Abstract
Objective:
This study aimed to determine the expression of lactate dehydrogenase (LDH)-A and LDH-D in patients with uterine myoma, cellular leiomyoma (CLM), and uterine sarcoma and to evaluate their prognostic significance.
Methods:
Protein expression levels of LDH-A and LDH-D were determined in tissue samples from 86 patients (26 uterine myoma, 10 CLM, 50 uterine sarcoma) by immunohistochemistry and their associations with clinicopathologic parameters and outcomes were analyzed in patients with uterine sarcoma.
Results:
The positivity rates for LDH-A and LDH-D were significantly higher in patients with uterine sarcoma compared with those with uterine myoma or CLM (P < .05). Patients with uterine sarcoma were classified as having uterine leiomyosarcoma (LMS), malignant endometrial stromal sarcoma, and malignant mixed Mullerian tumor, with 5-year overall survival rates of 59%, 71%, and 29%, respectively (P < .05). Univariate analysis showed that patients younger than 50 years and with stage I-II had better clinical prognoses. LDH-A-positive LMS patients had a poorer prognosis than LDH-A-negative patients (P = .03). The median survival time of LDH-A-positive patients was 35 months.
Conclusions:
We demonstrated that LDH-D was expressed in patients with uterine sarcoma. Furthermore, the overexpressions of LDH-A and LDH-D in uterine sarcoma patients may contribute to further understanding of the mechanism of LDH in tumor metabolism in uterine sarcoma. Positive expression of LDH-A in patients with LMS may act as a potential prognostic biomarker in these patients.
Keywords: lactate dehydrogenase-A, lactate dehydrogenase-D, immunohistochemistry, survival, uterine sarcoma
1. Introduction
Activation of glycolytic metabolism[1] is a significant chemical characteristic of malignant tumor cells, and lactate dehydrogenase (LDH) is an important coenzyme in glycolysis. Glycolysis occurs in malignant tumor cells even in microenvironments with adequate oxygen, which is a characteristic feature of tumor cells referred to as the Warburg effect.[2] Serum LDH levels were previously shown to be elevated in patients with uterine sarcoma.[3] LDH exists as either D- or LDH-L; numerous studies have investigated LDH-L, including its subunits LDH-A, LDH-B, and LDH-C, and its structure and function have been reported previously.[4] Many studies of tumor metabolism are currently focused on cell proliferation and apoptosis in the Warburg effect, and LDH-A may play an important role in this process.[5] Another study demonstrated that LDH-A was expressed in osteosarcoma patients.[6]The study found that LDH-A was commonly upregulated in four osteosarcoma cell lines compared with the normal osteoblast cells. Treatment with FX11, a specific inhibitor of LDH-A, significantly reduced LDH-A activity and inhibited cell proliferation and invasive potential in a dose-dependent manner. Taken together, upregulated LDH-A facilitates tumor progression of osteosarcoma. Glycolytic metabolism is also activated in uterine sarcoma, but there is currently no relevant research regarding the expression of LDH-A in patients with uterine sarcoma.
In contrast, although LDH-D has been studied in the mitochondria of yeast[7] and plants,[8,9] it has rarely been investigated in humans. Flick and Konieczny[10] identified and characterized putative human and murine LDH-D, which were shown to interact with the muscle-specific cysteine-rich protein CRP3/MLP. Expression analysis of mammalian proteins indicated that LDH-D was widespread in striated muscle tissues and a variety of other tissue types. Uterine sarcoma is rich in smooth muscle tissue, but the expression of LDH-D in human uterine leiomyoma and uterine sarcoma has not yet been investigated.
Uterine sarcoma accounts for 2.6% to 9.7% of uterine malignancies and 1% of malignant female genital system tumors, with a low morbidity but a poor prognosis.[11] The prognostic factors in uterine sarcoma are not well established. Although some studies have shown that p53 and Ki-67 overexpression may affect the prognosis of uterine sarcoma,[12] there is currently no consensus on their significance. The prognostic influence of LDH subunits in uterine sarcoma remains unknown.
The aim of this study was to evaluate the detection of LDH-D and the LDH-A subunit using immunohistochemistry (IHC) in human uterine myoma and uterine sarcoma, with implications for their diagnostic and treatment strategies. In addition, we also explored the association between the expression of LDH-A and LDH-D and overall survival (OS) in uterine sarcoma cases with known clinical follow-up.
2. Materials and methods
2.1. Patient selection
This retrospective study included patients diagnosed with primary uterine neoplasms who underwent surgical treatment from January 2008 to December 2015 at our university hospital. This study was approved by the hospital ethics committee. A total of 86 cases were assigned to 3 groups according to their pathological classification: uterine myoma (UM) (26 cases; age 23–80 years, mean 40.5 ± 7.6), cellular leiomyoma (CLM) (10 cases; age 36–53 years, mean 43.6 ± 5.8), and uterine sarcoma (50 cases; age 29–67 years, mean 52.8 ± 6.3). All cases were reviewed by 2 pathologists to ensure diagnostic accuracy. They were blinded to what the samples were when they scored these tissues. We included cases using the following criteria: diagnosed with uterine sarcoma according to the 2003 WHO diagnostic criteria; no preoperative antitumor treatment; and complete clinicopathologic and follow-up data available. We excluded cases diagnosed on autopsy or death certificate only.
The 2009 National Comprehensive Cancer Network guidelines classifications consider carcinosarcoma as a dedifferentiated or metaplastic endometrial carcinoma rather than a subtype of uterine sarcoma,[13] but most retrospective studies include it within uterine sarcoma because of its more aggressive behavior compared with endometrial carcinoma.[14] Clinical information and follow-up were collected by review of the medical records and pathology reports. The clinicopathologic characteristics of the patients included age at diagnosis, International Federation of Obstetrics and Gynecology (FIGO) stage, histopathologic subtype, surgical procedure, postoperative adjuvant therapy, pelvic lymphadenectomy, tumor size, distant metastasis, and recurrence. The follow-up data were last updated in August 2016. Patient death was the follow-up end point and the median follow-up period was 48 months (range 5–96 months).
2.2. IHC
IHC was performed by the envision plus method according to the manufacturer's instructions to evaluate the expression of LDH-A and LDH-D proteins in tissue sections from the 86 cases. First, paraffin-embedded sections (4-μm thick) were deparaffinized and rehydrated with double-distilled water. The antigens were retrieved using the heat-mediated method in citrate buffer (pH 6.0) for 20 minutes, and the sections were incubated with antibodies to LDH-A (GenBank accession number BC067223, Proteintech, Chicago, IL) (dilution:1:300) and LDH-D (GenBank accession number BC047902, Proteintech) (dilution:1:200) overnight at 4°C. Specific signals were incubated by incubation with peroxidase-coupled secondary antibody (PV-6001) for 60 minutes and visualized with 3,3’diaminobenzidine tetrachloride (Glostrup, Denmark). Counterstaining was performed with hematoxylin for 5 minutes, and the slides were coverslipped. Positive staining was located in the cytoplasm.
The immunoreactivity score was based on the positivity rate (percentage of positive tumor cells) and staining intensity (weak, moderate, strong). The positivity rate was represented by the average number of positive cells in 10 random views in each section at 400× magnification (100 cells counted in each view). Positivity rates were scored as 1 (0%–25% cells positive), 2 (25%–50% cells positive), 3 (51%–75% cells positive), and 4 (>75% cells positive). Staining intensity was scored as 0 (negative), 1 (canary yellow), 2 (clay yellow), and 3 (sepia yellow). The final result for each field was represented by the product of the positivity rate and staining intensity scores: 0 for negative (−), 1 to 4 for weakly positive (+), 5 to 8 for moderately positive (+ +), and 9 to 12 for strong positive (+ + +). The entire scoring process was performed independently by 2 pathologists.
2.3. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 20.0. Quantitative data were represented as the mean ± standard deviation. Comparisons between 2 groups were performed using t tests (for quantitative data) or χ2 tests (for qualitative data). Comparisons between multiple groups were performed by analysis of variance (for quantitative data) or rank sum tests (for qualitative data). OS was calculated from the date of surgery to last follow-up. Life tables were used to calculate survival rate and median survival time. Kaplan–Meier methods were used to compute survival analyses and curves were compared by log-rank tests. P < .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics of uterine sarcoma
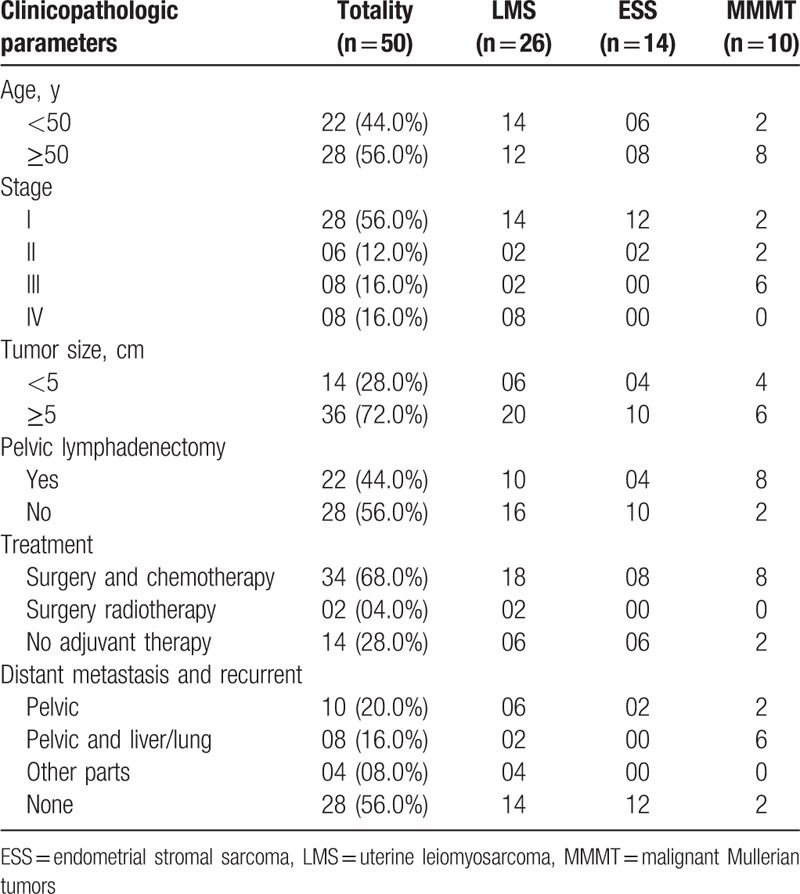
At the time of diagnosis, 44.0% of patients were younger than 50 years and 56.0% were aged 50 years or older. The tumor diameter was >5 cm in 72.0% patients. Among the 50 patients with uterine sarcoma, 26 (52.0%) had uterine leiomyosarcoma (LMS), 14 (28.0%) had malignant endometrial stromal sarcoma (ESS), and 10 (20.0%) had malignant Mullerian tumors (MMMT). Twenty-eight cases (56.0%) were stage I, 6 (12.0%) were stage II, 8 (16.0%) were stage III, and 8 (16.0%) were stage IV. Thirty-four (68.0%) patients underwent surgery and chemotherapy as initial treatment, 2 (4.0%) patients received surgery and radiotherapy, and 14 (28.0%) patients had no adjuvant therapy. Twenty-eight patients (56.0%) underwent pelvic lymphadenectomy (Table 1).
Table 1.
Clinicopathologic parameters.
3.2. Expression of LDH-A and LDH-D in tissues
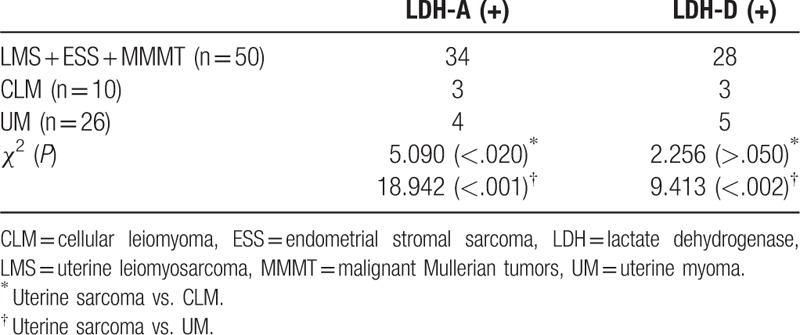
The expressions (the positivity rate and staining intensity scores) of LDH-A and LDH-D are indicated in Table 2 and Figures 1 and 2 . The positivity rates of LDH-A and LDH-D were significantly different in these 5 groups of patients with UM, CLM, and uterine sarcoma (χ2 = 28.068, P < .001; χ2 = 10.900, P < .05). The positivity rates and expression levels of LDH-A and LDH-D were higher in patients with uterine sarcoma compared with the patients with UM (χ2 = 18.942, P < .001; χ2 = 9.413, P < .002) (Table 3, Fig. 1). LDH-A expression levels were higher in MMMT patients compared with patients with other types of uterine sarcoma.
Table 2.
Immunohistochemical analysis of LDH-A and LDH-D in uterine sarcoma specimens.
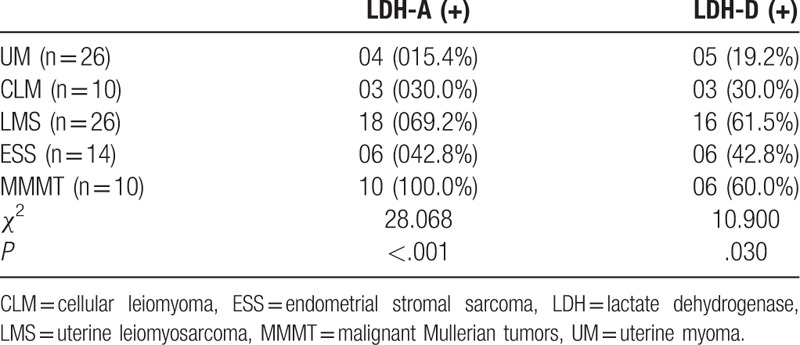
Figure 1.
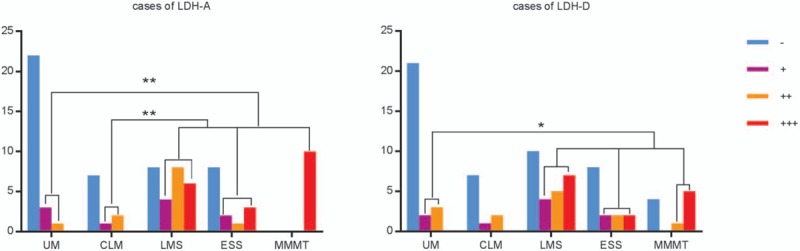
Expression (the positivity rate and staining intensity scores) of LDH-A and D-LDH. The positivity rates and expression levels of LDH-A and LDH-D were higher in patients with uterine sarcoma compared with the patients with UM (χ2 = 18.942, P < .001; χ2 = 9.413, P < .002). ∗, ∗∗ means P < .05. CLM = cellular leiomyoma, ESS = endometrial stromal sarcoma, LDH = lactate dehydrogenase, LMS = uterine leiomyosarcoma, MMMT = malignant Mullerian tumors, UM = uterine myoma.
Figure 2.
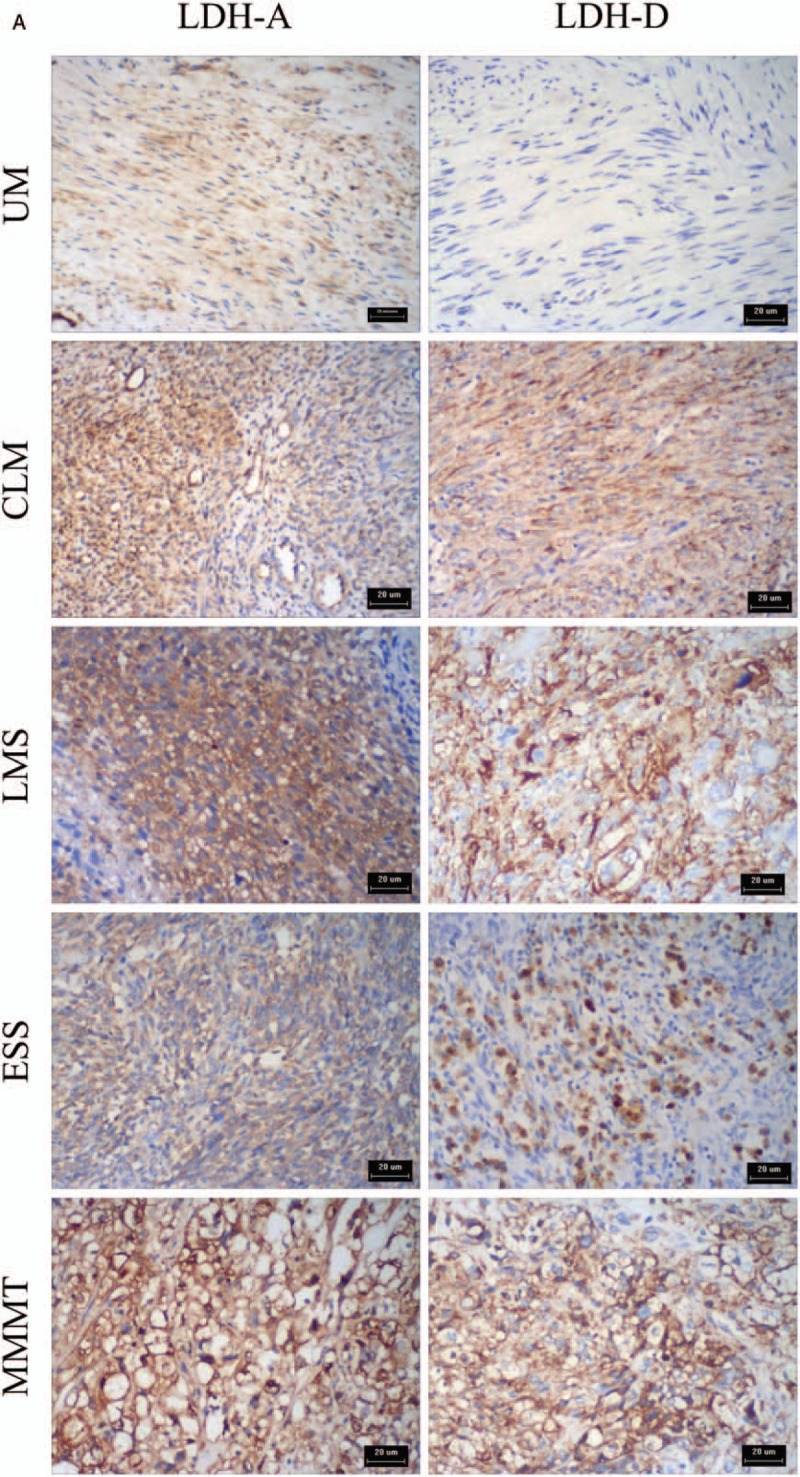
(A) Immunohistochemical staining for LDH-A and D-LDH expression in UM, CLM, LMS, ESS, and MMMT. LDH-A and D-LDH were located in the cytoplasm. LDH-A and D-LDH expression were negative in patients with UM but positive in patients with CLM, LMS, ESS, and MMMT. (B) Corresponding HE stained slices of UM, CLM, LMS, ESS, and MMMT. CLM = cellular leiomyoma, ESS = endometrial stromal sarcoma, LDH = lactate dehydrogenase, LMS = uterine leiomyosarcoma, MMMT = malignant Mullerian tumors, UM = uterine myoma.
Table 3.
Immunohistochemical analysis of LDH-A and LDH-D in uterine sarcoma and uterine myoma specimens.
3.3. Univariate analysis of factors affecting IS in uterine sarcoma
The 5-year OS rates of patients with LMS, ESS, and MMMT were 59%, 71%, and 29%, respectively (χ2 = 7.979, P = .02). ESS patients had a better prognosis than those with other histologic subtypes. Univariate analysis showed that patients younger than 50 years and with stage I-II had better clinical prognoses (Table 4). Tumor size and lymph node resection showed no obvious relationship to survival in patients with uterine sarcoma.
Table 4.
Univariate analysis of factors affecting overall survival in patients with uterine sarcoma (n = 50).
3.4. Prognostic significance of LDH-A and LDH-D expression
LDH-A-positive LMS patients had a poorer prognosis than LDH-A-negative ones (P = .03), with a median survival time of 35 months for LDH-A-positive patients. All carcinosarcoma patients showed LDH-A expression, and it was therefore not possible to perform a correlation analysis in these patients. There was no significant correlation between the expression of LDH-A and prognosis in the other groups. LDH-D expression was not correlated with prognosis in any of the groups (Fig. 3).
Figure 2 (Continued).
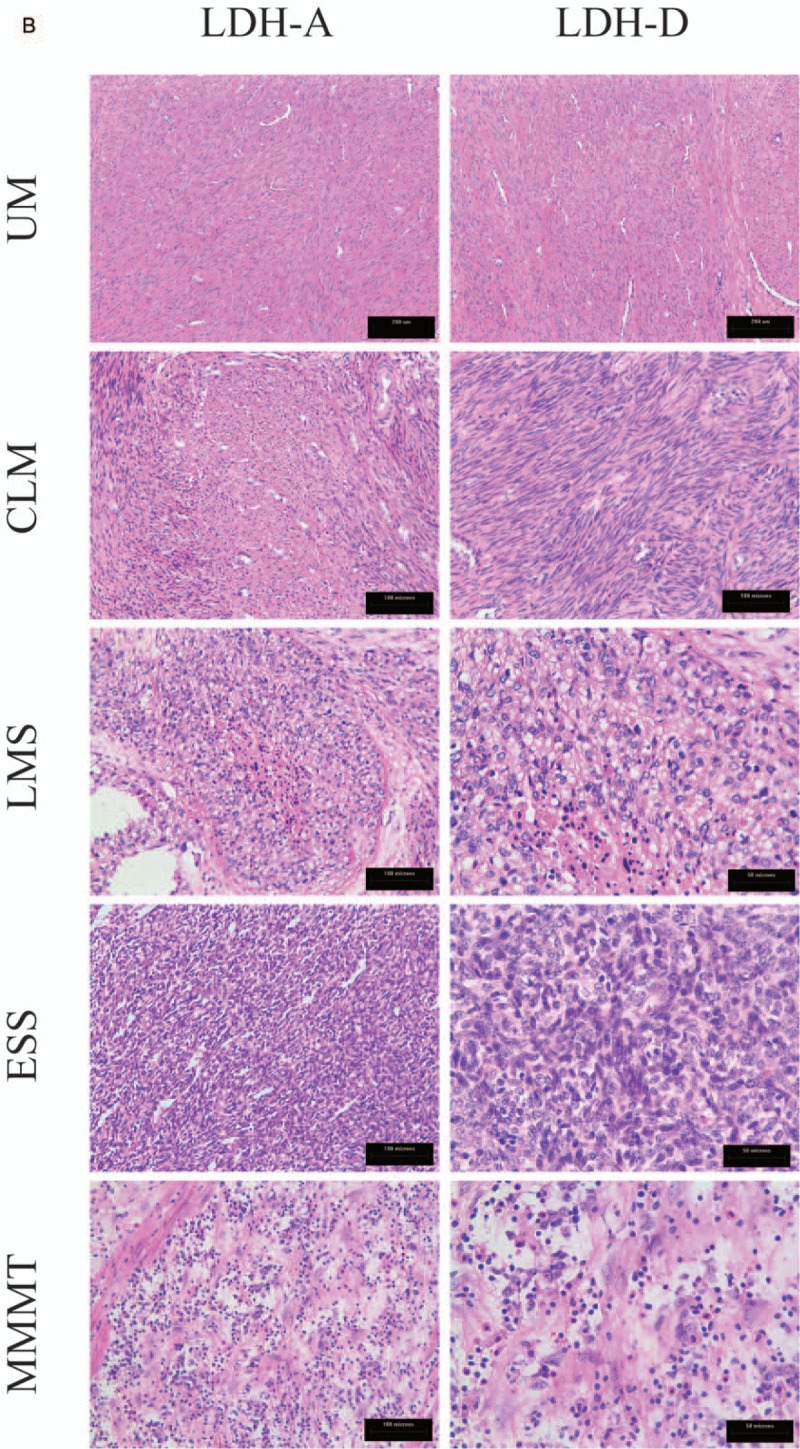
(A) Immunohistochemical staining for LDH-A and D-LDH expression in UM, CLM, LMS, ESS, and MMMT. LDH-A and D-LDH were located in the cytoplasm. LDH-A and D-LDH expression were negative in patients with UM but positive in patients with CLM, LMS, ESS, and MMMT. (B) Corresponding HE stained slices of UM, CLM, LMS, ESS, and MMMT. CLM = cellular leiomyoma, ESS = endometrial stromal sarcoma, LDH = lactate dehydrogenase, LMS = uterine leiomyosarcoma, MMMT = malignant Mullerian tumors, UM = uterine myoma.
Figure 3.
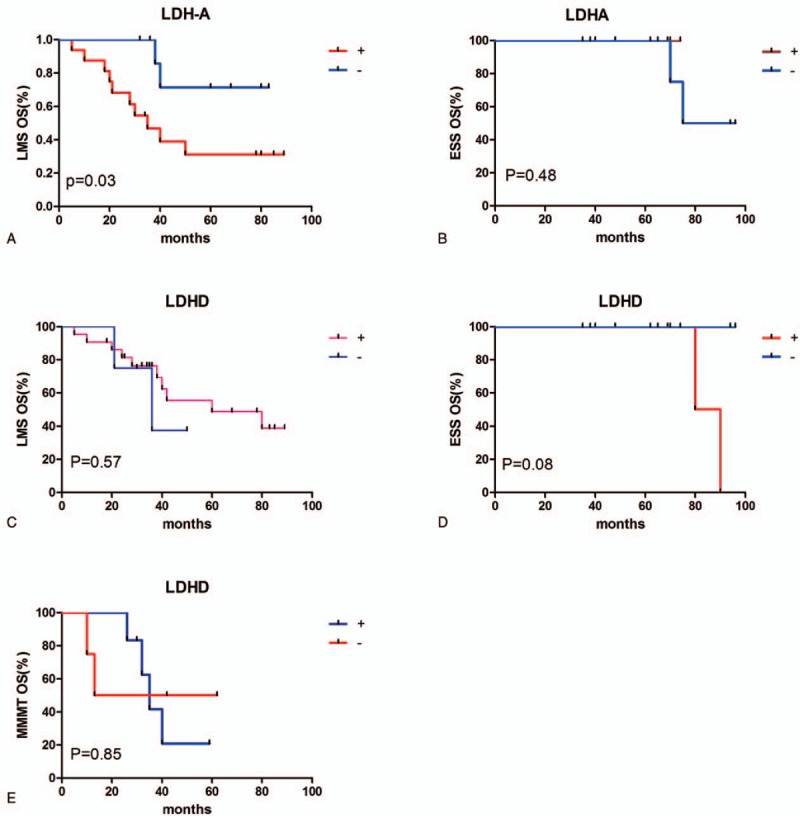
Prognostic significance of LDH-A and LDH-D expression. (A) Kaplan–Meier survival curve showing the trend of association between LDH-A protein expression and OS in patients with LMS. Patients with LMS and high LDH-A expression had significantly poorer outcomes (P = .03). (B) LDH-A expression showed no significant association with OS in ESS patients (P = .48). (C–E) D-LDH expression showed no significant association with OS in patients with LMS, ESS, or MMMT (P = 0.57, 0.08, and 0.85, respectively). ESS = endometrial stromal sarcoma, LDH = lactate dehydrogenase, LMS = uterine leiomyosarcoma, MMMT = malignant Mullerian tumors, OS = overall survival.
4. Discussion
Research into tumor metabolism has increased in recent years, as carcinoma was revealed to involve metabolic as well as genetic abnormalities. LDH is an important enzyme in glycolysis and in the Warburg effect,[15] and studies have shown that it plays an important role in tumor occurrence, development, invasion, metastasis, and in clinical tumor prognosis.[16,17] Expression of the four LDHs in human tissues differ. LDH-A is mainly expressed in hypoxic tissues, such as skeletal muscle, liver, and tumor tissues,[6] whereas LDH-B is mainly expressed in aerobic tissues, and LDH-C4 expression is restricted to the testicles.[18] However, although numerous studies have investigated L-LDH particularly LDH-A, D-LDH has rarely been studied.
LDH-A has been shown to be overexpressed in many tumors,[5,6] but its expression in human uterine sarcoma tissues remains unknown. The current IHC results confirmed that LDH-A was more highly expressed in LMS patients compared with UM and CLM patients. LDH-A plays an important role in tumor metabolism. Sheng et al.[5] showed that inhibiting LDH-A expression promoted apoptosis and reduced cell proliferation in vitro, suppressed infection and metastasis, and restored chemosensitivity in vivo.[19] Some drug-like inhibitors selective for human LDH-5, such as FX11 and NHI-1, have recently demonstrated promising anticancer activity both in vitro and in vivo, with potential clinical application prospects.[15] Goran et al[20] found that overexpression of LDH-A was an independent poor prognostic marker for survival of patients with pancreatic cancer. Overexpression of LDH-A in cholangiocarcinoma was also correlated with poor prognosis.[21] The present study provides the basis for the future use of LDH-A inhibitors in leiomyosarcoma of the uterus.
d-lactic acid is an important chiral intermediate in the synthesis of chiral materials. Previous research has focused on the food and chemical industries,[22] although malignant tumor cells have also been shown to release d-lactic acid[23]; however, few studies have investigated its role in human tumors. Bernard et al[24] reported the complete sequence of the LDH-D gene cloned from the Lactobacillus bulgaricus genome, whereas Taguchi et al[25,26] cloned the LDH-D gene and carried out studies using gene knockout technology. de Bari et al[19] examined the expression of LDH-D in prostate cancer cells and confirmed that it was expressed in human tumors. Uterine sarcoma is rich in smooth muscle tissue, and LDH-D can interact with muscle-specific protein CRP3/MLP.[10] We previously confirmed that total LDH expression was increased in human uterine sarcoma patients compared with UM patients, and the current results confirmed that LDH-D was also expressed in human uterine sarcoma patients, with significantly higher positivity in LMS compared with UM and CLM patients. There have been no studies of LDH-D metabolism in uterine sarcoma, and further studies are needed to explore its role in cell proliferation and apoptosis in these tumors. The diverse pathology of uterine sarcoma and atypical smooth muscle tumors makes their diagnosis difficult and presents a challenge to pathologists. The results of this study suggest that positive expression of LDH-A and LDH-D may aid in the diagnosis of uterine sarcoma in the future.
Although uterine sarcoma is rare, the degree of malignancy is high and the 5-year survival rate is low,[27,28] and it is associated with aggressive invasion and metastasis characteristics.[29] Furthermore, uterine sarcoma is insensitive to radiation and chemotherapy treatment, with easy relapse.[30] Although numerous studies have investigated the relationship between the clinical pathological features and prognosis of uterine sarcoma, the prognostic factors remain unclear, with no consensus on their significance. Patient age, clinical stage, tumor size, degree of nuclear atypia, and vascular invasion have all been identified as potential prognostic factors.[31] Clinical stage appeared to be the main factor affecting the prognosis of patients with uterine sarcoma, with a 5-year OS rate for patients with stage I of 50% to 70% compared with 0% to 20% for other stages.[32] The largest study to date analyzed the clinical and pathological characteristics of 13,089 cases of uterine sarcoma and showed that it was more common in older patients and among black women, with poor survival being associated with old age, black race, and advanced disease stage.[14] In our study, the 5-year OS rates for LMS, ESS, and MMMT were 59%, 71%, and 29% respectively, with ESS patients showing a better prognosis than those with other histologic subtypes. A possible reason for this may be the fact that ESS tends to present with less aggressive and metastatic features. The survival rate of patients with LMS in our study was higher than in other previous studies, which may be related to disease stage (56% stage I patients). We analyzed the relationship between the clinicopathologic parameters and survival rate using univariate analysis, which revealed that patients with uterine sarcoma younger than 50 years or diagnosed at stage I-II had better clinical prognoses, whereas histology and treatment plan had no effect on the prognosis. Tumor size showed no obvious relation with survival, possibly because of the differences in proportions of the different histological types of uterine sarcoma. The decision of whether or not to perform lymph node dissection remains controversial, given that uterine sarcoma generally metastasizes to the lungs and other organs via the blood vessels, but rarely through the lymphatic circulation. Our results found no significant correlation between lymph node resection and survival rate. However, further studies with larger sample sizes are needed to determine the benefits of lymph node dissection in uterine sarcoma patients.
In addition to clinicopathological features, tumor-related genes have also been shown to be related to prognosis. Koivistoet al[33] showed that p53, estrogen receptor α, and progesterone receptor (PR) A were significantly associated with survival of LMS patients, whereas Liang et al[34] demonstrated that high expression of p16 and pHH3 and low expression of PR were significant factors in the diagnosis of uterine sarcoma. Recently, Davidson et al[35] detected estrogen receptor and PR expression in 294 patients diagnosed with uterine sarcoma in Norway and showed that PR expression was an independent beneficial prognosticator in FIGO stage I LMS. However, although numerous studies have investigated the prognostic factors in uterine sarcoma, there is currently no consensus. LDH plays an important role in the Warburg effect in tumors, and the expression of the various LDH subunits differs in uterine sarcoma tissues. In our study, both LDH-A and LDH-D were overexpressed in uterine sarcoma compared with other uterine tumors, and survival analysis showed that LDH-A-positive LMS was associated with a poorer prognosis than LDH-A negative LMS, although there was no significant correlation between LDH-A expression and prognosis in the other groups and LDH-D expression was not correlated with prognosis. These results suggest that LDH-A is not only highly expressed in uterine sarcomas, but may also be used to predict the prognosis of patients with LMS. These results also suggest that the overexpression of LDH-A and LDH-D in uterine sarcoma is related to the biological characteristics of aggressive invasion and metastatic potential.
In summary, both LDH-A and LDH-D are expressed in uterine sarcomas and may thus be used to aid in the pathological diagnosis of this tumor type. These results also provide the basis for future research into the role of LDH-A in cell proliferation and apoptosis in relation to the Warburg effect in uterine sarcoma. LDH-A expression is associated with survival and prognosis in patients with uterine LMS. The limitation of our study is that the mechanism of LDH-A and LDH-D in LMS was not studied in detail. Further studies in a larger sample are needed to clarify the relationship between LDH-D and survival prognosis and to explore its role in the pathogenesis and treatment of uterine sarcoma. We aim to investigate the role of the different LDH subtypes in tumor metabolism in uterine sarcoma in the future.
Author contributions
Conceptualization: X. Yu, Q. Yao, Y. Wang.
Data curation: X. Yu.
Formal analysis: T. Lv.
Investigation: T. Lv.
Methodology: Y. Diao.
Project administration: K. Song.
Resources: K. Song.
Software: Y. Chen.
Supervision: Y. Diao, Y. Wang.
Validation: Y. Chen, Y. Wang.
Visualization: Y. Diao.
Writing – original draft: K. Song.
Writing – review & editing: K. Song, Q. Yao.
Footnotes
Abbreviations: CLM = cellular leiomyoma, ESS = endometrial stromal sarcoma, FIGO = International Federation of Obstetrics and Gynecology, IHC = immunohistochemistry, LDH = lactate dehydrogenase, LMS = uterine leiomyosarcoma, MMMT = malignant Mullerian tumors, OS = overall survival, PR = progesterone receptor, UM = uterine myoma.
K-jS and X-nY contributed equally to this study.
The authors report no conflicts of interest.
References
- [1].Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol 2012;24:62–7. [DOI] [PubMed] [Google Scholar]
- [2].van Horssen R, Freire Jorge P, van Dam GM, et al. [The Warburg effect and its role in tumour metabolism: opportunities for new cancer treatments]. Ned Tijdschr Geneeskd 2016;160:A9489. [PubMed] [Google Scholar]
- [3].Goto A, Takeuchi S, Sugimura K, et al. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 2002;12:354–61. [DOI] [PubMed] [Google Scholar]
- [4].Farrar WW, Bush FM. Isozymic structure and pyruvate inhibition of lactate dehydrogenase of ventricle, pectoralis muscle and cerebrum, and total LDH activity of cerebrum during morphogenesis of the house sparrow, Passer domesticus. Comp Biochem Physiol 1969;29:89–108. [DOI] [PubMed] [Google Scholar]
- [5].Sheng SL, Liu JJ, Dai YH, et al. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J 2012;279:3898–910. [DOI] [PubMed] [Google Scholar]
- [6].Gao S, Tu DN, Li H, et al. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. Biomed Pharmacother 2016;81:388–93. [DOI] [PubMed] [Google Scholar]
- [7].Pallotta ML, Valenti D, Iacovino M, et al. Two separate pathways for d-lactate oxidation by Saccharomyces cerevisiae mitochondria which differ in energy production and carrier involvement. Biochim Biophys Acta 2004;1608:104–13. [DOI] [PubMed] [Google Scholar]
- [8].Atlante A, de Bari L, Valenti D, et al. Transport and metabolism of D-lactate in Jerusalem artichoke mitochondria. Biochim Biophys Acta 2005;1708:13–22. [DOI] [PubMed] [Google Scholar]
- [9].de Bari L, Valenti D, Pizzuto R, et al. Jerusalem artichoke mitochondria can export reducing equivalents in the form of malate as a result of D-lactate uptake and metabolism. Biochem Biophys Res Commun 2005;335:1224–30. [DOI] [PubMed] [Google Scholar]
- [10].Flick MJ, Konieczny SF. Identification of putative mammalian D-lactate dehydrogenase enzymes. Biochem Biophys Res Commun 2002;295:910–6. [DOI] [PubMed] [Google Scholar]
- [11].Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer 1993;71:1702–9. [DOI] [PubMed] [Google Scholar]
- [12].Mayerhofer K. Ki-67 and vascular endothelial growth factor expression in uterine leiomyosarcoma. Gynecol Oncol 2004;92:175–9. [DOI] [PubMed] [Google Scholar]
- [13].D’Angelo E, Prat J. Pathology of mixed Mullerian tumours. Best Pract ResClin Obstet Gynaecol 2011;25:705–18. [DOI] [PubMed] [Google Scholar]
- [14].Hosh M, Antar S, Nazzal A, et al. Uterine sarcoma: analysis of 13,089 cases based on Surveillance, Epidemiology, and End Results Database. Int J Gynecol Cancer 2016;26:1098–104. [DOI] [PubMed] [Google Scholar]
- [15].Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 2015;358:1–7. [DOI] [PubMed] [Google Scholar]
- [17].Faloppi L, Bianconi M, Giampieri R, et al. The value of lactate dehydrogenase serum levels as a prognostic and predictive factor for advanced pancreatic cancer patients receiving sorafenib. Oncotarget 2015;6:35087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gupta GS. LDH-C4: a target with therapeutic potential for cancer and contraception. Mol Cell Biochem 2012;371:115–27. [DOI] [PubMed] [Google Scholar]
- [19].de Bari L, Moro L, Passarella S. Prostate cancer cells metabolize d-lactate inside mitochondria via a D-lactate dehydrogenase which is more active and highly expressed than in normal cells. FEBS Lett 2013;587:467–73. [DOI] [PubMed] [Google Scholar]
- [20].Mohammad GH, Olde Damink SW, Malago M, et al. Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. PLoS One 2016;11:e0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thonsri U, Seubwai W, Waraasawapati S, et al. Overexpression of lactate dehydrogenase A in cholangiocarcinoma is correlated with poor prognosis. Histol Histopathol 2017;32:503–10. [DOI] [PubMed] [Google Scholar]
- [22].Tanaka T, Hoshina M, Tanabe S, et al. Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour Technol 2006;97:211–7. [DOI] [PubMed] [Google Scholar]
- [23].Santel T, Pflug G, Hemdan NY, et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS One 2008;3:e3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bernard N, Ferain T, Garmyn D, et al. Cloning of the D-lactate dehydrogenase gene from Lactobacillus delbrueckii subsp. bulgaricus by complementation in Escherichia coli. FEBS Lett 1991;290:61–4. [DOI] [PubMed] [Google Scholar]
- [25].Taguchi H, Ohta T. D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, sequencing, and expression in Escherichia coli of the D-lactate dehydrogenase gene of Lactobacillus plantarum. J Biol Chem 1991;266:12588–94. [PubMed] [Google Scholar]
- [26].Kochhar S, Hottinger H, Chuard N, et al. Cloning and overexpression of Lactobacillus helveticus D-lactate dehydrogenase gene in Escherichia coli. Eur J Biochem 1992;208:799–805. [DOI] [PubMed] [Google Scholar]
- [27].Sehnal B, Driak D, Kmonickova E, et al. [Current classification of malignant tumours in gynecological oncology—part I]. Ceska Gynekol 2011;76:279–84. [PubMed] [Google Scholar]
- [28].Sehnal B, Driak D, Kmonickova E, et al. [Current classification of malignant tumours in gynecological oncology—part II]. Ceska Gynekol 2011;76:360–6. [PubMed] [Google Scholar]
- [29].Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol 2012;36:641–53. [DOI] [PubMed] [Google Scholar]
- [30].Lee EJ, Kim TJ, Choi CH, et al. Uterine endometrial carcinoma: 10 years’ experience with long-term follow-up at a single Korean institution. Gynecol Obstet Invest 2012;74:313–9. [DOI] [PubMed] [Google Scholar]
- [31].Zhongqiu Lin, Jinxiao Liang, Rongchun Lin. «The report of the women's cancer FIGO 2015» read serial four -a guideline to diagnosis and treatment of uterine sarcoma. Chin J Pract Gynecol Obstet 2015;1082–7. [Google Scholar]
- [32].Pautier P, Genestie C, Rey A, et al. Analysis of clinicopathologic prognostic factors for 157 uterine sarcomas and evaluation of a grading score validated for soft tissue sarcoma. Cancer 2000;88:1425–31. [PubMed] [Google Scholar]
- [33].Koivisto-Korander R, Butzow R, Koivisto AM, et al. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: expression and prognostic importance of ten different markers. Tumour Biol 2011;32:451–9. [DOI] [PubMed] [Google Scholar]
- [34].Liang Y, Zhang X, Chen X, et al. Diagnostic value of progesterone receptor, p16, p53 and pHH3 expression in uterine atypical leiomyoma. Int J Clin Exp Pathol 2015;8:7196–202. [PMC free article] [PubMed] [Google Scholar]
- [35].Davidson B, Kjaereng ML, Forsund M, et al. Progesterone receptor expression is an independent prognosticator in FIGO Stage I uterine leiomyosarcoma. Am J Clin Pathol 2016;145:449–58. [DOI] [PubMed] [Google Scholar]


