Abstract
This study aimed to evaluate the clinical significance of pretreatment red cell distribution width (RDW), monocyte/lymphocyte ratio (MLR), neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) in patients with urothelial carcinoma of the bladder (UCB).
Hematological parameters of 127 consecutive patients with UCB and 162 healthy controls were retrospectively analyzed. Receiver operating characteristic curve was plotted to determine the optimal cut-off value of RDW, MLR, NLR, and PLR to predict UCB. Whether these parameters could be independent predictors of UCB and had an association with the demographics and clinical characteristics of patients were also assessed.
Patients with UCB had higher pretreatment RDW, MLR, NLR, and PLR compared with the healthy controls. With the tumor progression, MLR, NLR, and PLR rose consistently, whereas no significant difference was observed in RDW across tumor stages. NLR and PLR were associated with tumor size and tumor grade, while MLR was correlated with tumor size only. The best threshold of RDW, MLR, NLR, and PLR to predict UCB was 13.50%, 0.26, 2.16, and 128.46, respectively. Multivariate logistic regression model identified NLR ≥ 2.16 (odds ratio [OR] = 2.914; P < .001) and PLR ≥ 128.46 (OR = 2.761; P < .001) as independent predictors of UCB. High NLR and PLR were also associated with tumor markers, such as carcinoembryonic antigen and α-fetoprotein.
Pretreatment NLR and PLR could be significant independent predictors of UCB. These simple and readily available inflammatory markers therefore might be used to manage the disease.
Keywords: bladder, monocyte/lymphocyte ratio, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, red cell distribution width, urothelial carcinoma
1. Introduction
Bladder cancer is a catch-all term for several types of carcinoma arising from the epithelial lining of the urinary bladder. Urinary bladder cancer was the 6th most common carcinoma and the 9th main cause of cancer death among males throughout the world, with an estimated 330,400 new cases and 123,100 deaths in 2012.[1] In developed countries, both estimated new cases and estimated deaths of urinary bladder cancer are higher than in developing countries.[1] Bladder cancer is the 4th most frequent malignancy in males in the United States.[2] According to estimates, 79,030 new cases of bladder cancer are predicted to occur in the United States in 2017, with approximately 60,490 new cases among men.[2] In China, bladder cancer was the 6th and 16th most common cancer in men and women, respectively, in 2015.[3] Urothelial carcinoma of the bladder (UCB), also called transitional cell carcinoma of the bladder, accounts for more than 90% of cancers in the bladder[4] and is consequently the focus of this study.
The initiation, development, invasion, and metastasis of a tumor are always accompanied by inflammation and immune response, which have a complex interaction with the tumor microenvironment.[5,6] Recently, hematological inflammatory markers that are easily and quickly measured in the clinic, such as red cell distribution width (RDW), monocyte/lymphocyte ratio (MLR), neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR), are drawing increased attention because they could be prognostic or predictive factors for numerous cancers, for example, gastric cancer, pancreatic adenocarcinoma, and nonsmall cell lung cancer.[7–9] Although many studies have reported the roles of NLR and PLR in patients with bladder cancer, which consisted of UCB, squamous cell carcinoma, and adenocarcinoma, those studies either have not independently focused on UCB[10–12] or just elucidated the value of one of peripheral blood-based biomarkers in patients with UCB.[13,14] In addition, the relationship between RDW, MLR, and UCB has not been well studied.
Therefore, the objective of the present study was to evaluate the clinical value of pretreatment RDW, MLR, NLR, and PLR in patients with UCB.
2. Materials and methods
2.1. Study subjects
This retrospective study consisted of 705 patients who were admitted to the First Affiliated Hospital of Guangxi Medical University for a newly diagnosed UCB between December 2011 and March 2017. A 2-step method was adopted to enroll the subjects. First, all newly diagnosed UCB patients who with available reports of pretreatment hematological parameters were selected. Those bladder cancer patients diagnosed with the following 4 types of carcinoma were enrolled: infiltrating urothelial carcinoma; urothelial carcinoma in situ; high grade non-invasive papillary urothelial carcinoma; and low grade non-invasive papillary urothelial carcinoma. Second, patients who met any one of the following criteria were excluded: other carcinoma except for UCB, chronic inflammatory diseases, chronic obstructive pulmonary disease, history of diabetes or hypertension, obesity, psychogenic diseases, cardiovascular diseases, hepatitis B or C, autoimmune diseases, renal diseases, or hematological diseases. Other exclusion criteria were the existence of active inflammation, iron supplementation therapy, recent blood transfusion (past 3 months), and recent phlebothrombosis (past 6 months). We randomly chose 162 matched healthy subjects who visited the hospital over the same period for physical examination as controls.
All of the chosen 127 patients underwent radical cystectomy (RC) or transurethral resection of bladder tumor (TURBT) for the first time and their urocystic samplings were performed in the Urology Department and evaluated in the Pathology Department of the First Affiliated Hospital of Guangxi Medical University. Moreover, all of the patients were histologically diagnosed with UCB only. Tumor staging was performed in accordance with the 2009 tumor node metastasis (TNM) classification (7th edition) approved by Union for International Cancer Control (UICC). Tumor grade was assigned based on the 2004 World Health Organization system.
2.2. Data collection and definitions
For UCB patients, we collected patient characteristics, pretreatment hematological parameters, and pathological features from their medical records in April 2017. Pretreatment hematological parameters were assessed in fresh EDTA-K2 anticoagulated blood collected between 08:00 and 10:00 within a week before any treatment. For healthy controls, peripheral blood-based parameters were recorded according to their healthy check reports.
All the blood samples were analyzed using COULTER LH780 hematology analyzer (Beckman Coulter, Inc, Kraemer Boulevard Brea, CA), and a well-established quality management system accredited by China National Accreditation Board for Laboratories was run to provide accurate and reliable data. We performed internal quality control and ensured that quality control results passed Westgard Rules before analyzing patient's sample every day. In addition, external quality assessment was implemented in 3 levels: regional, national, and international to measure the laboratory performance. All of the strategies ensured the accuracy of hematology analysis system.
RDW was displayed with coefficient of variation. MLR was defined as absolute monocyte count divided by absolute lymphocyte count, NLR was obtained with absolute neutrophil count divided by absolute lymphocyte count, and absolute platelet count divided by absolute lymphocyte count was recorded as PLR. Comparison of hematological parameters was performed between UCB patients and healthy controls and among patients with different stages and different grades.
Our study was carried out in accordance with the permission of the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University, and the committee approved our application for exemption of informed consent.
2.3. Statistical analysis
SPSS version 20.0 (SSPS Inc, Chicago, IL) and GraphPad Prism 5 (http://www.graphpad.com/scientifc-software/prism/) were used for statistical analysis. With respect to smoking history and gender, the matching of patient group and control group was checked with Pearson chi-squared test. The Mann–Whitney U tests were utilized to evaluate the difference in complete blood count-based parameters and age between 2 groups. The Kruskal–Wallis tests and the Mann–Whitney U tests were applied to appraise the associations of hematological parameters with tumor stages and pretreatment RDW, MLR, NLR, and PLR with clinicopathological characteristics, respectively. The abilities of RDW, MLR, NLR, and PLR to distinguish patients with cancer from healthy subjects were assessed using receiver operating characteristic (ROC) curve analysis. Multivariate logistic regression analysis was performed for the variables identified as statistically significant in univariate analysis. The association between higher levels of NLR and PLR with the demographics and other clinical characteristics of patients was assessed with Pearson chi-squared test or Mann–Whitney U test. Statistical significance was set at P < .05 (2-tailed).
3. Results
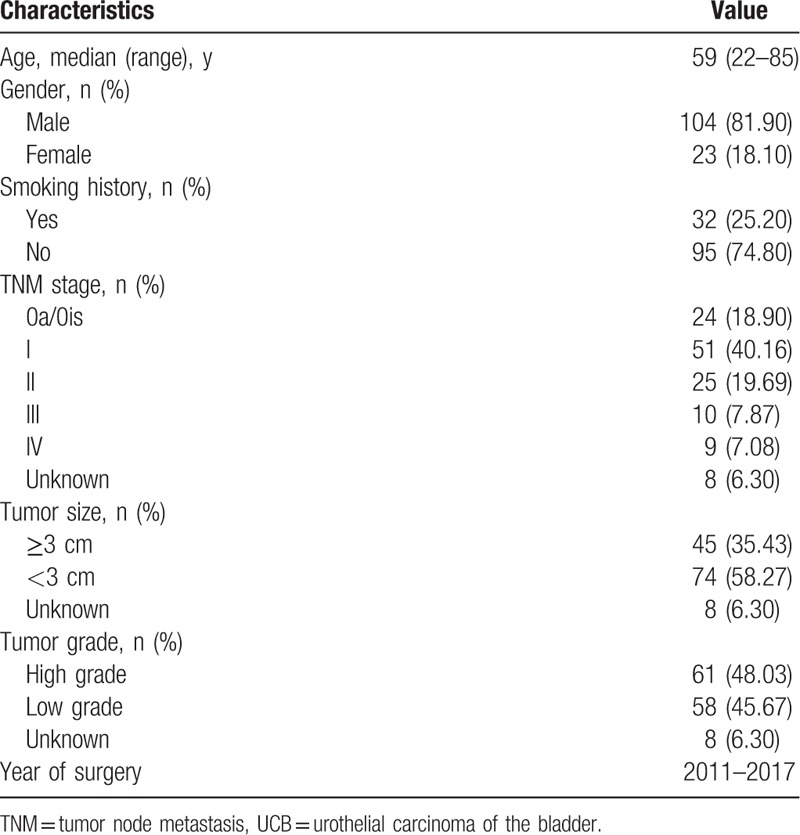
After inclusion and exclusion criteria were applied, 127 patients with median age of 59 years (range, 22–85 years) remained. Of these 127 patients, 24 (18.90%), 51 (40.16%), 25 (19.69%), 10 (7.87%), and 9 (7.08%) patients were diagnosed with UICC stage 0a/0is, I, II, III, and IV, respectively; and 8 (6.30%) patients were excluded when assessing the link between hematological variables and tumor stage, tumor grade, and tumor size due to lack of detailed information on these 3 clinicopathological features (Table 1).
Table 1.
Characteristics of 127 UCB patients.
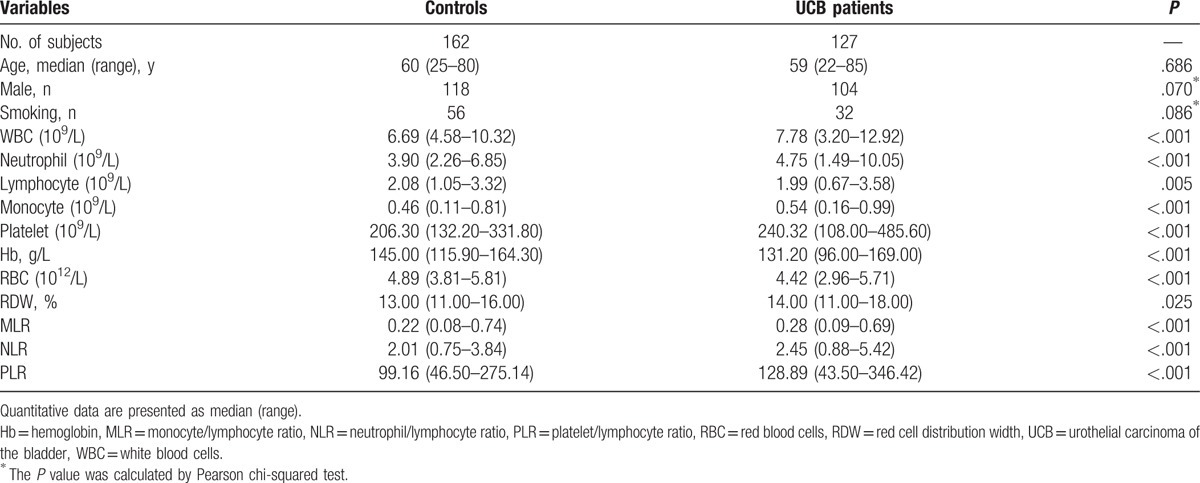
The data in Table 2 display laboratory parameters of healthy controls and patients with cancer. The total number of peripheral blood leukocytes, neutrophil count, monocyte count, and platelet count were higher in UCB patients than in healthy controls. By contrast, patients with cancer had significantly lower hemoglobin (Hb) level and peripheral lymphocyte level than the controls. Compared to healthy controls, the median of pretreatment RDW, MLR, NLR, and PLR was significantly elevated in patients with cancer.
Table 2.
Comparison of demographics and hematological parameters in UCB patients and controls.
The results of Kruskal–Wallis analysis, utilized to assess the links between hematological variables and tumor stages, are presented in Fig. 1. MLR, NLR, and PLR increased significantly with advanced UCB TNM stage. Similarly, level of neutrophil, monocyte, and platelet was associated with advanced UCB TNM stage as well. Differing from above parameters, lymphocyte count decreased slightly across TNM stages and no significant difference was observed. Decreasing Hb level was significantly associated with advanced UCB TNM stage. Changes in RDW across tumor stages were not significant.
Figure 1.
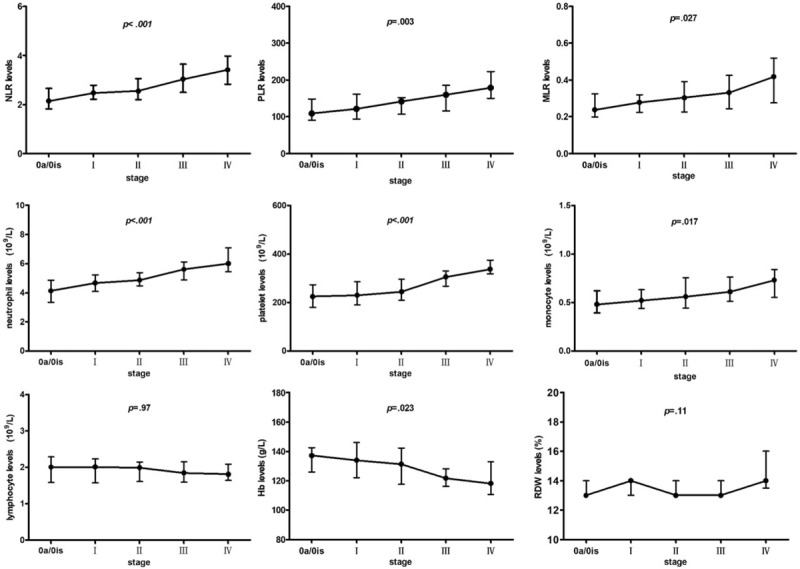
Comparison of hematological parameters across stages in UCB patients. Hb = hemoglobin, MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, PLR = platelet/lymphocyte ratio, RDW = red cell distribution width, UCB = urothelial carcinoma of the bladder.
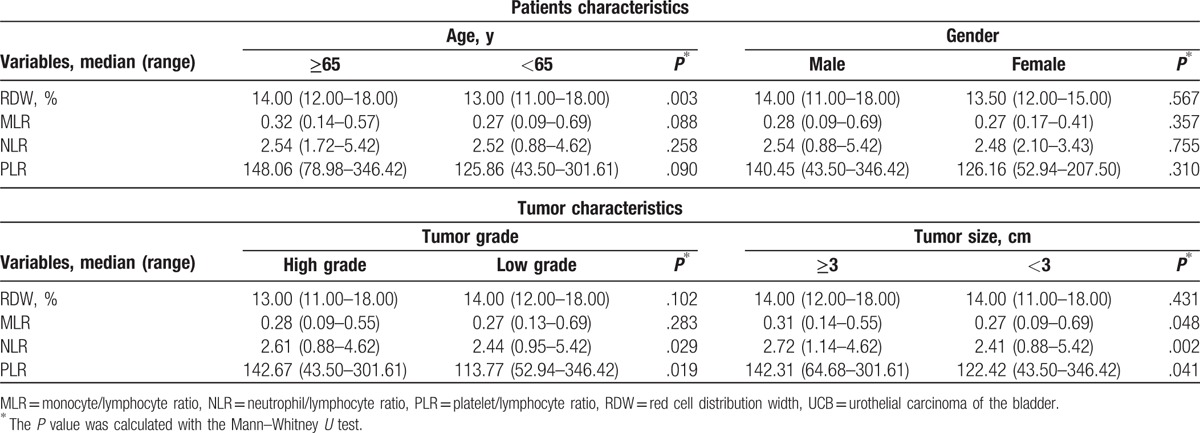
Grouping by age, gender, tumor grade, and tumor size, we used Mann–Whitney U test (Table 3) to detect the correlations between the clinicopathological features with pretreatment RDW, MLR, NLR, and PLR. Our results showed that MLR was associated with tumor size while NLR and PLR were correlated with tumor grade and tumor size. MLR, NLR, and PLR were not correlated with gender and age.
Table 3.
The relationship of RDW, MLR, NLR, and PLR with clinicopathological characteristics in patients with UCB.
Using UCB as a classification variable, ROC curves for the ability of RDW, MLR, NLR, and PLR to predict UCB are showed in Fig. 2. The area under the ROC curve for pretreatment RDW were 0.572 (P = .037, cut-off = 0.135), 0.697 for MLR (P < .001, cut-off = 0.26), 0.725 for NLR (P < .001, cut-off = 2.16), and 0.706 for PLR (P < .001, cut-off = 128.46).
Figure 2.
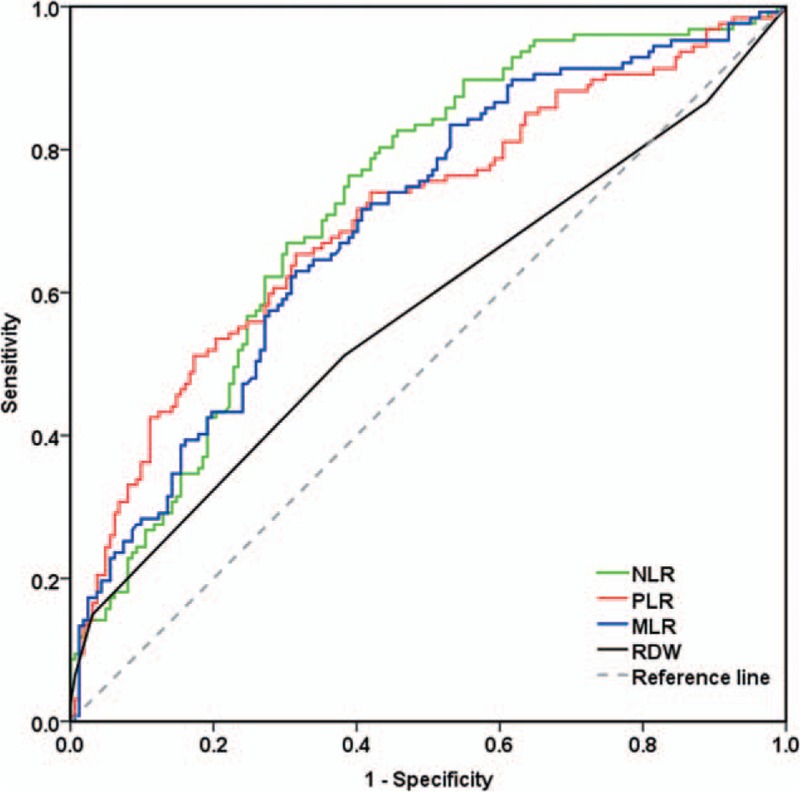
ROC curves for pretreatment RDW, MLR, NLR, and PLR to predict UCB. MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, PLR = platelet/lymphocyte ratio, RDW = red cell distribution width, ROC = receiver operating characteristic, UCB = urothelial carcinoma of the bladder.
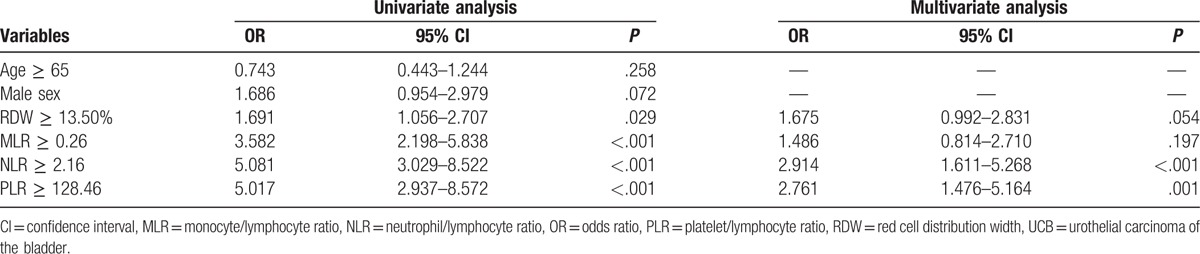
Given that the RDW, MLR, NLR, and PLR were different between the control group and the UCB group, we sought to determine whether these 4 inflammation-based markers could be predictors of UCB. Univariate analysis identified high RDW, MLR, NLR, and PLR levels, which were determined by ROC curve analysis, as significant predictors of UCB while the multivariate logistic regression model identified NLR ≥ 2.16 (odds ratio [OR], 2.914; 95% CI, 1.611–5.268; P < .001) and PLR ≥ 128.46 (OR, 2.761; 95% CI, 1.476–5.164; P = .001) as independent predictors of UCB (Table 4). Patients with NLR ≥ 2.16 were 2.914 times more likely to be diagnosed with UCB than those with NLR < 2.16. Similarly, patients with PLR ≥ 128.46 were 2.761 times more likely to be diagnosed with UCB than those with PLR < 128.46.
Table 4.
Independent predictors of UCB in binary logistic regression analysis.
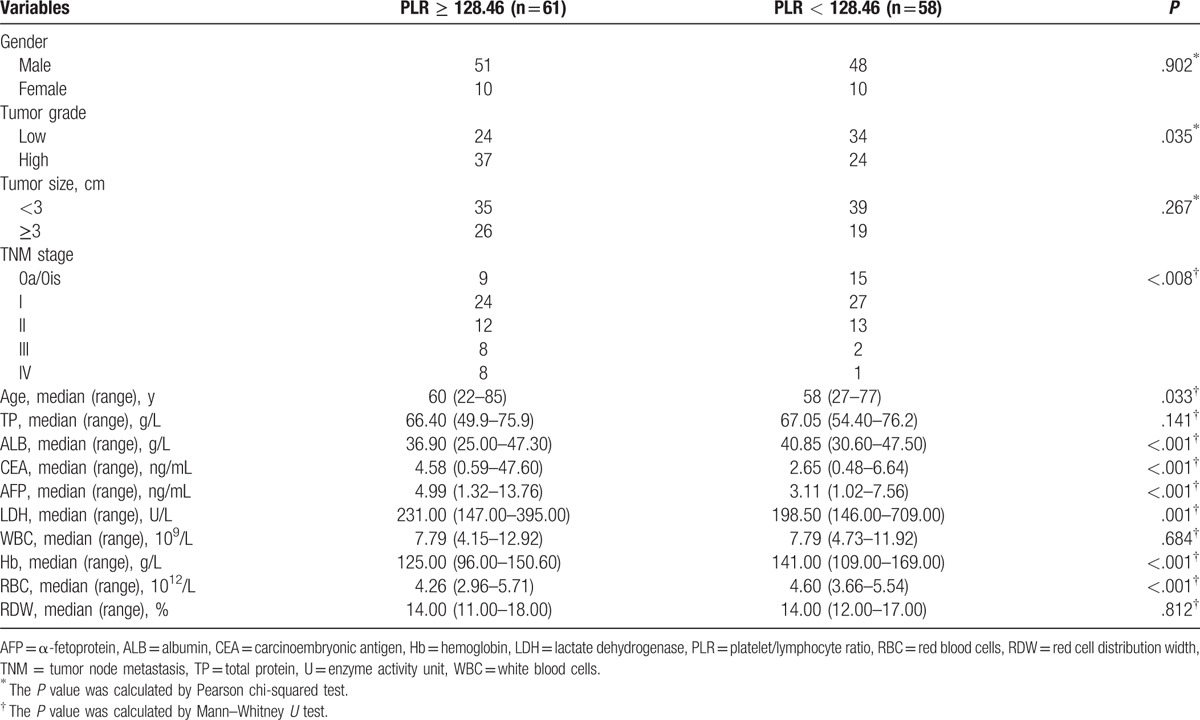
To investigate whether there was a direct evidence link between higher levels of NLR and PLR with the cancer risk and behavior, the association between these 2 parameters and clinicopathological features and other clinical characteristics of patients was assessed. Compared to patients with low NLR, patients with NLR ≥ 2.16 were characterized by significantly increased carcinoembryonic antigen (CEA), α-fetoprotein (AFP), and lactate dehydrogenase (LDH), and significantly decreased serum total protein and albumin (ALB), and Hb (Table 5). High NLR was associated with larger tumor size when compared to patients with lower NLR but no significant association with high tumor grade. NLR ≥ 2.16 was also associated with tumor stage. Patients with increased PLR were characterized by significantly increased levels of CEA, AFP, and LDH and significantly decreased level of ALB, Hb, and red blood cells (RBC) (Table 6). In PLR ≥ 128.46 group, patients with high tumor grade and tumor stage accounted for a larger proportion, but there was no significant difference in regard to tumor size.
Table 5.
Comparison of 119 urothelial carcinoma of the bladder patient's clinicopathological features and baseline clinical data stratified by NLR.
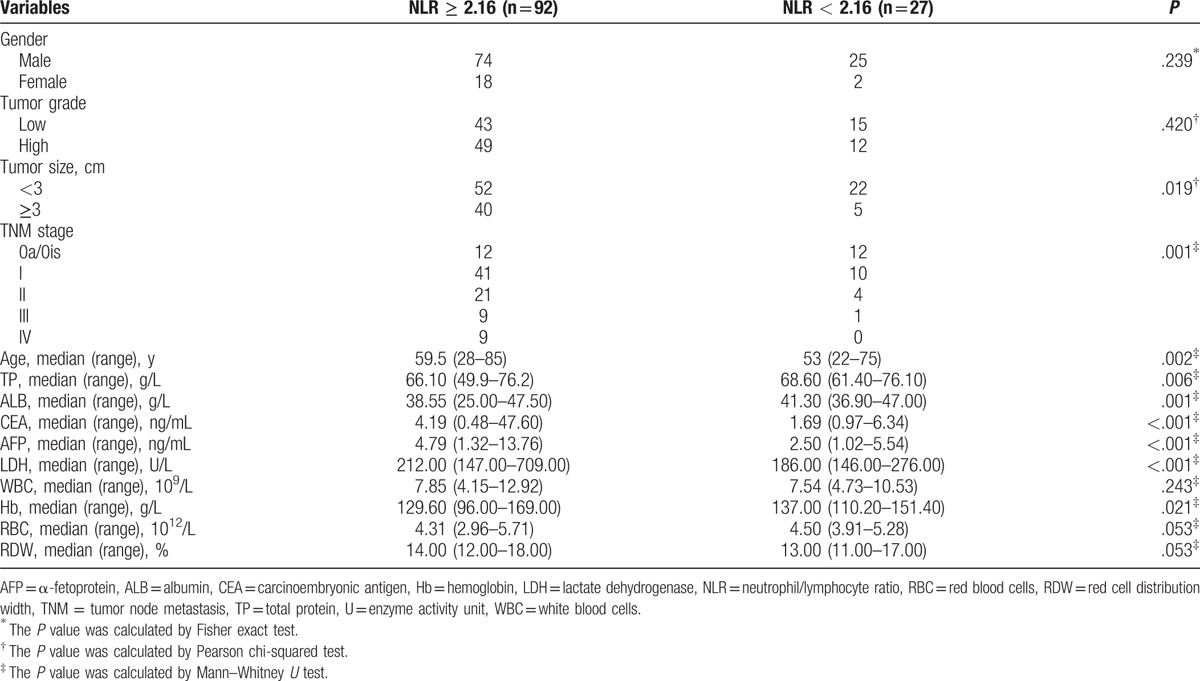
Table 6.
Comparison of 119 urothelial carcinoma of the bladder patient's clinicopathological features and baseline clinical data stratified by PLR.
4. Discussion
Our study assessed the clinical significance of inflammation-related parameters derived from peripheral blood in UCB, including RDW, NLR, PLR, and MLR. Higher pretreatment value of these 4 inflammation-related hematological parameters was detected in patients with UCB compared with healthy subjects. In addition, there were significant increases in MLR, NLR, and PLR from TNM stage 0a/0is to stage IV. Our present case–control data showed that patients with UCB were different from healthy controls in inflammatory condition and patients with different stages had different inflammatory states. From these results, we can infer that an inflammatory response continuously progresses in patients as disease advances. In addition, NLR and PLR were found to be independent predictors of UCB, while RDW and MLR were not. This is the first study to our knowledge to investigate the potential roles of RDW and MLR in patients with UCB.
In the tumor microenvironment, inflammatory cell-producing chemokines and cytokines affect the overall tumor progression by regulating the differentiation, growth, migration, and apoptosis of all cell types, forming a complex interaction between cancer cells and inflammatory cells.[15,16] Therefore, inflammatory pathways have been investigated for cancer treatment.[17,18] In addition, inflammatory reaction to tumor growth leads to changes in relative levels of circulating leukocytes and other inflammation-related markers, making this response measurable.[5,16] Recently, hematological markers of inflammation, such as RDW, NLR, and PLR, are attracting increased interest as they have proven to be potential prognostic factors in certain cancers.[19–21] Consequently, research on the clinical value of these inflammatory response markers is needed in bladder cancer, especially in UCB.
In the present study, we found a significant difference in pretreatment RDW value between patients with UCB and the control group. This result is consistent with previous studies that concluded that RDW value in patients with prostate, breast, or renal carcinoma was higher than in the healthy subjects.[22–24] However, there were no associations between RDW and tumor stage, tumor size, or tumor grade in our study. Likewise, Wang et al[19] did not observe a significant association between RDW and tumor stage. By contrast, some researchers detected a correlation between elevated RDW and advanced tumor stage or higher tumor grade in patients with certain solid tumors.[24,25] With respect to the discrepancies between our observations and those of earlier studies, we suggest 2 possible explanations. First, the function of RDW may vary among types of cancer. Second, differences in study design and patient ethnic background may lead to diversity in the results of the studies. We note that strict inclusion and exclusion criteria were performed to ensure the representativeness of samples. Therefore, we conclude that there is not enough scientific evidence to prove that RDW could be a predictor of UCB.
In our research, the pretreatment MLR in the UCB group significantly increased relative to the control group and it was associated with tumor size and tumor stage. However, it is not an independent predictor for UCB. This observation is in line with the report of Lee et al[26] that lymphocyte/monocyte ratio (LMR), which is 1 divided by MLR, was significantly correlated with invasive bladder cancer stage, whereas LMR was not an independent predictor of muscle invasive bladder cancer (MIBC). Xiang et al[27] compared MLR between 133 ovarian cancer patients and healthy controls and concluded that an elevated MLR reflected the immune condition of patients and was a strong risk factor for advanced ovarian cancer stages and pathologic grades. Previous studies have reported that MLR was associated with survival in patients with malignant tumors, such as gastric cancer[28] and primary pulmonary lymphoepithelioma-like carcinoma.[29] The role of LMR has currently been studied in patients with bladder cancer. Some investigators reported that pretreatment LMR was significantly correlated with survival in bladder cancer patients after RC and could be an independent prognostic factor for UCB patients underwent RC.[11,30] Although MLR has been studied in many solid tumors, its role in cancer has not been unanimously approved. The findings about MLR in UCB patients we observed in our paper still need to be substantiated due to the limitation of the study. Therefore, it is necessary put more efforts in studying the function of MLR in managing UCB.
Our results also showed, via the logistic regression, that an increased NLR or PLR was a predictor for UCB. Both NLR and PLR were associated with tumor stage, tumor size, and tumor grade. In recent years, NLR has been extensively researched as useful prognostic markers in cancers, with unfavorable oncological outcomes, such as more advanced stage and more aggressive tumor behavior, providing prognostic information for these patients.[14,31] Despite inconsistent results, significant diagnostic and prognostic values of NLR have been reported in diverse cancers, including bladder cancer.[13,14,30] In non-muscle invasive bladder cancer (NMIBC) patients, Kang et al[10] revealed that preoperative NLR was a predictor for oncological outcomes. Mano et al[12] retrospectively analyzed 122 consecutive NMIBC patients and reported that NLR was an independent predictor of tumor progression and recurrence. Further, a positive correlation of NLR with tumor invasiveness had also been observed in NMIBC patients, indicating that NLR could be a promising biomarker in bladder cancer.[32,33] In most studies based on MIBC, elevated NLR has also been considered as an independent predictor of survival and pathological outcomes. Hermanns et al[14] evaluated 424 bladder cancer patients undergoing RC, and found that higher NLR ≥ 3.0 was significantly associated with worse recurrence-free survival (hazard ratio [HR] = 1.49), cancer-specific survival (HR = 1.88), and overall survival (average HR = 1.67). Moreover, Viers et al[13] revealed a positive association of pretreatment NLR and advanced tumor stage and increased cancer-specific mortality among patients with UCB undergoing RC. Our results indicated that NLR ≥ 2.16 was associated with decreased Hb and ALB levels, thus revealing that high NLR was an important factor in UCB. In addition, increased NLR was associated with elevated CEA, AFP, and LDH which have been previously reported as tumor and inflammation and immune markers.[34–36] Moreover, patients with NLR ≥ 2.16 tended to be diagnosed with higher TNM stage and tumor size. Thus, the pretreatment high NLR may be an indicator of increased inflammation and immune responses in UCB.
The prognostic value of pretreatment PLR in bladder cancer patients has less been examined than NLR. Bhindi et al[32] and Zhang et al[37] reported that elevated PLR was associated with shorter overall survival in bladder cancer patients undergoing RC. However, after adjusting for confounding factors, preoperative PLR was found not to be an independent predictor of prognosis in both studies. Another study performed by Kang et al[10] drew a similar conclusion that though PLR (≥124) was associated with poor overall survival in NMIBC undergoing TURBT, high PLR was not independently associated with worse overall survival. Consistent with these findings, our study further demonstrated that increased PLR was associated with high levels of CEA, AFP, LDH, and advanced UCB stage and high tumor grade. In addition, patients with high PLR exhibited decreased ALB, Hb, and RBC. These findings, similarly to NLR, equally indicated that PLR may be a predictor of inflammation and immune status in UCB. However, as literature of pretreatment PLR on UCB is relatively insufficient, further validation with large sample sizes is required, and the exact mechanism through which PLR associates with UCB has yet to be elucidated.
The mechanisms underlying our observations are unclear. The occurrence, development, and prognosis of neoplasm involve multiple factors, including inflammation and host immunity response. The link between inflammation and cancer can be explained in 2 pathways: extrinsic pathway and intrinsic pathway. The former is driven by inflammatory states that increase cancer risk while the latter is driven by genetic alterations that induce inflammation and neoplasia.[5] Both extrinsic and intrinsic inflammation can affect the progression of premalignant lesion, which is the key rate-limiting step in cancer development.[38] The immune system–tumor interaction was summarized as cancer immunoediting, a dynamic process comprised of 3 phases: elimination (classical concept of cancer immunosurveillance), equilibrium, and escape.[39] According to the notion of “cancer immunoediting,” the immune system plays dual roles throughout tumor development: the host-protection through eradicating the developing tumor cells and the tumor-sculpture by selecting tumors with reduced immunogenicity.[40] During both processes of tumor-associated inflammation and tumor-related immune response, a polyfactorial network of biochemical signals can be stimulated and maintain the host response to tumor behaviors. This involves initiation and directed migration of leukocytes from blood system to target sites.[38,41] Tumor cells, in turn, can produce various cytokines and chemokines that attract leukocytes, all of which produce an assorted array of damaging cytokines and cytotoxic mediators.[16]
Leukocyte plays crucial role in cancer formation. Macrophages, especially tumor-associated macrophages, which differentiate in part from monocytes, contribute to cancer initiation and promotion: on one hand, the tumor microenvironment educates macrophages; on the other hand, macrophages boost angiogenesis, matrix breakdown, and tumor-cell motility by producing many compounds, ranging from mutagenic oxygen and nitrogen radicals to angiogenic factors.[42] In addition, neutrophils play a significant role in tumor growth and progression via possible mechanisms as follows: First, neutrophils are involved in the process of carcinogenesis through releasing reactive oxygen species and nitric oxide derivatives.[43] Second, neutrophils also express a large set of angiogenic factors capable of modulating tumor angiogenesis, such as vascular endothelial growth factor (VEGF).[44] Furthermore, the cytokines and proteins stored within granules of neutrophils may also function as tumor promoter. For example, neutrophil elastase has also been an independent poor prognostic factor for breast cancer patients.[45] Pretreatment thrombocytosis was an independent predictor of shorter survival, Takahashi et al[46] reported. Mezouar et al[47] held that interactions between cancer and platelet were bidirectional: tumor cells affect platelet physiology through several molecular pathways, including thrombin, thromboxane A2, and ADP; in turn, activated platelets further cancer growth, angiogenesis, metastasis, and cancer-associated thrombosis through releasing numerous mitogenic proteins and growth factors including-but not limited to VEGF and platelet-derived growth factor. Therefore, platelets have been suggested to be a potential target against cancer. Lymphocytes have been considered as one of the most significant components of the host's immunity, and the foundation of elimination phase of immunoediting against tumor cells.[39] CD8+ cytotoxic T lymphocytes, CD4+ T helper (Th)1 cells, and NK cells, together with their characteristic cytokine interferon function as main antitumor effector cells.[40] Activated B cells generate cancer-specific antibody inducing antibody-mediated cancer cell killing and cytokines coordinating other immune cells, particularly cytotoxic T-cells.[48] Hence, lymphocytopenia may result in a weak, insufficient immunological response to a tumor, leading to a poor outcome.[49] It is widely accepted that a defense barrier established by tumor-infiltrating lymphocytes contributes to controlling cancer cell dissemination.[50]
Taken together, the levels of monocyte, neutrophil, and platelet reflect inflammatory states that act as evil in tumor microenvironment of patients with cancer and lymphocyte levels indicate an antitumor response. MLR, NLR, and PLR are parameters that take both inflammatory cells and lymphocytes into account, so their variation in UCB patients reflects a state of inflammatory responses and antitumor immune responses in cancer progression.
There are some limitations in this study. First, selection biases cannot be avoided because of the retrospective and hospital-based characteristics of the study. Second, the sample size in our study is small and the proportion of advanced UCB is low as well, which may lead to incorrect results in evaluating the significance of the parameters. Third, hematological parameters evaluated in this paper are transient and susceptible to many factors and we cannot eliminate all of potential factors that impact these parameters. Finally, RDW, MLR, NLR, and PLR are all based on machine automatic count, which makes it difficult to validate the correctness of our data. Therefore, the studies below are required to confirm our observations: a large-scale prospective study with repetitive measurements data that considers as many as possible confounding factors, a set of validated study like flowcytometry with cell markers compared with machine reading data. Despite these important limitations, our study presents the possible role of NLR and PLR in clinical practice of UCB. This is the first study to convey the role of RDW and MLR in patients with UCB.
5. Conclusions
Our study demonstrated that pretreatment NLR and PLR are strongly associated with advanced tumor stages, pathologic grades, and larger tumor size in patients with UCB and are possibly independent predictors for UCB. NLR and PLR, which are easily calculated, readily accessible and inexpensive, could be simple-to-use predictors of UCB severity and might be used to manage the disease.
Acknowledgments
This study was accomplished with help from the Department of Clinical Laboratory, the First Affiliated Hospital of Guangxi Medical University, China.
Author contributions
Conceptualization: W. Mo, Y. Luo, Z. Yang.
Data curation: L. Qin, X. Shi.
Formal analysis: L. Mo.
Investigation: Y. Luo.
Methodology: Y. Luo, Z. Yang.
Project administration: W. Mo.
Software: W. Li, X. Shi.
Supervision: W. Mo.
Visualization: W. Li.
Writing – original draft: Y. Luo.
Writing – review & editing: L. Mo, L. Qin, X. Li, X. Shi, Y. Luo.
Footnotes
Abbreviations: AFP = α-fetoprotein, ALB = albumin, CEA = carcinoembryonic antigen, Hb = hemoglobin, LDH = lactate dehydrogenase, LMR = lymphocyte/monocyte ratio, MIBC = muscle invasive bladder cancer, MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, NMIBC = non-muscle invasive bladder cancer, OR = odds ratio, PLR = platelet/lymphocyte ratio, RBC = red blood cells, RC = radical cystectomy, RDW = red cell distribution width, ROC = receiver operating characteristic, TNM = tumor node metastasis, TURBT = transurethral resection of bladder tumor, UCB = urothelial carcinoma of the bladder, UICC = Union for International Cancer Control, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Griffiths TR. Current perspectives in bladder cancer management. Int J Clin Pract 2013;67:435–48. [DOI] [PubMed] [Google Scholar]
- [5].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [6].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark 2017;18:19–25. [DOI] [PubMed] [Google Scholar]
- [8].Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 2012;29:3092–100. [DOI] [PubMed] [Google Scholar]
- [9].Osugi J, Muto S, Matsumura Y, et al. Prognostic impact of the high-sensitivity modified Glasgow prognostic score in patients with resectable non-small cell lung cancer. J Cancer Res Ther 2016;12:945–51. [DOI] [PubMed] [Google Scholar]
- [10].Kang M, Jeong CW, Kwak C, et al. Preoperative neutrophil–lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget 2017;8:12891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yoshida T, Kinoshita H, Yoshida K, et al. Prognostic impact of perioperative lymphocyte–monocyte ratio in patients with bladder cancer undergoing radical cystectomy. Tumour Biol 2016;37:10067–74. [DOI] [PubMed] [Google Scholar]
- [12].Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol 2015;33:67e1–7. [DOI] [PubMed] [Google Scholar]
- [13].Viers BR, Boorjian SA, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol 2014;66:1157–64. [DOI] [PubMed] [Google Scholar]
- [14].Hermanns T, Bhindi B, Wei Y, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer 2014;111:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szebeni GJ, Vizler C, Kitajka K, et al. Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm 2017;2017:9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Munn LL. Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med 2017;9:e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Phila) 2016;9:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nesi G, Nobili S, Cai T, et al. Chronic inflammation in urothelial bladder cancer. Virchows Arch 2015;467:623–33. [DOI] [PubMed] [Google Scholar]
- [19].Wang L, Jia J, Lin L, et al. Predictive value of hematological markers of systemic inflammation for managing cervical cancer. Oncotarget 2017;8:44824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kara M, Uysal S, Altinisik U, et al. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol 2017;274:535–42. [DOI] [PubMed] [Google Scholar]
- [21].Pietrzyk L, Plewa Z, Denisow-Pietrzyk M, et al. Diagnostic power of blood parameters as screening markers in gastric cancer patients. Asian Pac J Cancer Prev 2016;17:4433–7. [PubMed] [Google Scholar]
- [22].Albayrak S, Zengin K, Tanik S, et al. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev 2014;15:7781–4. [DOI] [PubMed] [Google Scholar]
- [23].Schairer C, Li Y, Frawley P, et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. J Natl Cancer Inst 2013;105:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang FM, Xu G, Zhang Y, et al. Red cell distribution width is associated with presence, stage, and grade in patients with renal cell carcinoma. Dis Markers 2014;2014:860419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kemal Y, Demirag G, Bas B, et al. The value of red blood cell distribution width in endometrial cancer. Clin Chem Lab Med 2015;53:823–7. [DOI] [PubMed] [Google Scholar]
- [26].Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol 2015;56:749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol 2017;10:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Feng F, Sun L, Zheng G, et al. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget 2017;8:5281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang L, Long W, Li PF, et al. An elevated peripheral blood monocyte-to-lymphocyte ratio predicts poor prognosis in patients with primary pulmonary lymphoepithelioma-like carcinoma. PLoS ONE 2015;10:e0126269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].D’Andrea D, Moschini M, Gust KM, et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol 2017;115:455–61. [DOI] [PubMed] [Google Scholar]
- [31].Faria SS, Fernandes PC, Jr, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bhindi B, Hermanns T, Wei Y, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer 2016;114:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaynar M, Yildirim ME, Badem H, et al. Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumour Biol 2014;35:6601–5. [DOI] [PubMed] [Google Scholar]
- [34].Yuan C, Yang K, Tang H, et al. Diagnostic values of serum tumor markers Cyfra21-1, SCCAg, ferritin, CEA, CA19-9, and AFP in oral/oropharyngeal squamous cell carcinoma. Onco Targets Ther 2016;9:3381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhao S, Mei Y, Wang Y, et al. Levels of CEA, CA153, CA199, CA724 and AFP in nipple discharge of breast cancer patients. Int J Clin Exp Med 2015;8:20837–44. [PMC free article] [PubMed] [Google Scholar]
- [36].Yu S-L, Xu L-T, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep 2017;7:45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol 2015;36:8537–43. [DOI] [PubMed] [Google Scholar]
- [38].Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest 2015;125:3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [40].Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, et al. Immune system: a double-edged sword in cancer. Inflamm Res 2013;62:823–34. [DOI] [PubMed] [Google Scholar]
- [41].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254–65. [DOI] [PubMed] [Google Scholar]
- [43].Treffers LW, Hiemstra IH, Kuijpers TW, et al. Neutrophils in cancer. Immunol Rev 2016;273:312–28. [DOI] [PubMed] [Google Scholar]
- [44].Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013;218:1402–10. [DOI] [PubMed] [Google Scholar]
- [45].Sato T, Takahashi S, Mizumoto T, et al. Neutrophil elastase and cancer. Surg Oncol 2006;15:217–22. [DOI] [PubMed] [Google Scholar]
- [46].Takahashi R, Mabuchi S, Kuroda H, et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial cancer. Int J Gynecol Cancer 2017;27:1399–407. [DOI] [PubMed] [Google Scholar]
- [47].Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res 2016;139:65–76. [DOI] [PubMed] [Google Scholar]
- [48].Tsou P, Katayama H, Ostrin EJ, et al. The emerging role of b cells in tumor immunity. Cancer Res 2016;76:5597–601. [DOI] [PubMed] [Google Scholar]
- [49].Kuss I, Hathaway B, Ferris RL, et al. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 2004;10:3755–62. [DOI] [PubMed] [Google Scholar]
- [50].Leibowitz-Amit R, Israel A, Gal M, et al. Association between the absolute baseline lymphocyte count and response to neoadjuvant platinum-based chemotherapy in muscle-invasive bladder cancer. Clin Oncol (R Coll Radiol) 2016;28:790–6. [DOI] [PubMed] [Google Scholar]


