Supplemental Digital Content is available in the text
Keywords: cardiovascular disease, isolated systolic hypertension, low sodium salt, salt restriction, systolic blood pressure
Abstract
Evidence has shown that long-term sodium reduction can not only reduce blood pressure, but also provide cardiovascular benefits. To date, there is little evidence related to the effects of salt reduction on isolated systolic hypertension (ISH).
A total of 126 hypertensive patients were divided into an ISH group (n = 51) and a non-ISH (NISH) group (n = 75). The members of each group were then randomly assigned to low sodium salt (LSSalt) or normal salt (NSalt) diets for 6 months. Their blood pressure was measured every 2 months. Serum plasma renin-angiotensin activity, blood biochemical assays and urinary measurements were determined at the baseline and at the end of the 6 months.
At the end of the study, the mean systolic blood pressure (SBP) of the ISH LSSalt group had significantly decreased by 10.18 mm Hg (95% confidence interval (CI): 3.13 to 17.2, P = .006) compared with that of the ISH NSalt group, while the mean SBP only decreased by 5.10 mm Hg (95% CI: −2.02 to 12.2, P = .158) in the NISH LSSalt group compared with that of the NISH NSalt group. The mean diastolic blood pressure (DBP) had no significant differences in the ISH and NISH groups. No obvious renin angiotensin system activation was found after LSSalt intervention. Regarding the urinary excretion of electrolytes and blood biochemical assays, the LSSalt treatment had the same effects on the ISH group as on the NISH group.
The present study showed that the SBP of ISH patients was significantly decreased with the LSSalt intervention, while neither the SBP of the NISH patients nor the DBP of either group were similarly decreased, which indicated that ISH patients were more sensitive to salt restriction.
1. Introduction
Hypertension is a major risk factor for cardiovascular events, such as stroke, myocardial infarction, heart failure, and renal disease.[1–3] Strong evidence has shown linear relationships between cardiovascular risk and both systolic and diastolic blood pressures (BPs).[4] systolic BP (SBP) is one of the main risk factors affecting cardiovascular events in elderly people.[5] Unlike diastolic BP (DBP), SBP increases gradually with age,[6] and in aging societies, isolated SBP increase is the most common form of hypertension [7,8] However, few clinical trials on isolated systolic hypertension (ISH) have been conducted.
Numerous clinical studies have clearly demonstrated a positive relationship between salt intake and BP elevation, and substantial evidence has shown that daily salt restriction may be a useful lifestyle modification for hypertensive patients.[9,10] Moreover, several large clinical trials have supported the hypothesis that long-term sodium reduction, previously manifested to lower BP, may also provide cardiovascular benefits.[11] As arterial stiffness is one of the most important inducing factors in the development and maintenance of ISH, stiffer arteries are more sensitive to BP changes induced by liquid volume variation.[12,13] Salt restriction could reduce volume load, thereby lowering BP and providing cardiovascular benefits to ISH patients. However, until now, the effect of salt reduction on ISH has been still unclear.
Although moderate salt restriction has proved beneficial in cardiovascular disease, it is difficult to perform studies in large population because long-term established salt intake habits are hard to change. In the treatment of Mild Hypertension Study, the mean salt intake was decreased by 2 to 3 g/day during the first year but regressed to less than 1 g/day after 4 years.[14] In order to change this situation, we used a novel low sodium salt (LSSalt) in the present study. This LSSalt is composed of 65% sodium chloride, 30% potassium chloride, 5% calcium salts (4% calcium citrate tetrahydrate and 1% calcium carbonate), and 12 mg/kg folic acid.[15] Compared to normal salt (NSalt), this substitute can significantly decrease sodium intake (a decrease of approximately 40% compared with normal salt) with no obvious difference in the salty flavor. In the present study, we enrolled 126 hypertensive participants who were divided into an ISH group and a non-ISH (NISH) group to investigate the effects of salt restriction on ISH individuals.
2. Methods
2.1. Subjects
This study was a single blind, randomized, controlled trial, conducted in 10 communities in the rural Hedong District, Tianjin, China. Informed consent was obtained from all participants, and the research protocol was approved by the Ethical Committee of Pingjin Hospital in accordance with the principles of the Declaration of Helsinki.
A total of 138 participants, male and female Han participants, who were 50–80 years of age, and who had mild to moderate hypertension (meeting one of the following criteria: average SBP≥140 mm Hg and/or DBP≥90 mm Hg or being treated with antihypertensive drugs) were recruited in the present study. To minimize the influences of confounding factors on the study, the following inclusion criteria were applied: patients who ate no more than one meal per week outside of their home, did not use potassium-sparing medication, were willing to commit to long-term intake of LSSalt, and had serum potassium levels < 5.5 mmol/L and a net elevation of serum potassium < 1.0 mmol/L at the end of the run-in period. The exclusion criteria included history of a heart attack or stroke within the preceding 6 months, current angina pectoris, congestive heart failure, diabetes mellitus, serious liver and kidney dysfunction, serious mental or physical illness, malignancy, and definite secondary hypertension at end of the run-in period.
All the participants were asked to provide information regarding age, education, marital status, physical activity, history of smoking and alcohol consumption, and family history. Baseline body weights, BP, and levels of blood urea nitrogen, creatinine, and serum potassium were recorded. In addition, 24 hours urine was collected for the determination of sodium, potassium, and calcium excretion. After a 4-week run-in period, the participants were divided into an ISH group (meeting one of the following criteria: SBP≥140 mm Hg and DBP < 90 mm Hg or previously diagnosed with ISH and being treated with antihypertensive drugs) and a NISH group (meeting one of the following criteria: DBP≥90 mm Hg or previously diagnosed with NISH and being treated with antihypertensive drugs). Each group was then randomized to LSSalt or NSalt diets for 6 months. A flowchart is displayed in Figure 1.
Figure 1.
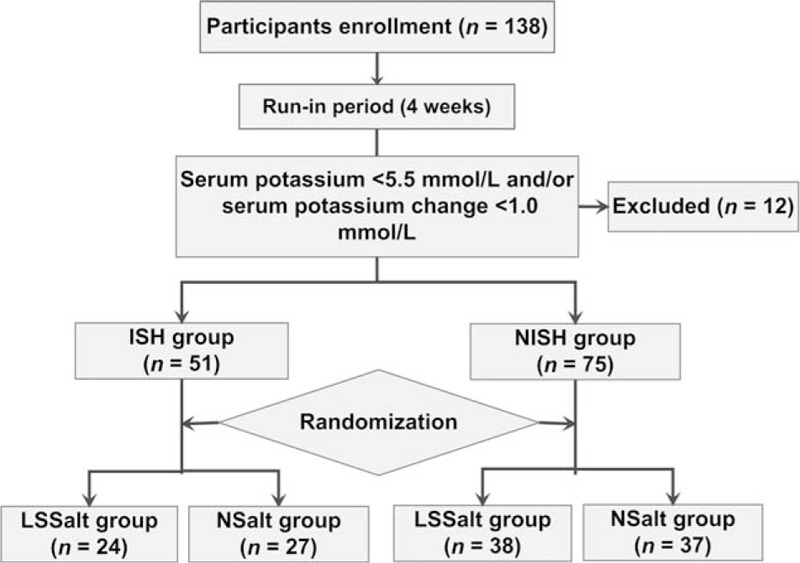
Flowchart of the study. ISH = isolated systolic hypertension, LSSalt = low sodium salt, NISH = nonisolated systolic hypertension, NSalt = normal salt.
2.2. Follow-up and measurements
All the hypertensive participants were followed-up every 2 months. Our primary outcomes were changes in SBP and DBP in the hypertensive patients. And the secondary outcomes were changes in the 24 hours urine excretion of electrolytes and related blood sample analyses.
SBP and DBP were defined by Korotkoff sound phase 1 and phase 5, respectively. The measurement was performed by 2 experienced physicians and was measured twice after a test measurement. The measurement was considered valid if the difference between the 2 measurements was < 10 mm Hg; otherwise, a third measurement was taken, and the average value was used for analysis. At the end of 6 months, 24 hours urine and blood samples were collected. The urinary and intraerythrocyte Na+, K+, and Ca2+ levels were determined by atomic absorbance spectrophotometry. The serum assays included total cholesterol, high density lipoprotein, triglyceride, glucose, and insulin. Plasma renin activity, angiotensin II level, and atrial natriuretic peptide (ANP) level were measured by radioimmunoassay as previous described.[15]
2.3. Statistical analysis
The target sample size of 100, with 25 in each subgroup, was estimated to provide 80% power at a two-tailed P = .05 to detect a difference of SBP≥8.0 mm Hg between the LSSalt and the NSalt groups in each cohort (ISH and NISH groups). The normal distribution of the data was estimated using the Kolmogorov–Smirnov test. To estimate the overall effects over time of LSS (time × treatment) on BP, two-way analysis of variance repeated measurements was used. Within-group differences were evaluated by paired t-test. Considering the influence of seasonal changes on BP,[16] between-group differences in BP and serum and urinary assays were reanalyzed by comparing the net change at the time of randomization and end of the study using unpaired t-tests. Net change was calculated as follows: (V6 − V0)LSS − (V6 − V0)NS, where V represents a certain variable and the numbers indicate the months after randomization.[15] Categorical data were compared using χ2-test and Fisher's exact test when expected cell values were < 5. The relationships between BP and the blood/urinary parameters were evaluated with Pearson's correlation (if the data passed the normality test) or Spearman's rank correlation (if the data failed the normality test). A two-tailed P < .05 was considered statistically significant. All analyses were performed using SPSS, version 18.0 (SPSS, Chicago, IL).
3. Results
3.1. Baseline characteristics
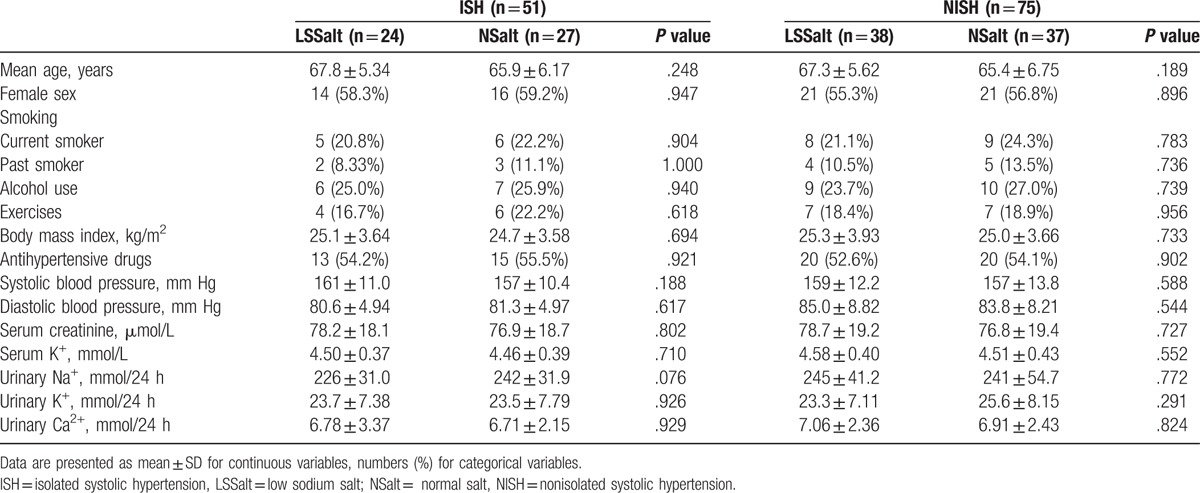
As shown in Table 1 and Supplemental Table 1, 126 hypertensive participants were enrolled in the study. No adverse cardiovascular events happened during the follow-up period. No significant differences in baseline characteristics were observed among the different groups (all P > .05).
Table 1.
Baseline characteristics of all participants.
3.2. The BP levels of different groups
As shown in Figure 2 and Supplemental Table 2, in the ISH subjects, there was a continuous decrease of SBP over time with LSSalt treatment (P = .003) while no significant differences were found in the NSalt group, and no apparent differences in DBP were found in either the LSSalt or NSalt groups. In the NISH subjects, no marked changes in SBP and DBP were observed in either the LSSalt or NSalt treatment groups, although SBP displayed a decreasing trend in the LSSalt group (all P > .05). Compared with mean of the NSalt group, the mean SBP of ISH LSSalt group was significantly decreased by 10.18 mm Hg (95% confidence interval (CI): 3.13 to 17.2, P = .006), while it only decreased 5.10 mm Hg (95% CI: −2.02 to 12.2, P = .158) in the NISH LSSalt group. No marked differences in the administration of antihypertensive drugs were observed in the ISH and NISH LSSalt and NSalt groups at the end of the study (Supplemental Table 1, all P > .05).
Figure 2.
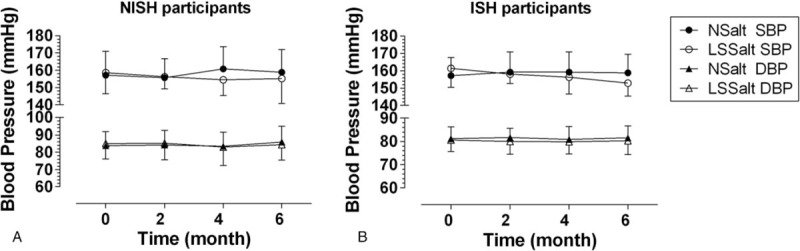
Systolic and diastolic blood pressure changes in ISH and NISH participants after 6 months follow-up. Data are reported as mean ± SD. ISH, isolated systolic hypertension; NISH = nonisolated systolic hypertension; LSSalt = low sodium salt; NSalt = normal salt; SBP = systolic blood pressure; DBP = diastolic blood pressure.
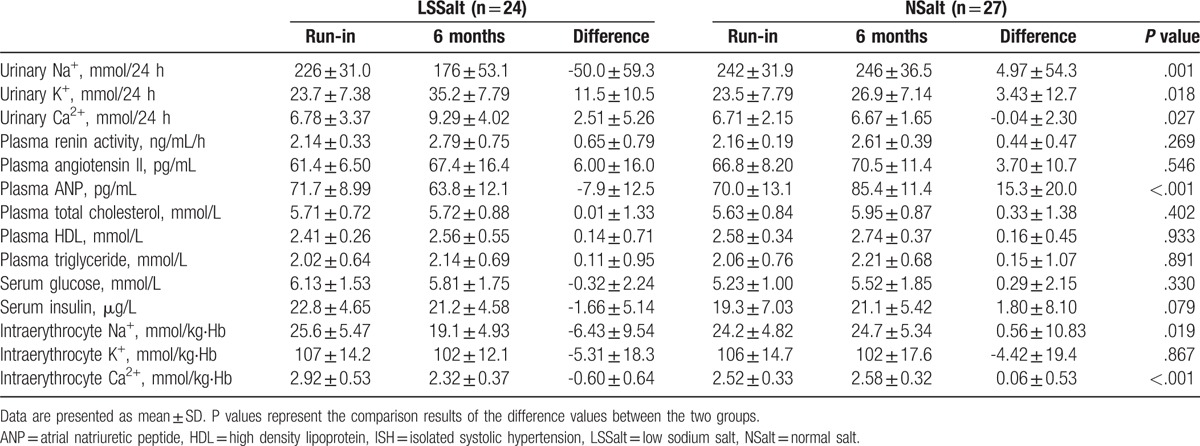
3.3. Blood/urinary parameters in ISH participants
As illustrated in Table 2, compared with the NSalt group, the LSSalt group had to average changes in the excretion urinary electrolytes of −55.0 mmol/24 h for Na+ (95% CI: −86.9 to −23.0, P = .001), 8.07 mmol/24 h for K+ (95% CI: −1.50 to 17.6, P = .018), and 2.55 mmol/24 h for Ca2+ (95% CI: 0.31 to 4.78, P = .027). These changes were accompanied by decreases in intraerythrocyte Na+ and Ca2+ levels. In addition, the plasma ANP level was significantly decreased after LSSalt intervention (−23.2 pg/mL, 95% CI: −32.7 to −13.7, P < .001). There were no significant differences in other blood parameters, such as plasma renin activity, plasma angiotensin II, plasma lipid parameters, serum glucose, serum insulin, and the intraerythrocyte K+ level, between the NSalt and LSSalt groups (all P > .05).
Table 2.
24 h urinary excretion of electrolytes and blood biochemical assays at end of run-in period and 6 month after randomization in isolated systolic hypertension group.
3.4. Blood/urinary parameters in NISH participants
As illustrated in Table 3, compared to the NSalt group, the LSSalt group showed average changes in the excretion of urinary electrolytes, with−75.7 mmol/24 h for Na+ (95% CI:−108 to−43.9, P < .001), 10.3 mmol/24 h for K+ (95% CI: 4.34 to 16.3, P = .001), and 4.15 mmol/24 h for Ca2+ (95% CI: 2.25 to 6.04, P < .001). These changes were accompanied by an apparent decrease in the intraerythrocyte levels of Na+ and Ca2+. Furthermore, the ANP level was significantly decreased after LSSalt intervention (−20.9 pg/mL, 95% CI: −28.1 to −13.7, P < .001). No significant differences in other blood biochemical assays were observed between the NSalt and LSSalt groups (all P > .05).
Table 3.
24 h urinary excretion of electrolytes and blood biochemical assays at end of run-in period and 6 month after randomization in nonisolated systolic hypertension group.
3.5. Correlation analysis between BP and blood/urinary parameters
Correlation analyses were then carried out to evaluate the association between BP and the blood/urinary parameters. The results showed that the 24 hours urinary Na+ and intraerythrocyte K+ levels were positively correlated with SBP and that the 24 hours urinary K+ level was negatively correlated with SBP in ISH participants, while the 24 hours urinary Na+ level was positively correlated with DBP in these participants (Figs. 3 and 4). In the NISH participants, no correlations between BP and the blood/urinary parameters were found (Supplemental Figure 1 and Supplemental Figure 2).
Figure 3.
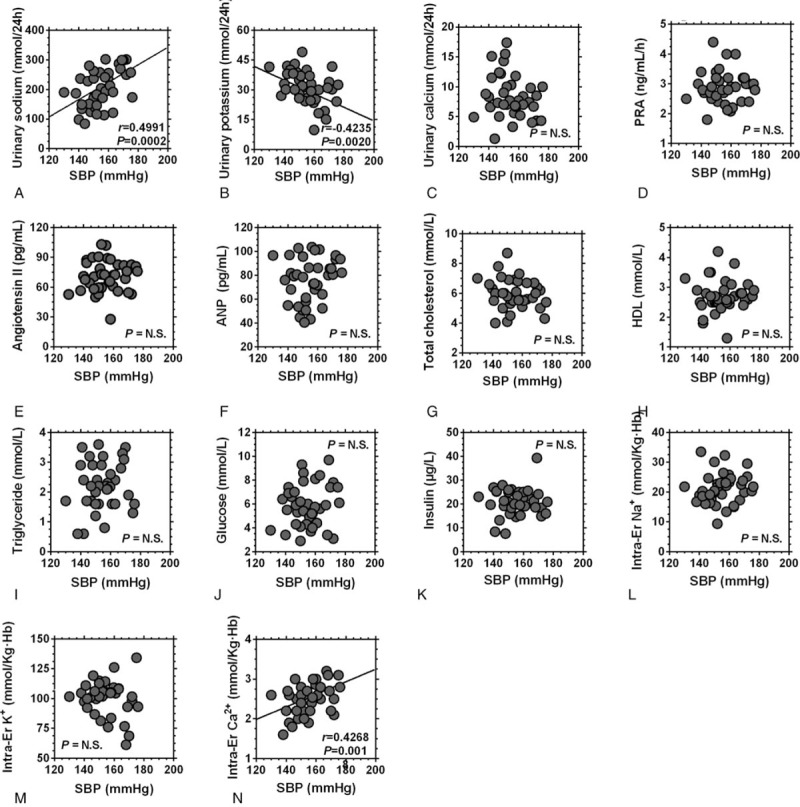
Correlation analysis between the systolic blood pressure and the urinary/blood parameters of ISH participants. Abbreviations = ISH, isolated systolic hypertension; SBP = systolic blood pressure; PRA = plasma renin activity; ANP = atrial natriuretic peptide; HDL = high density lipoprotein, Er = erythrocyte.
Figure 4.
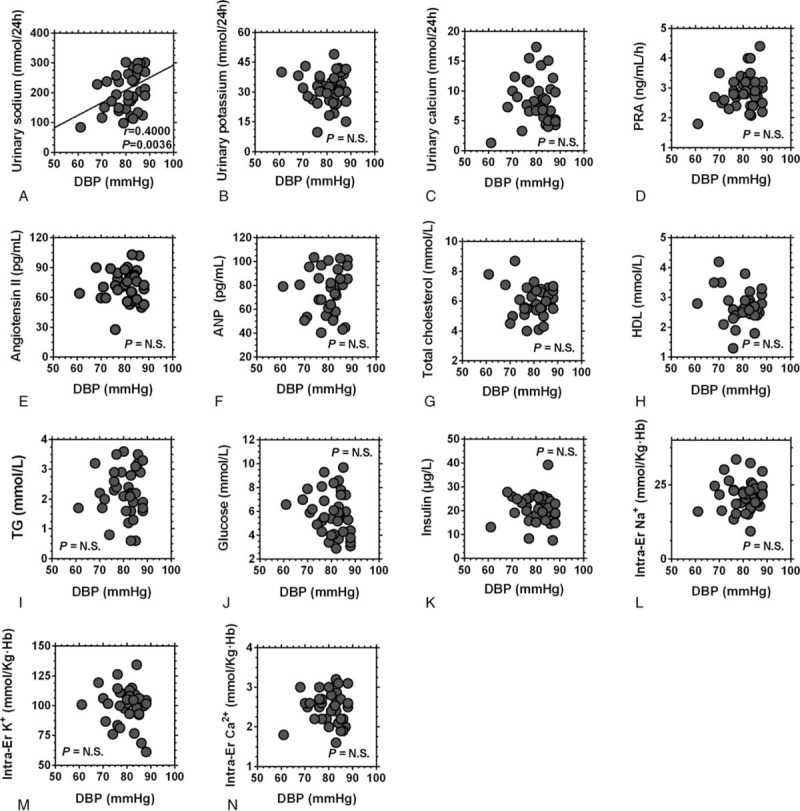
Correlation analysis between the diastolic blood pressure and The urinary/blood parameters of ISH participants. Abbreviations = ISH, isolated systolic hypertension; DBP = diastolic blood pressure; PRA = plasma renin activity; ANP = atrial natriuretic peptide; HDL = high density lipoprotein, Er = erythrocyte.
4. Discussion
The present study used salts with different sodium chloride contents to observe the effects of low sodium salt intake on the BP of ISH patients. The results showed that in the ISH participants, the SBP of the LSSalt group was significantly decreased compared with that of the NSalt group after 6 months of intervention, while no changes were observed for DBP or in NISH participants. Furthermore, the correlation analyses showed that the SBP of ISH participants was positively correlated with the 24 h urinary Na+ level and negatively correlated with the 24 hours urinary K+ level, indicating that the ISH patients were more sensitive to the effects of LSSalt.
Numerous clinical studies have demonstrated the benefits of reducing BP in ISH patients.[8,17,18] A recent study demonstrated that after 1 month of low salt intervention, the average SBP of ISH patients was decreased from 166 to 156 mm Hg (P < .001), but no significant reduction was found in DBP, which agrees with the results of the present study.[19] However, a reanalysis of NHANES III and IV showed that salt intake was only related to isolated diastolic hypertension, and there was an inverse effect in the BP levels of ISH patients.[20] This disparity in the effects of salt intake on the BP of ISH individuals may be partly attributed to differences in the dietary intake of various components, such as energy, protein, total fat, carbohydrates, potassium, magnesium, and calcium, which may also influence the BP levels.[19,21] The other interpretation is that location and ethnicity may result in different reactivities of BP levels to salt intake.[22,23]
The low sodium content of the LSSalt could be the major contributor to the decrease in BP. The 24 hours urinary Na+ excretion was a good marker of the daily sodium intake.[15] In the present study, compared with the ISH NSalt group, the sodium intake decreased by 55.0 mmol/24 h in the ISH LSSalt group, and the SBP decreased by 10.18 mm Hg. In the NISH participants, the SBP of the LSSalt group only decreased 5.10 mm Hg compared with that of the NSalt group, when the sodium intake decreased by 75.7 mmol/24 h, which indicated that the ISH patients were more sensitive to salt restriction, as found in a previous study.[19] Additionally, because salt restriction leads to an increase in Na+ reabsorption, the excretion of other urinary electrolytes could also be influenced.[15] In the present study, the decrease in the urinary excretion of Na+ was accompanied by apparent increases in the urinary excretion of K+ and Ca2+, which indicated that there was an increase in the active reabsorption of Na+.
The present study also showed that the plasma ANP level was significantly decreased by LSSalt intervention in both the ISH and NISH groups. ANP is secreted by mammals and released into circulation in response to acute or chronic atrial stretch, playing an important role in circulatory homeostasis.[24] It can regulate BP by modulating fluid homeostasis and vascular function,[25,26] which may be a mechanism of BP reduction by low salt intake in hypertensive patients.
The detailed mechanism underlying the lowering of BP due to salt restriction is still unclear, although several possible mechanisms have been elucidated.[27] First, high salt intake could lead to increases in blood volume and peripheral vascular resistance; thus, salt restriction could weaken these effects and reduce BP accordingly.[28] The second proposed mechanism suggests that compared with high salt intake, salt restriction could inhibit the production of reactive oxygen species, reducing the activity of nitric oxide, improving peripheral vascular resistance and lowering BP.[29] Another possible mechanism is that the low level of inflammation induced by inflammatory cells and factors is an important factor inducing increased BP.[30] Salt restriction could inhibit inflammatory cell infiltration and decrease the production of inflammatory factors, thereby lowering BP.[31]
The reason for the enhanced sensitivity of ISH patients to salt restriction needs further study to be elucidated. One possible reason is that ISH is the most dominant form of hypertension with increasing age,[6] among people aged 70 and older, the prevalence is 8%, and it rises to > 25% among those aged 80 years or older.[8] Previous studies have shown that most ISH patients have a more severe arterial stiffness than is found in patients with other types of hypertension, and that stiffer arteries are more sensitive to BP changes induced by variation in liquid volume.[12,13] Salt restriction could reduce the volume load, leading to lower BP. A second possible reason is that inflammation is one of the most important causes of arterial stiffness,[32,33] and salt restriction can inhibit inflammatory cell infiltration, decreasing the production of inflammatory factors, improving arterial stiffness and lowering BP. Another possible reason is that most ISH patients may have salt sensitive hypertension, resulting in a stronger effect on BP of salt restriction. This may be supported by the fact that only ISH patients had a positive correlation between 24 hours urinary sodium levels and BP. However, this proposed mechanism still needs further study to be confirmed.
There were several novel findings in the present study. First, although studies have investigated the relationship between low sodium salt intake and BP of ISH patients, the results have been controversial.[19,20] In He's study,[19] the SBP of the ISH group decreased 10 mm Hg after 1 month of low sodium intervention while another study showed that salt intake had an inverse relationship with BP levels in ISH patients.[20] Therefore, we further investigated the relationship of low sodium salt intake and BP in ISH patients and found that the ISH patients experienced larger decreased in SBP after moderate salt restriction. The second finding was that an increasingly lower salt intake does not continue to confer additional benefits, high salt intake ISH population, moderate salt restriction is beneficial. In the present study, the excretion of sodium in the ISH LSSalt group decreased from a relatively high level (226 mmol/L) to a low level (176 mmol/L), and the SBP decreased 10.10 mm Hg without obvious RAS activation. But in He's study,[19] the sodium excretion of the ISH group decreased from a relatively low level (175 mmol/L) to a lower level (87 mmol/L), and the SBP decreased 10 mm Hg, accompanied by significant RAS activation. Another previous study has shown that salt restriction with RAS and sympathetic nerve system activation may offset the benefits of salt restriction on high BP and may even cause adverse effects.[34] The third finding was that the novel low sodium salt used in this study can not only decrease sodium intake with no obvious difference in salty flavor but also replenish potassium which may also lower BP, as the correlation analysis in the present study showed an inverse relationship between 24 hours urinary of excretion of K+ and SBP in ISH participants.
Several limitations should be acknowledged in our study. First, this community-based ISH salt restriction trial was a single-blinded, single-center study, which may lead to systematic bias in BP measurements. Second, we selected LSSalt as the low salt intervention, and it may be exaggerate the effect of salt restriction because several other components of LSSalt (potassium, calcium, and folic acid) may also lower BP.[35–38] Third, the measurement of BP in the study was office BP, not ambulatory blood pressure monitoring or home monitoring data, which may be different from the patients’ usual BP. Fourth, to enrol participants with good compliance, we recruited an relatively old population and a small sample; thus, to extrapolate the results to a more general population, further studies are needed.
5. Conclusions
The present study showed that the SBP of ISH patients, but not that of NISH patients or the DBP of either group, was significantly decreased under LSSalt intervention, which indicated that ISH patients were more sensitive to salt restriction. More benefits in the secondary prevention of hypertension might be obtained in ISH patients from moderate salt restriction.
Author contributions
Conceptualization: Yu-Ming. Li.
Data curation: WenJie Ji, JunXiang Liu, R. Shi.
Formal analysis: GuoHong Yang, X. Zhou.
Funding acquisition: GuoHong Yang, Yu-Ming. Li.
Investigation: JunXiang Liu, TieMin Jiang.
Methodology: GuoHong Yang, X. Zhou, WenJie Ji, R. Shi.
Project administration: TieMin Jiang, Yu-Ming. Li.
Software: J. Sun.
Supervision: WenJie Ji, R. Shi, TieMin Jiang.
Visualization: J. Sun.
Writing original draft: GuoHong Yang, X. Zhou.
Supplementary Material
Footnotes
Abbreviations: 95%CI = 95%confidence interval, ACEI = angiotensin-converting enzyme inhibitor, ANP = atrial natriuretic peptide, ARB = angiotensin receptor blockers, BP = blood pressure, CCB = calcium channel blockers, DBP = diastolic blood pressure, Er = erythrocyte, HDL = high density lipoprotein, ISH = isolated systolic hypertension, LSSalt = low sodium salt, NISH = nonisolated systolic hypertension, NSalt = normal salt, PRA = plasma renin activity, SBP = systolic blood pressure.
GHY and XZ contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (grant number 81600328), Tianjin Municipal Science, and Technology Committee (grant numbers 16JCQNJC11800, 15ZXJZSY00010, and 16ZXMJSY00130) and intramural research program from Logistics University of Chinese People's Armed Police Forces (grant numbers 2015ZXKF11 and FYM201533).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [2].Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- [3].Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J 1999;138:205–10. [DOI] [PubMed] [Google Scholar]
- [4].Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol 2000;85:251–5. [DOI] [PubMed] [Google Scholar]
- [5].Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355:865–72. [DOI] [PubMed] [Google Scholar]
- [6].Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995;25:305–13. [DOI] [PubMed] [Google Scholar]
- [7].Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001;358:1305–15. [DOI] [PubMed] [Google Scholar]
- [8].Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757–64. [DOI] [PubMed] [Google Scholar]
- [9].Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 2007;356:1966–78. [DOI] [PubMed] [Google Scholar]
- [10].Meneton P, Jeunemaitre X, de Wardener HE, et al. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 2005;85:679–715. [DOI] [PubMed] [Google Scholar]
- [11].Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yano Y, Neeland IJ, Ayers C, et al. Hemodynamic and mechanical properties of the proximal aorta in young and middle-aged adults with isolated systolic hypertension: the Dallas Heart Study. Hypertension 2017;70:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr Hypertens Rep 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neaton JD, Grimm RH, Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA 1993;270:713–24. [PubMed] [Google Scholar]
- [15].Zhou X, Liu JX, Shi R, et al. Compound ion salt, a novel low-sodium salt substitute: from animal study to community-based population trial. Am J Hypertens 2009;22:934–42. [DOI] [PubMed] [Google Scholar]
- [16].Fujiwara T, Kawamura M, Nakajima J, et al. Seasonal differences in diurnal blood pressure of hypertensive patients living in a stable environmental temperature. J Hypertens 1995;13:1747–52. [PubMed] [Google Scholar]
- [17].Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens 1998;16:1823–9. [DOI] [PubMed] [Google Scholar]
- [18].Group SCR. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255–64. [PubMed] [Google Scholar]
- [19].He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure in isolated systolic hypertension and combined hypertension. Hypertension 2005;46:66–70. [DOI] [PubMed] [Google Scholar]
- [20].Townsend MS, Fulgoni VL, III, Stern JS, et al. Low mineral intake is associated with high systolic blood pressure in the Third and Fourth National Health and Nutrition Examination Surveys. Am J Hypertens 2005;18:261–9. [DOI] [PubMed] [Google Scholar]
- [21].Briefel RR, Sempos CT, McDowell MA, et al. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr 1997;65suppl:S1203–9. [DOI] [PubMed] [Google Scholar]
- [22].He FJ, Marciniak M, Markandu ND, et al. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension 2010;56:253–9. [DOI] [PubMed] [Google Scholar]
- [23].de Brito-Ashurst I, Perry L, Sanders TA, et al. The role of salt intake and salt sensitivity in the management of hypertension in South Asian people with chronic kidney disease: a randomised controlled trial. Heart 2013;99:1256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rubattu S, Calvieri C, Pagliaro B, et al. Atrial natriuretic peptide and regulation of vascular function in hypertension and heart failure: implications for novel therapeutic strategies. J Hypertens 2013;31:1061–72. [DOI] [PubMed] [Google Scholar]
- [25].Chen W, Gaßner B, Börner S, et al. Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway. Cardiovasc Res 2012;93:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bolli P, Müller F, Linder L, et al. The vasodilator potency of atrial natriuretic peptide in man. Circulation 1987;75:221–8. [DOI] [PubMed] [Google Scholar]
- [27].Ha SK. Dietary salt intake and hypertension. Electrolyte Blood Press 2014;12:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15:545–52. [DOI] [PubMed] [Google Scholar]
- [29].Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res 2013;50:458–67. [DOI] [PubMed] [Google Scholar]
- [30].Chan CT, Moore JP, Budzyn K, et al. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 2012;60:1207–12. [DOI] [PubMed] [Google Scholar]
- [31].Yang GH, Zhou X, Ji WJ, et al. Overexpression of VEGF-C attenuates chronic high salt intake-induced left ventricular maladaptive remodeling in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2014;306:H598–609. [DOI] [PubMed] [Google Scholar]
- [32].Mattace-Raso FU, Verwoert GC, Hofman A, et al. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. J Hypertens 2010;28:892–5. [DOI] [PubMed] [Google Scholar]
- [33].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:932–43. [DOI] [PubMed] [Google Scholar]
- [34].Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011;305:1777–85. [DOI] [PubMed] [Google Scholar]
- [35].Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011;123:2434–506. [DOI] [PubMed] [Google Scholar]
- [36].Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med 1999;159:285–93. [DOI] [PubMed] [Google Scholar]
- [37].Whelton PK, He J, Cutler JA, et al. Effects of oral potassium on blood pressure. JAMA 1997;277:1624–32. [DOI] [PubMed] [Google Scholar]
- [38].Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


