Abstract
To study the outcomes following concurrent chemoradiotherapy (CCRT) and subsequent radical surgery for locally advanced cervical cancer (LACC), analyze the relationship between imaging-diagnosed and postoperative-diagnosed lymph node (LN) involvement, and identify patients who would benefit from individualized pelvic lymphadenectomy.
We retrospectively reviewed records of 410 patients who underwent CCRT followed by radical surgery for International Federation of Gynecology and Obstetrics Stage Ib2-IIIb disease. Correlations of LN size on imaging before CCRT with pathological responses after CCRT, overall survival (OS), distant metastasis-free survival (DMFS), and complications were analyzed.
During a median follow-up of 51.3 months, the respective 5-year OS and DMFS were 86.7% and 88.6%, respectively. Pathological primary tumor type, LN size on imaging before CCRT, and pathologic response after CCRT were independent prognostic factors for OS. Patients with a LN ≥0.8 cm had a significantly higher residual carcinoma rate versus those with LN <0.8 cm (33% vs 22.6%, P = .032). Postoperative pathological positive LN frequencies differed significantly by LN size on imaging (LN <0.8 cm vs LN ≥0.8 cm, 3% vs 19.3%, P < .0001). Grade 1–3 lower extremity edema occurred in 23.9% of cases; no grade 3–4 gastrointestinal and genitourinary toxicities were observed.
CCRT followed by radical surgery for LACC yielded encouraging outcomes without unacceptable complications. Additionally, patients with a LN <0.8 cm on imaging before CCRT had a very low risk of postoperative pathological positive LN identification. Individualized pelvic lymphadenectomy (e.g., omitting or limiting the extent of LN dissection) might be an alternative option for some patients with a low risk of LN metastasis.
Keywords: individualized pelvic lymphadenectomy, locally advanced cervical cancer, lymph node metastasis, neoadjuvant concurrent chemoradiotherapy, subsequent radical surgery
1. Introduction
Cervical cancer is the most common type of gynecological malignancy affecting women worldwide, and the third leading cause of cancer-related death among women in developing countries.[1] In 2012, an estimated 527,600 new cervical cancer cases were identified worldwide, and 265,700 deaths were attributed to this type of cancer.[1] Approximately, 80% of newly diagnosed cases and nearly 85% of related deaths occur in developing countries.[1] Worse, more than 80% of new cervical cancer cases are detected at a locally advanced stage (International Federation of Gynecology and Obstetrics [FIGO] stage ≥Ib2), and in developing countries, >50% of these cases involve stage III–IV disease.[2]
Since 1999, radical concurrent chemoradiotherapy (CCRT) has been recommended as the standard treatment for locally advanced cervical cancer (LACC), based on the results of 5 randomized studies.[3–7] Radical CCRT for LACC generally includes pelvic external beam radiotherapy, concomitant chemotherapy, and intracavitary brachytherapy.[8] Unfortunately, a lack of brachytherapy equipment restricts the use of radical CCRT in most developing countries. In Mainland China, only 31% (439/1413) of the radiation oncology centers are equipped with brachytherapy machine, with the number of well-equipped centers having risen only marginally, from 400 in 2005 to 439 in 2015.[9] Therefore, roughly two-thirds of the patients cannot be treated conveniently with brachytherapy. Accordingly, the majority of patients must alternatively undergo preoperative chemoradiotherapy and radical surgery as a replacement for radical CCRT.[10] Many studies have shown that for LACC, CCRT followed by radical surgery could yield encouraging results and a favorable long-term toxicity profile.[11–13] The performance of radical surgery after CCRT has a couple of notable advantages. First, preoperative CCRT can shrink bulky tumors and therefore improve the successful resection rate[14,15] and counteract the negative effects of brachytherapy omission. Second, radical surgery after CCRT could allow clinicians to remove potentially chemoresistant and radioresistant foci,[11,15] leading to improved local control and overall survival.
Although many previous studies have reported acceptable treatment-related urinary and gastrointestinal complications associated with CCRT followed by radical surgery,[11,12,14–17] postoperative complications such as ureterohydronephrosis and lymphatic sequelae should not be ignored.[12,17] From our previous single-center experience [12] and the clinical research observations of Fanfani et al,[13] lymphatic sequelae such as leg edema comprise the main type of adverse effect after CCRT followed by radical surgery, and the incidence of leg edema with this combined therapy is significantly greater than with radical CCRT alone.
Because lower extremity edema results from damage to the lower limb lymphatic circulation, which is a risk of pelvic lymphadenectomy,[18,19] we wondered whether we could reduce the risk of this complication by avoiding or limiting the extent of pelvic lymphadenectomy, and whether such limitation would increase the rate of local recurrence or reduce overall survival. The present study therefore analyzed prognostic factors such as primary tumor size, pathological primary tumor type, squamous cell carcinoma antigen (SCC-Ag) level at diagnosis, LN size on diagnostic imaging, and pathologic response after preoperative CCRT in an attempt to identify patients in whom pelvic lymphadenectomy could be reduced in extent or omitted to decrease the incidence of leg edema without sacrificing local control and survival.
2. Methods and materials
2.1. Patient selection and data collection
Patients with clinical evidence of cervical involvement and treated with neoadjuvant CCRT and subsequent radical surgery, from January 2009 to December 2014 at our center, were retrospectively reviewed. The exclusion criteria in this study were as follows: FIGO stage Ia–Ib1 and IV disease (FIGO stage Ib2–IIIb according to gynecological examination and confirmation by at least 2 experts were enrolled), Eastern Cooperative Oncology Group (ECOG) performance status ≥3, history of other malignancies or cancer therapies, para-aortic lymph node (PALN) metastasis according to computed tomography (CT) and magnetic resonance (MR) images, and failing to complete preoperative radiotherapy and radical surgery.
This study was approved by the Research Ethics Committee of the local hospital, and informed consent was obtained from all included patients. The consent forms were preserved in the patients’ medical records.
The pretreatment evaluation included a gynecological examination, chest radiography, cardiovascular evaluation, abdominopelvic CT, and MR imaging (except for patients who could not undergo MR because of an intrauterine coil or other metal in the body), complete blood cell count, transvaginal ultrasound, serological evaluation of liver and kidney functions, and SCC-Ag level evaluation. In brief, the cervical tumor size and LN size were determined using anteroposterior, lateral, and proximodistal tumor measurements (cm) from MR images (or transvaginal ultrasound images in the absence of MR). Patients were classified into 2 groups according to the pelvic LN size as determined on contrast-enhanced CT and MR images at diagnosis: enlarged LN short diameter <0.8 cm, and enlarged short diameter ≥0.8 cm.
2.2. Treatment protocol
2.2.1. Preoperative chemoradiotherapy or radiotherapy alone
Preoperative whole pelvic radiotherapy was delivered via a 6- or 15-MV photon beam according to a 3-dimensional conformal radiation protocol; a linear accelerator (Clinac 23EX or 600 CD or Clinac iX, Varian Medical Systems) was used for therapy. During treatment planning, all patients were immobilized on a custom vacuum mattress in the supine position and subjected to an enhanced CT simulation scan (Philips, Amsterdam, the Netherlands) with a slice thickness of 5 mm. The clinical target volume (CTV) was defined as the gross tumor, cervix, uterus, parametria, upper part of the vagina to 3 cm below the level of tumor invasion, and regional LN (external iliac, internal iliac, obturator, and presacral LN). The planning target volume (PTV) was defined as a uniform 3-dimensional expansion around the CTV, with 7-mm margins around the LNs, 10-mm margins around the vagina and parametria, and 15-mm margins around the gross cervical tumor and uterus. In patients undergoing intensity-modulated radiotherapy (IMRT), treatment was delivered via a 6-MV photon beam through 5 to 7 fields. The total planned dose for a pelvic field was 50 Gy/25 fractions.
Concurrent chemotherapy (CCT) regimens comprised either cisplatin every 3 weeks (75 mg/m2) or weekly (40 mg/m2). If grade 3 or 4 toxicity necessitated a delay in chemotherapy, we re-evaluated the toxicity status after 1 week and withheld CCT until the white blood cell and platelet counts recovered to >3000/mm3 and >75,000/mm3, respectively.
2.2.2. Surgery and histopathological examination
At 3 to 4 weeks after CCRT completion, patients again underwent pelvic MR or CT imaging and transvaginal ultrasound to evaluate the objective response. We, then, consulted at least 2 gynecological experts to determine eligibility of patients for radical surgery. Accordingly, all the eligible candidates would undergo type B radical hysterectomy, and pelvic lymphadenectomy via laparotomy, laparoscopy, or robotic techniques. Pathological responses to neoadjuvant therapy were evaluated based on a histopathological examination of resected specimens (e.g., uterus, vaginal cuff, parametria, pelvic LN). Pathological primary tumor responses were classified as a pathological complete response (pCR; no microscopic residual cancer cells), microscopically heterotypic cells (MHC; noncancerous but abnormal cells, including degeneration or necrosis of heterotypic cell nests), or residual carcinoma cells (RCC). Pathological responses of resected LNs were described as LN positive or LN negative.
2.2.3. Data collection and statistical analysis
2.2.3.1. Response, recurrence, and survival
Local failure was defined as either pathological proof of cancer in the vaginal stump or the reappearance or enlargement of a tumor or any pelvic LN on imaging studies. Distant metastasis was confirmed using pathological, cytological, and/or radiological evidence. In this study, local or distant failure was defined according to the locations of lesions detected at the time of the first postoperative relapse. Patients were considered to have both local and distant failure when different lesions were detected synchronously or within a 1-month interval. Pelvic recurrence-free survival (PRFS), distant metastasis-free survival (DMFS), and overall survival (OS) were defined as the intervals from the end of CCRT to the time of pelvic recurrence, distant metastasis, and cancer-related death or last follow-up, respectively.
2.3. Toxicity and follow-up
Acute and chronic toxicities, including hematologic, gastrointestinal (GI), and genitourinary (GU) toxicities, were evaluated according to the Acute Radiation Morbidity Scoring Criteria and Late Radiation Morbidity Scoring Scheme of the Radiation Therapy Oncology Group (RTOG). Lower extremity edema was graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Acute toxicity was evaluated weekly during treatment. After completing treatment, patients were followed-up after 1 month, and every 3 months thereafter during the first year. Subsequently, patients were followed up every 3 to 12 months. Toxicities were graded basing on the clinical findings described in the medical records.
2.4. Statistical analysis
The normal distribution of the continuous data was described with mean ± standard deviation (SD), while the skewed distribution data were described with the median and interquartile range (IQR). Categorical data were presented as the number of patients and a percentage. OS, DMFS, and PRFS were calculated using the Kaplan–Meier method, and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards model. Comparative analyses were performed using Chi-square or Fisher's exact test. SPSS, version 18.0 (IBM, NY) was used for the statistical analyses. A P value < .05 was considered statistically significant.
3. Results
3.1. Baseline of patient characteristics
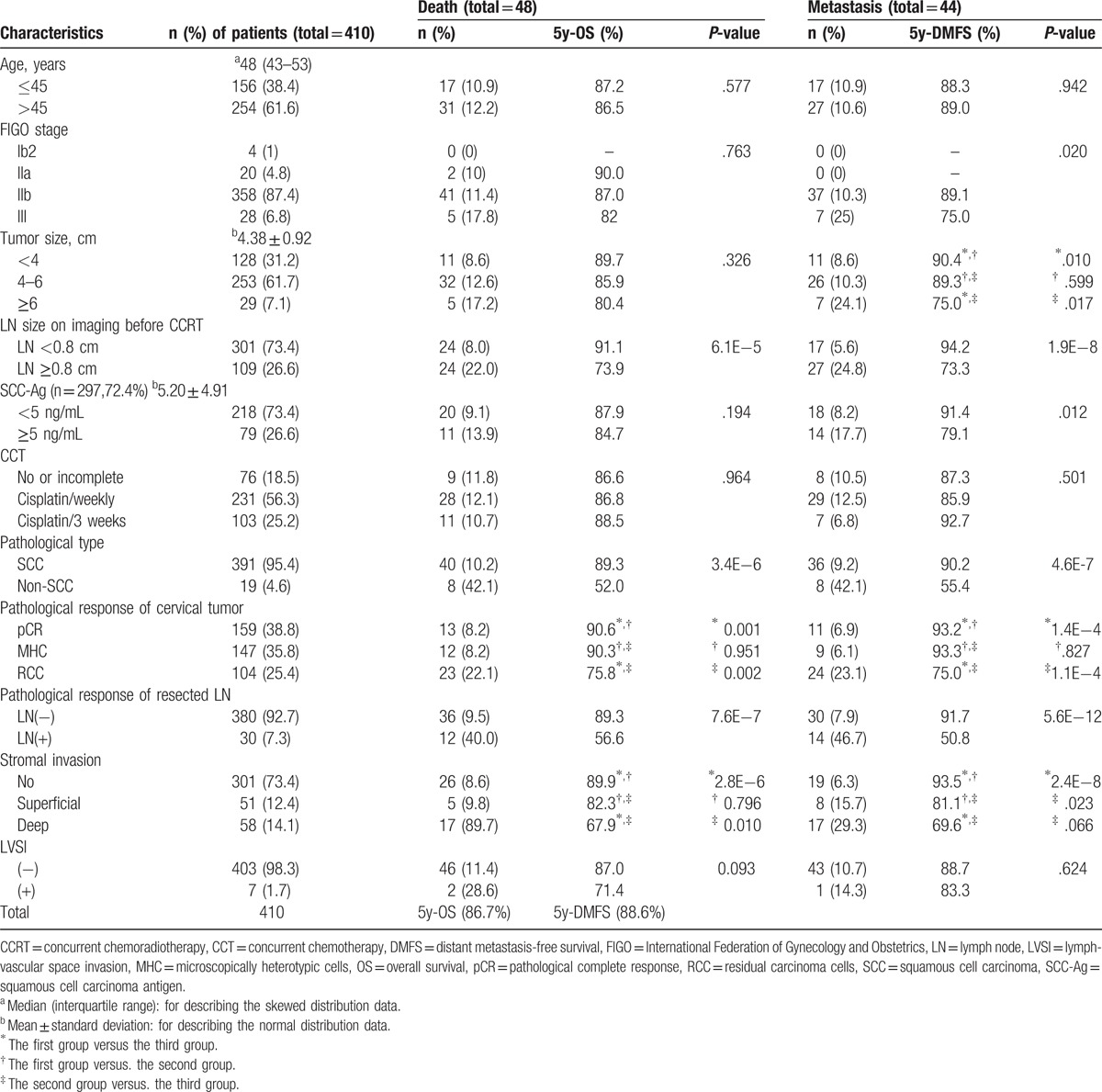
A total of 472 (31.5%) of the 1497 LACC patients received the combined treatment during the study period, while others received the standard treatment of CCRT alone; 38 patients did not undergo subsequent surgery for various reasons and complete data were not available for 24 patients. Ultimately, 410 patients with FIGO stage Ib2–IIIb disease were observed in this study. The median age at diagnosis was 48 years, and the tumor diameter was 4.38 (± 0.92) cm. All patients underwent radical surgery, including type B hysterectomy and systematic pelvic lymphadenectomy via laparotomy, laproscopy, or robotic techniques. Squamous cell carcinoma (SCC) was the predominant pathologic subtype (391/410, 95.4%); non-SCC cases, including adenocarcinoma (AC) and adenosquamous carcinoma (ASC), accounted for only 4.6% of all cases. Patient classification according to an enlarged LN short diameter on CT and MR images yielded the following information: 26.6% (109/410) had an enlarged LN ≥0.8 cm. Regarding preoperative treatment, 18.5% (76/410) of the patients received no or incomplete CCT (Table 1).
Table 1.
Clinical characteristics and postoperative pathological details of the study population.
3.2. Assessment of pathological responses to CCRT
During a postoperative pathological evaluation, 38.8% of patients (159/410) were found to have achieved a pCR to preoperative CCRT, 35.8% (147/410) had MHC, and 25.4% (104/410) patients exhibited RCC. A pathological analysis of metastatic involvement in the resected pelvic LNs revealed that 92.7% of cases (380/410) were LN negative, whereas only 7.3% (30/410) were LN positive. Superficial, deep, and no stromal invasion were observed in 12.4% (51/410), 14.1% (58/410), and 73.4% (301/410) of cases, respectively. Negative and positive lymphovascular space invasion (LVSI) were reported in 98.3% (403/410) and 1.7% (7/410) of patients, respectively (Table 1).
3.3. Survival outcomes and prognostic factors
During a median follow-up of 51.3 months (range: 4–97 months), from September 2009 to December 2016, 48 patients died, 44 developed distant metastases, and 10 experienced local failures (including vaginal stump recurrence in 9 patients and pelvic recurrence in 1 patient). The 5-year OS, DMFS, and PRFS rates were 86.7%, 88.6%, and 96.9%, respectively. The relationships of the patient clinicopathological characteristics at baseline with OS and DMFS are presented in Table 1.
A univariate analysis identified the pathological primary tumor type, LN size on imaging before CCRT, pathological responses of the primary tumor and resected LN after CCRT, and stromal invasion status as factors related to OS. Factors found to associate with DMFS included FIGO stage, tumor size, LN size on imaging before CCRT, SCC-Ag level before CCRT, pathological primary tumor type, pathological responses of the primary tumor and resected LN after CCRT, and stromal invasion status (Table 1).
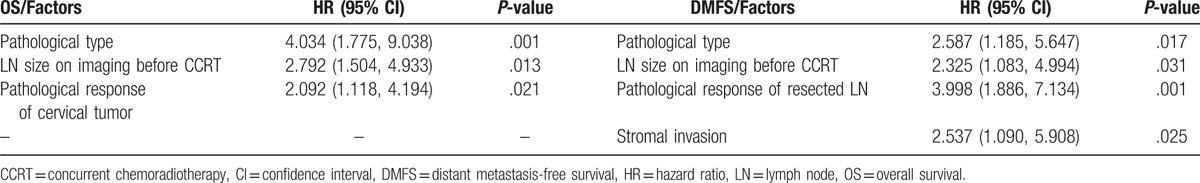
A multivariate analysis identified the following prognostic factors as strongly predictive of OS: pathological type of primary tumor (non-SCC vs SCC, 5-year OS: 52.0% vs 89.3%; hazard ratio [HR] = 4.034, P = .001), LN size on CT/MR imaging before CCRT (LN ≥0.8 cm vs LN <0.8 cm, 5-year OS: 73.9% vs 91.1%; HR = 2.792, P = .013), and postoperative pathologic response of the primary tumor (RCC vs pCR, 5-year OS: 75.8% vs 90.6%; HR = 2.092, P = .021). DMFS was found to associate significantly with the pathological primary tumor type (non-SCC vs SCC, 5-year DMFS: 55.4% vs 90.2%; hazard ratio [HR] = 2.587, P = .017), the LN size on CT/MR imaging before CCRT (LN ≥0.8 cm vs LN <0.8 cm, 5-year DMFS: 73.3% vs 94.2%; HR = 2.325, P = .031), pathological response of the resected LN (LN positive vs LN negative, 5-year DMFS: 50.8% vs 91.7%; HR = 3.998, P = .001), and stromal invasion status (deep invasion vs no invasion, 5-year DMFS: 69.6% vs 93.5%; HR = 2.537, P = .025) (Table 2).
Table 2.
Multivariate Cox proportional hazard regression model analysis for OS and DMFS.
3.4. Relationship between pretreatment LN size on imaging and pathological responses
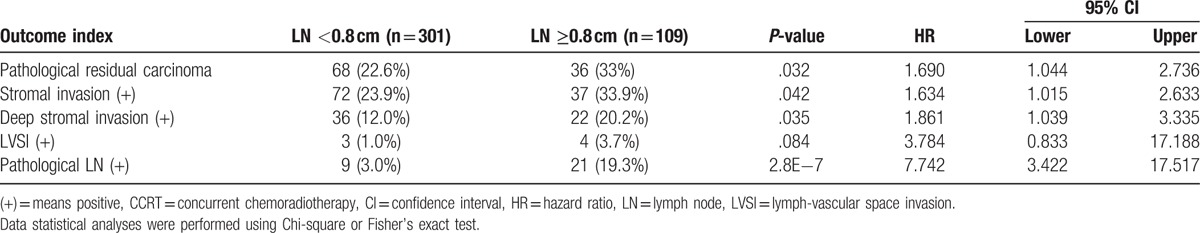
We identified a strong correlation between the postoperative pathologic response and LN size on CT/MR imaging before CCRT. Patients with a LN ≥0.8 cm had a significantly higher RCC rate when compared to those with a LN <0.8 cm (33% vs 22.6%, P = .032). Patients with a LN ≥0.8 cm also had a significantly higher rate of stromal invasion when compared to those with a LN <0.8 cm (33.9% vs 23.9%, P = .042), and a significant difference in deep stromal invasion was also observed (LN ≥0.8 cm vs LN <0.8 cm, 20.2% vs 12.0%, P = .035). Furthermore, the frequency of postoperative pathological LN positivity differed significantly between the 2 LN size groups (LN <0.8 cm vs LN ≥0.8 cm, 3.0% vs 19.3%, P < .0001). In brief, the risk of a postoperative pathological positive LN was very low among patients with a LN <0.8 cm on CT/MR imaging (Table 3).
Table 3.
Comparative analysis of the outcome indices according to the LN size on imaging before CCRT.
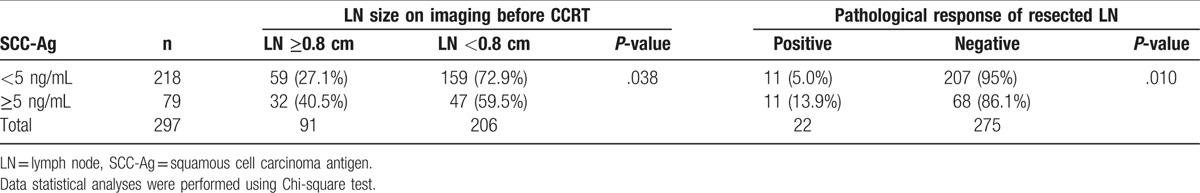
The pretreatment SCC-Ag level is known to be a predictor of LN metastasis. Accordingly, we studied the relationship between SCC-Ag levels and LN in the 72.4% (297/410) of patients for whom precise SCC-Ag data at diagnosis were available. Of these patients, 73.4% (218/297) had a SCC-Ag level <5 ng/mL, and 26.6% (79/297) had a SCC-Ag level ≥5 ng/mL. In the former and latter subgroups, 27.1% (59/218) and 40.5% (32/79) of patients, respectively, had a LN ≥0.8 cm on CT/MR imaging (P = .038), and approximately 5.0% (11/218) and 13.9% (11/79), respectively, had a pathologically positive LN (P = .010) (Table 4). In summary, SCC-Ag levels may predict LN positivity.
Table 4.
Relationship between pretreatment SCC-Ag level and LN metastasis.
3.5. Identifying patients at low risk for LN metastasis according to the prognosis factors
Using the above results from an analysis of relationships among prognosis factors, we defined patients with SCC pathologic subtype, a pretreatment LN diameter <0.8 cm on CT/MR imaging, and SCC-Ag level <5 ng/mL as the low-risk group, while classifying the remaining patients as the high-risk group. Among our cohort, 51.5% (153/297) of patients were classified as low risk, and 48.5% (144/297) were classified as high risk. The low-risk group had very good outcomes. In contrast, patients in the high-risk group had a much worse prognosis. The corresponding 5-year OS and DMFS rates were 91.9% versus 81.1% and 95.9% versus 80.1%, respectively (P < .05). The rates of pathological LN positivity in the low-risk and high-risk groups were 2.0% (3/153) and 13.2% (19/144), respectively (P < .0001; Table 5; survival curves are included in Fig. 1).
Table 5.
Different outcomes between the high- and low-risk groups which were classified according to the prognosis factors.
Figure 1.
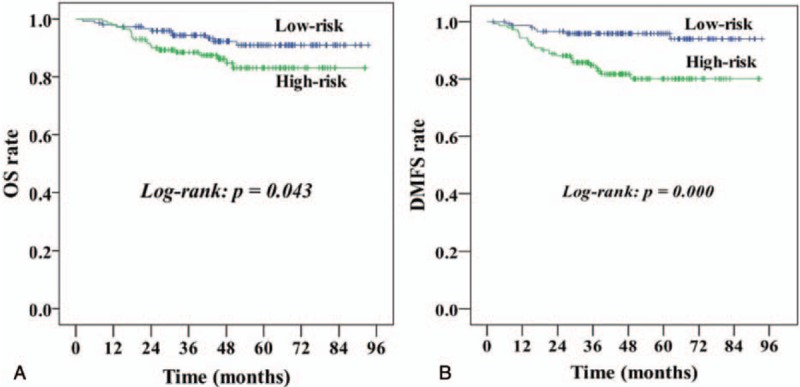
Comparison of Kaplan–Meier curves for OS (A) and DMFS (B) between the high-risk and low-risk groups. DMFS = distant metastasis-free survival, OS = overall survival.
3.6. Toxicities
The median interval between CCRT completion and surgery was 26 days (range: 14–71 days). The overall incidences of grade 3 and 4 acute hematological toxicities were 18.5% and 1.8%, respectively. The overall incidences of grade 2 and 3 acute gastrointestinal (GI) toxicities were 26.3% and 2.4%, respectively, and no grade 4 GI toxicities were observed. The incidences of grade 1 and 2 acute genitourinary (GU) toxicities were 11.2% and 1.8%, respectively, and no grade 3 or 4 acute GU toxicities were observed. Only 7.3% of patients developed chronic GI toxicities, and 11.0% of patients developed chronic GU toxicities (grade 1, 10.5%; grade 2, 0.5%). The incidence of lower extremity edema was 23.8% (grade 1, 14.6%; grade 2, 8.5%, grade 3, 0.7%). All such cases involved unilateral leg edema, and only 2 (0.7%) patients involved thrombus of a lower extremity vein (Table 6).
Table 6.
Treatment-related toxicities (N = 410).
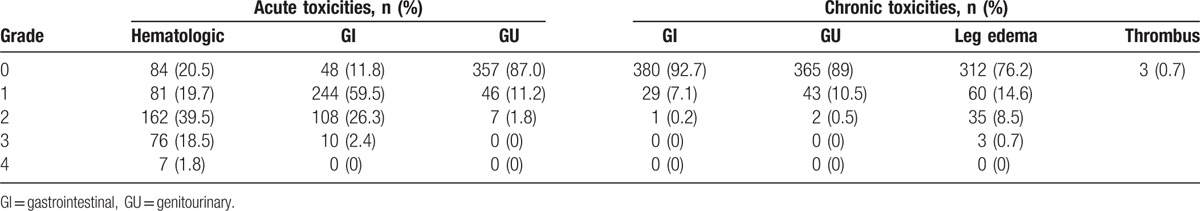
We used the Chi-square test to analyze factors that might contribute to the incidence of lower extremity edema. Among the 380 patients with complete data regarding LN resection, a median of 15 pelvic LNs were resected (range: 4–49). Our data showed that leg edema occurred more frequently when ≥15 pelvic LNs were resected (25.9% versus 16.9%) than that for <15 LNs (P = .022). However, the incidence of lower extremity edema was not associated with the choice of surgical method (e.g., transabdominal or laparoscopic hysterectomy or da Vinci surgical system).
4. Discussion
Many studies have reported encouraging results and favorable long-term toxicity profiles associated with CCRT followed by radical surgery for LACC.[11–13] The conclusions of our study are in agreement with these results, and our respective 5-year OS, DMFS, and PRFS rates of 86.7%, 88.6%, and 96.9% were not inferior to the reported rates from previous studies (OS: 57–85%, disease-free survival: 64–90%).[20,21] Furthermore, our toxicity analysis indicated acceptable levels of acute and chronic toxicities with this treatment combination.
The satisfactory OS outcomes achieved with this combination of treatment modalities may be attributed to the considerable improvements in local control, as described in the introduction section. For example, our study revealed a good overall prognosis status and very low local recurrence rate (10/410; vaginal stump recurrence in 9 patients and pelvic recurrence in one patient), with distant metastasis as the main cause of death. Notably, residual tumor after radiotherapy or chemotherapy is a major risk factor for recurrence and poor OS.[11,14,22–24] Our results demonstrated very encouraging pathological responses of cervical tumors after CCRT and subsequent surgery, as well as a very low positive LN rate (7.3%). Gadducci et al[22] reported that patients who did not achieve an overall optimal response had a 2.757-fold higher risk of recurrence and 5.413-fold higher risk of death, compared with those who achieved such a response (5-year RFS: 87.4% vs 47.5%, OS: 96% vs 53.7%, P < .0001). Moreover, in the present study, significantly better survival results were noted among patients who achieved a primary tumor pCR, compared to those who harbored residual carcinoma cells after CCRT. We further note that the pathological response of the resected LN was found to play an important prognostic role, consistent with previous reports by Ferrandina et al[11] and Classe et al.[14]
Our study also observed that the pelvic LN status on imaging could be used to estimate the possibility of LN metastasis. Klerkx et al[25] have concluded that best sensitivity and specificity rates are acquired when short axis diameter of pelvic LN on the MR image is >8 mm, resulting in a sensitivity of 42.9% and specificity 96.6%. In one meta-analysis [26], the authors recommended LN diameter ≥8 mm as the best cut-off value when short axis diameter was adopted as a positive criterion in MRI examination. According to the above research, we classified the patients into 2 groups: enlarged LN short diameter <0.8 cm, and enlarged short diameter ≥0.8 cm. Specifically, patients with a pretreatment LN size ≥0.8 cm on imaging had a positive LN rate of 19.3% and very poor prognosis (5-year OS, 56.6%), whereas those with LN <0.8 cm had a positive LN rate of only 3.0%. In addition, a pretreatment LN size ≥0.8 cm was associated with a significantly higher likelihood of a pathological residual primary tumor and deep stromal invasion. These results suggest that the pelvic LN status on imaging could be used to predict the eventual prognosis. Ohara and colleagues[27] noted that the CT-determined LN status might be a strong and useful predictor of cervical cancer confinement to the pelvis. In addition, despite the lack of a fixed prognostic cut-off value, SCC-Ag is considered an important predictor of overall survival, particularly with regard to distant metastasis.[28,29] According to our data, a SCC-Ag level ≥5 ng/mL was significantly associated with an increased risk of a pathological positive LN and reduced 5-year DMFS.
The pathological primary tumor type is unquestionably the most important prognostic factor for a cervical cancer. Adenocarcinoma (AC) is widely considered to have a poorer survival outcome than adenosquamous carcinoma (ASC) or SCC.[30,31] In our study, the pathological primary tumor type was found to be an independent prognostic factor for OS. Notably, only 15.8% (3/19) of non-SCC patients achieved a pCR after CCRT, indicating a high level of resistance to CCRT that was accompanied by a low rate of survival (5-year OS, 52.0%).
Finally, our toxicity analysis indicated acceptable levels of both acute and chronic toxicities, and we noted that only the incidence of leg edema after CCRT followed by radical surgery remained unimproved. Regarding our previous single-center experience, Wang et al[12] reported that the rate of leg edema was significantly higher with this therapeutic combination than with radical CCRT alone (35.29% vs 4.96%, P < .001). These authors also demonstrated that leg edema occurred more frequently when ≥20 pelvic LNs were dissected (55.56% vs 29.79% for <20 LNs, P = .022). Additionally, Fanfani et al[13] observed that in contrast to radical CCRT alone, vascular complications (including lymphocele and leg edema) were observed only in patients treated with CCRT followed by radical surgery (16.4%, P < .001). Todo et al[32] analyzed the relationship between the removal of circumflex iliac nodes distal to external iliac nodes (CINDEIN) and lower extremity lymphedema after systematic lymphadenectomy and concluded that the elimination of CINDEIN dissection could help to reduce the incidence of leg edema. These authors also concluded that patients with ≥31 LNs resected had a significantly higher incidence of edema than patients with ≤ 30 LNs resected (26.0% vs 16.5%, P = .0237). Although our study set a cut-off value of 15 resected LNs, we similarly concluded that a higher number of removed LNs correlated with a higher incidence of leg edema (25.9% versus 16.9%, P = .022). In addition, the number of removed LNs indirectly represents the region and extent of pelvic lymphadenectomy, which are associated with the occurrence of leg edema. Our results suggest that patients with a LN <0.8 cm on imaging before CCRT, SCC histology, and a SCC-Ag level <5 ng/mL at diagnosis have a low risk of LN metastasis and can expect good OS and DMFS outcomes. We therefore speculate that in these patients, pelvic lymphadenectomy could be avoided or that the extent of LN dissection could be limited to eliminate the risk of leg edema. While patients with any LN ≥0.8 cm, a SCC-Ag level ≥5 ng/mL and/or non-SCC histology must undergo standard pelvic lymphadenectomy. However, prospective randomized trials are needed to confirm the feasibility of individualized pelvic lymphadenectomy.
One limitation of the present study is the short follow-up period. At the time of the analysis, the median follow-up period for patients who remained alive was 51.3 months (range 4–97 months). Moreover, we used SPSS 18.0 software to calculate the 5-year OS according to the Kaplan–Meier method. Therefore, the estimated 5-year OS in this study could be a representation of the overall survival trend. In addition, our study is a retrospective analysis of patients who were treated at a single center. However, to the best of our knowledge, this is the first and probably the largest study which demonstrated that CCRT followed by radical surgery is an effective therapeutic approach for LACC, especially, in those countries that have a shortage of brachytherapy equipment. Moreover, we identified the patient group that would benefit from the individualized pelvic lymphadenectomy, by analyzing the relationship between imaging at diagnosis and postoperative pathological LN involvement. However, prospective randomized trials are needed to compare the effectiveness of this combined treatment with the standard radical CCRT alone.
5. Conclusion
Preoperative CCRT and radical surgery are feasible treatment options for LACC, particularly in economically underdeveloped areas. The outcomes of CCRT followed by radical surgery in this study were encouraging, and moreover this treatment was not associated with unacceptable complications. Postoperative pathologic responses correlated strongly with the pretreatment LN size on CT/MR imaging, such that patients with a LN <0.8 cm on diagnostic imaging, SCC histology, and a SCC-Ag level <5 ng/mL at diagnosis had a very low risk of a postoperative pathologically positive LN. Accordingly, individualized pelvic lymphadenectomy such as omitting or limiting the extent of LN dissection might be an alternative option for the patients with a low risk of LN metastasis.
Acknowledgments
The authors thank Xiao-Meng Wang, MB for her assistance in the work of follow-up visits.
Author contributions
Conceptualization: Chun Li Wei, Xin Li, Wei Wei Li, Mei Shi.
Data curation: Chun Li Wei, Xin Li, Mei Shi.
Formal analysis: Chun Li Wei.
Funding acquisition: Chun Li Wei.
Investigation: Xin Li, Ying Zhang, Wei Wei Li, Ping Jian Li, Na Li Zhao.
Methodology: Xin Li, Ying Zhang, Zhi Yun Dang, Wei Wei Li, Ping Jian Li, Na Li Zhao.
Project administration: Mei Shi.
Resources: Chun Li Wei, Juan shu Liu, Xia Li.
Software: Xin Li.
Supervision: Chun Li Wei, Ying Zhang, Zhi Yun Dang, Mei Shi.
Validation: Juan shu Liu, Xia Li, Mei Shi.
Visualization: Xin Li, Mei Shi.
Writing – original draft: Xin Li.
Writing – review & editing: Mei Shi.
Footnotes
Abbreviations: AC = adenocarcinoma, ASC = adenosquamous carcinoma, CCRT = concurrent chemoradiotherapy, CCT = concurrent chemotherapy, CINDEIN = circumflex iliac nodes distal to external iliac nodes, CIs = confidence intervals, CT = computed tomography, CTV = clinical target volume, DMFS = distant metastasis-free survival, FIGO = International Federation of Gynecology and Obstetrics, GI = gastrointestinal, GU = genitourinary, HR = hazard ratio, IMRT = intensity modulated radiotherapy, LACC = locally advanced cervical cancer, LN = lymph node, LVSI = lymphovascular space invasion, MHC = microscopically heterotypic cells, MR = magnetic resonance, OS = overall survival, PALN = para-aortic lymph node, pCR = pathological complete response, PRFS = pelvic recurrence-free survival, PTV = planning target volume, RCC = residual carcinoma cells, SCC = squamous cell carcinoma, SCC-Ag = squamous cell carcinoma antigen.
Li-Chun Wei and Xin Li shared first co-authorship.
This work is supported by National Natural Science Foundation of China (NSFC,81272346).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Kokka F, Bryant A, Brockbank E, et al. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev 2015;4:CD010260. [DOI] [PubMed] [Google Scholar]
- [3].Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–61. [DOI] [PubMed] [Google Scholar]
- [4].Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–53. [DOI] [PubMed] [Google Scholar]
- [5].Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339–48. [DOI] [PubMed] [Google Scholar]
- [6].Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–43. [DOI] [PubMed] [Google Scholar]
- [7].Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- [8].Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2000;48:201–11. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Lu JJ, Yin W, et al. Perspectives on patient access to radiation oncology facilities and services in Mainland China. Semin Radiat Oncol 2017;27:164–8. [DOI] [PubMed] [Google Scholar]
- [10].Chuang L, Kanis MJ, Miller B, et al. Treating locally advanced cervical cancer with concurrent chemoradiation without brachytherapy in low-resource countries. Am J Clin Oncol 2016;39:92–7. [DOI] [PubMed] [Google Scholar]
- [11].Ferrandina G, Margariti PA, Smaniotto D, et al. Long-term analysis of clinical outcome and complications in locally advanced cervical cancer patients administered concomitant chemoradiation followed by radical surgery. Gynecol Oncol 2010;119:404–10. [DOI] [PubMed] [Google Scholar]
- [12].Wang N, Li WW, Li JP, et al. Comparison of concurrent chemoradiotherapy followed by radical surgery and high-dose-rate intracavitary brachytherapy: a retrospective study of 240 patients with FIGO stage IIB cervical carcinoma. Onco Targets Ther 2014;7:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fanfani F, Vizza E, Landoni F, et al. Radical hysterectomy after chemoradiation in FIGO stage III cervical cancer patients versus chemoradiation and brachytherapy: complications and 3-years survival. Eur J Surg Oncol 2016;42:1519–25. [DOI] [PubMed] [Google Scholar]
- [14].Classe JM, Rauch P, Rodier JF, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer). Gynecol Oncol 2006;102:523–9. [DOI] [PubMed] [Google Scholar]
- [15].Houvenaeghel G, Lelievre L, Buttarelli M, et al. Contribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinoma. Eur J Surg Oncol 2007;33:498–503. [DOI] [PubMed] [Google Scholar]
- [16].Tummers P, Makar A, Vandecasteele K, et al. Completion surgery after intensity-modulated arc therapy in the treatment of locally advanced cervical cancer: feasibility, surgical outcome, and oncologic results. Int J Gynecol Cancer 2013;23:877–83. [DOI] [PubMed] [Google Scholar]
- [17].Ferrandina G, Ercoli A, Fagotti A, et al. Completion surgery after concomitant chemoradiation in locally advanced cervical cancer: a comprehensive analysis of pattern of postoperative complications. Ann Surg Oncol 2014;21:1692–9. [DOI] [PubMed] [Google Scholar]
- [18].Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 2006;103:714–8. [DOI] [PubMed] [Google Scholar]
- [19].Beesley V, Janda M, Eakin E, et al. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer 2007;109:2607–14. [DOI] [PubMed] [Google Scholar]
- [20].Ferrandina G, Legge F, Fagotti A, et al. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measures. Gynecol Oncol 2007;107(1 suppl 1):S127–32. [DOI] [PubMed] [Google Scholar]
- [21].Mariagrazia D, Anna F, Gabriella F, et al. Preoperative chemoradiotherapy in locally advanced cervical cancer: long-term outcome and complications. Gynecol Oncol 2005;99(3 suppl 1):S166–70. [DOI] [PubMed] [Google Scholar]
- [22].Gadducci A, Sartori E, Maggino T, et al. Pathological response on surgical samples is an independent prognostic variable for patients with Stage Ib2-IIb cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: an Italian multicenter retrospective study (CTF Study). Gynecol Oncol 2013;131:640–4. [DOI] [PubMed] [Google Scholar]
- [23].Ferrandina G, Gambacorta A, Gallotta V, et al. Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study. Int J Radiat Oncol Biol Phys 2014;90:778–85. [DOI] [PubMed] [Google Scholar]
- [24].Huang K, Sun H, Chen Z, et al. Optimal pathological response indicated better long-term outcome among patients with stage IB2 to IIB cervical cancer submitted to neoadjuvant chemotherapy. Sci Rep 2016;6:28278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Klerkx WM, Veldhuis WB, Spijkerboer AM, et al. The value of 3.0 Tesla diffusion-weighted MRI for pelvic nodal staging in patients with early stage cervical cancer. Eur J Cancer 2012;48:3414–21. [DOI] [PubMed] [Google Scholar]
- [26].Gong Y, Wang Q, Dong L, et al. Different imaging techniques for the detection of pelvic lymph nodes metastasis from gynecological malignancies: a systematic review and meta-analysis. Oncotarget 2017;8:14107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ohara K, Tanaka YO, Tsunoda H, et al. Nonoperative assessment of nodal status for locally advanced cervical squamous cell carcinoma treated by radiotherapy with regard to patterns of treatment failure. Int J Radiat Oncol Biol Phys 2003;55:354–61. [DOI] [PubMed] [Google Scholar]
- [28].Huang EY, Huang YJ, Chanchien CC, et al. Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiat Oncol 2012;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hong JH, Tsai CS, Lai CH, et al. Risk stratification of patients with advanced squamous cell carcinoma of cervix treated by radiotherapy alone. Int J Radiat Oncol Biol Phys 2005;63:492–9. [DOI] [PubMed] [Google Scholar]
- [30].Noh JM, Park W, Kim YS, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13-10). Gynecol Oncol 2014;132:618–23. [DOI] [PubMed] [Google Scholar]
- [31].Huang YT, Wang CC, Tsai CS, et al. Clinical behaviors and outcomes for adenocarcinoma or adenosquamous carcinoma of cervix treated by radical hysterectomy and adjuvant radiotherapy or chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;84:420–7. [DOI] [PubMed] [Google Scholar]
- [32].Todo Y, Yamazaki H, Takeshita S, et al. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus malignant tumors. Gynecol Oncol 2015;139:160–4. [DOI] [PubMed] [Google Scholar]


