI] [PubMed] [Google Scholar]
- [11].Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 2000;111:413–16. [DOI] [PubMed] [Google Scholar]
- [12].Kennedy WR, Vanhove GF, Lu SP, Tobias J, Bley KR, Walk D, Wendelschafer-Crabb G, Simone DA, Selim MM. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain 2010;11:579–87. [DOI] [PubMed] [Google Scholar]
- [13].Khalili N, Wendelschafer-Crabb G, Kennedy WR, Simone DA. Influence of thermode size for detecting heat pain dysfunction in a capsaicin model of epidermal nerve fiber loss. PAIN 2001;91:241–50. [DOI] [PubMed] [Google Scholar]
- [14].Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C; European Federation of Neurological S. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–58. [DOI] [PubMed] [Google Scholar]
- [15].Lefaucheur JP, Ahdab R, Ayache SS, Lefaucheur-Menard I, Rouie D, Tebbal D, Neves DO, Ciampi de Andrade D. Pain-related evoked potentials: a comparative study between electrical stimulation using a concentric planar electrode and laser stimulation using a CO2 laser. Neurophysiol Clin 2012;42:199–206. [DOI] [PubMed] [Google Scholar]
- [16].Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol 1996;75:1503–14. [DOI] [PubMed] [Google Scholar]
- [17].Maihöfner CG, Heskamp ML. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain 2013;18:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mainka T, Malewicz NM, Baron R, Enax-Krumova EK, Treede RD, Maier C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur J Pain 2016;20:116–29. [DOI] [PubMed] [Google Scholar]
- [19].Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. PAIN 2004;111:360–7. [DOI] [PubMed] [Google Scholar]
- [20].Martini CH, Yassen A, Krebs-Brown A, Passier P, Stoker M, Olofsen E, Dahan A. A novel approach to identify responder subgroups and predictors of response to low- and high-dose capsaicin patches in postherpetic neuralgia. Eur J Pain 2013;17:1491–501. [DOI] [PubMed] [Google Scholar]
- [21].Mueller D, Obermann M, Koeppen S, Kavuk I, Yoon MS, Sack F, Diener HC, Kaube H, Katsarava Z. Electrically evoked nociceptive potentials for early detection of diabetic small-fiber neuropathy. Eur J Neurol 2010;17:834–41. [DOI] [PubMed] [Google Scholar]
- [22].Muller MD, Muller SM, Ryan EJ, Bellar DM, Kim CH, Glickman EL. Pain and thermal sensation in the cold: the effect of interval versus continuous exercise. Eur J Appl Physiol 2011;111:979–87. [DOI] [PubMed] [Google Scholar]
- [23].Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. PAIN 1999;81:135–45. [DOI] [PubMed] [Google Scholar]
- [24].Obermann M, Katsarava Z, Esser S, Sommer C, He L, Selter L, Yoon MS, Kaube H, Diener HC, Maschke M. Correlation of epidermal nerve fiber density with pain-related evoked potentials in HIV neuropathy. PAIN 2008;138:79–86. [DOI] [PubMed] [Google Scholar]
- [25].Özgül OS, Maier C, Enax-Krumova EK, Vollert J, Fischer M, Tegenthoff M, Höffken O. High test-retest-reliability of pain-related evoked potentials (PREP) in healthy subjects. Neurosci Lett 2017;647:110–16. [DOI] [PubMed] [Google Scholar]
- [26].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [27].Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. [DOI] [PubMed] [Google Scholar]
- [28].Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharmacol 2013;720:55–62. [DOI] [PubMed] [Google Scholar]
- [29].Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci 1998;18:8947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 1999;51:159–212. [PubMed] [Google Scholar]
- [31].Treede RD, Wagner T, Kern KU, Husstedt IW, Arendt G, Birklein F, Cegla T, Freynhagen R, Gockel HH, Heskamp ML, Jager H, Joppich R, Maier C, Leffler A, Nagelein HH, Rolke R, Seddigh S, Sommer C, Stander S, Wasner G, Baron R. Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr Med Res Opin 2013;29:527–38. [DOI] [PubMed] [Google Scholar]
- [32].Truini A, Panuccio G, Galeotti F, Maluccio MR, Sartucci F, Avoli M, Cruccu G. Laser-evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. Eur J Pain 2010;14:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Üçeyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, Sommer C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010;74:1806–13. [DOI] [PubMed] [Google Scholar]
- [34].Üçeyler N, Kahn AK, Kramer D, Zeller D, Casanova-Molla J, Wanner C, Weidemann F, Katsarava Z, Sommer C. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case-control study. BMC Neurol 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:1857–67. [DOI] [PubMed] [Google Scholar]
- [36].Wohlrab J, Neubert RH, Heskamp ML, Michael J. Cutaneous drug delivery of capsaicin after in vitro administration of the 8% capsaicin dermal patch system. Skin Pharmacol Physiol 2015;28:65–74. [DOI] [PubMed] [Google Scholar]