Supplemental Digital Content is available in the text
Keywords: hemorrhagic glaucoma, iris neovascularization, meta-analysis, neovascular glaucoma, randomized controlled trials
Abstract
Purpose:
Neovascular glaucoma (NVG) is a severe secondary glaucoma with uncontrolled intraocular pressure that leads to serious eye pain and vision loss. Presently, the therapeutic strategies for NVG are diverse, but the therapeutic effects are still not ideal. We performed a network analysis to assess the effect of multiple therapeutic strategies on the treatment of NVG patients.
Methods:
We searched public electronic databases through April 2017 using the following keywords “neovascular glaucoma,” “iris neovascularization,” “hemorrhagic glaucoma,” and “random” without language restrictions. The outcome considered in the present analysis was treatment success rate. A network meta-analysis and multilevel mixed-effects logistic regression were used to compare regimens.
Results:
We included 27 articles assessing a total of 1884 NVG patients in our analysis. According to the network analysis, interferon and mitomycin plus trabeculectomy (94.9%), glaucoma valve implantation (86.9%), and iris photocoagulation plus trabeculectomy (81.9%) were the most likely to improve treatment success rate in NVG patients. The multilevel logistic regression analysis showed that glaucoma valve, bevacizumab, interferon, cyclophotocoagulation, trabeculectomy, iris photocoagulation, ranibizumab, and mitomycin had advantages in terms of improving treatment success rate in NVG patients. However, the application of retinal photocoagulation and vitrectomy reduced patient treatment success rate.
Conclusion:
The regimen including mitomycin, interferon, and trabeculectomy was the most likely to improve the treatment success rate in NVG patients. The application of glaucoma valve and bevacizumab were more beneficial for improving patient treatment success rate as a surgery and as an agent, respectively.
1. Introduction
Glaucoma is a common and difficult ophthalmic disease that is characterized by intermittent or persistently increased intraocular pressure (IOP). Neovascular glaucoma (NVG) is a severe form of secondary glaucoma, which usually occurs secondary to central retinal vein (artery) occlusion, diabetic retinopathy, and retinal periphlebitis. Among these pathologies, central retinal vein occlusion and diabetic retinopathy account for nearly 70% of cases. NVG is a substantial threat to the eye, causing uncontrolled IOP, which leads to severe eye pain and vision loss.
NVG involves the proliferation of fibrovascular tissue in the anterior chamber angle, which is commonly caused by retinal hypoxia leading to insufficient oxygen supply to retinal cells and the release of vascular endothelial growth factor (VEGF).[1] The imbalance between VEGF and antiangiogenic factors, such as pigment epithelium-derived growth factor (PEDF), occurs when VEGF increases. High levels of VEGF promote the activation, migration, and proliferation of endothelial cells, leading to neovascularization of the anterior segment, fibrous membrane formation, peripheral anterior synechia, and progressive angle closure.[2,3] Therefore, as a type of refractory glaucoma, NVG has several characteristics. Neovascularization can cause extensive anterior synechia and can destroy the normal anatomical structure, thereby increasing surgical difficulty. Due to neovascularization, bleeding and fibrin exudation can occur during NVG operations. The neovascular membrane will grow, ultimately blocking the drainage channel and causing recurrent adhesion atresia.
Presently, NVG therapeutic strategies are diverse, but the therapeutic effects are still not ideal, and the application of a general antiglaucoma drug is inappropriate for this disease.[4] In a previous meta-analysis, the effect of anti-VEGF drugs, especially bevacizumab, has been analyzed for use in NVG treatment. A comprehensive analysis of case reports and series reports showed that the effective rate of bevacizumab-related treatment is 68.7%, and the recurrence rate is 18.6% at 4.2 months of follow-up.[5] A later review could not evaluate the efficacy of anti-VEGF drugs because of the lack of randomized controlled trials (RCTs).[6] Two meta-analyses reported the effect of intravitreal bevacizumab injection before Ahmed glaucoma valve (AGV) implantation. The results indicated no significant difference in IOP reduction with bevacizumab application, but the surgical success rate was found to be higher after bevacizumab application and fewer side effects, such as hyphema, occurred.[7,8]
Previous systematic reviews only analyzed the effect of anti-VEGF drugs. However, the types of surgery as well as the combined effects of surgery and drugs also play a very important role in NVG treatment. Currently, there are many types of surgery in clinical application, and the combination of different operations and drugs further increases the diversity of treatment strategies. Therefore, traditional meta-analyses cannot fully reflect the effect of different therapeutic strategies for NVG treatment. In this study, we comprehensively analyzed different therapeutic strategies for NVG by network meta-analysis and aimed to determine the best strategy through direct and indirect comparisons.
2. Methods
2.1. Search strategy and selection criteria
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA). Our study was performed on the basis of previous studies; therefore, ethical approval and informed consent were not required. For this network analysis, we searched PubMed, Embase, the Cochrane Central Register of Controlled Trials, and Chinese databases, including the China National Knowledge Infrastructure, the China Science Periodical Database (the Wanfang Database), the VIP journal integration platform, and China Biology Medicine database RCTs published from the date of database inception to April 2017 using the following keywords: neovascular glaucoma, iris neovascularization, hemorrhagic glaucoma, and random∗. We put no restrictions on language. The bibliographies of the obtained publications and relevant reviews were also assessed to ensure that no relevant studies were inadvertently omitted.
Publications were included in the present study when they met the following criteria: prospective RCT design; patients with a clinical diagnosis of NVG; controlled study of different therapeutic strategies related to different surgeries and (or) drugs; outcome assessments that included treatment success rate based on the number of patients who achieve normal IOP during the follow-up period. The exclusion criteria included the following: nonprospective RCTs; unknown or other types of glaucoma patients; several surgery types in a group without randomization; comparative studies of similar surgical procedures, such as trabeculectomy versus modified trabeculectomy; drug dose-related study; studies where the results were unclear or inconsistent with the evaluation criteria; traditional Chinese medicine-related studies, which were excluded due to the unclear compositions of the treatments. In addition, since most included studies did not limit the use of antibiotics, steroids after surgery, and IOP-lowering agents during follow-up, controlled studies of these 3 types of drug were also excluded. Due to issues of unreliability, conference reports and dissertations including nonpeer-reviewed studies were also excluded.
2.2. Data extraction and quality assessment
Two investigators independently extracted the following information from each eligible study: name of the first author, publication year, sample size, number of eyes, stages of NVG, intervention treatment, control treatment, and follow-up. We evaluated the rate of treatment success during the follow-up period. The success rate criterion differed slightly due to the use of different reference standards. The main evaluation criterion was the return of IOP to the normal level, and the measurement range included 6 to 21 mm Hg, 7 to 22 mm Hg, and 10 to 21 mm Hg. The IOPs of glaucoma patients were generally higher than normal; thus, criteria that included measurement outcomes less than 21 or 22 mm Hg or IOP reductions of more than 30% were also accepted in our analysis. Additionally, IOP lowering agents were not restricted during the follow-up period. We assessed the methodological quality of the included trials using the Cochrane Collaboration tool. Studies were graded as having a “low risk,” “unclear risk,” or “high risk” of bias across the 7 specified domains.[9]
2.3. Statistical analysis
We conducted a random-effects network meta-analysis, which used a frequentist framework, with STATA (Version 14.0).[10] Inconsistency between direct and indirect sources of evidence was statistically assessed both globally (by comparing the fit and the parsimony of consistency and inconsistency models) and locally (by calculating the difference between direct and indirect estimates in all closed loops in the network). We estimated the ranking probabilities for all treatment regimens of being at each possible rank for each intervention. The treatment hierarchy was summarized, and the results are reported as surface under the cumulative ranking curve (SUCRA). We also plotted a comparison-adjusted funnel plot for the network meta-analysis to detect the presence of any dominant publication bias in our network meta-analysis. For multiple therapeutic regimens, we attempted to use a multilevel mixed-effects logistic regression model for each type of surgery and drug, which is an expansion of the logistic regression.[11] The ingredients of different therapeutic strategies were considered as fixed effects, and those of different studies were considered random effects. All tests were 2-tailed, and P values of less than .05 were considered statistically significant.
3. Results
Overall, 393 citations were identified from English databases, and 682 citations were identified from Chinese databases after duplicates were removed. A total of 1009 articles were excluded after the titles and abstracts were screened. The full texts of the remaining 66 articles were assessed, and studies were removed due to the following issues: no desired outcomes (22); included other types of patients (5); not prospective RCTs (4); unclear types of surgery in the group (3); undesired agents related to controlled studies (2); comparison of similar operations (1); dose-related research (1); and duplicate publications (1). Finally, 27 RCTs assessing a total of 1884 NVG patients were included in our analysis[12–38] (Table 1).
Table 1.
Characteristics of subjects in eligible studies.
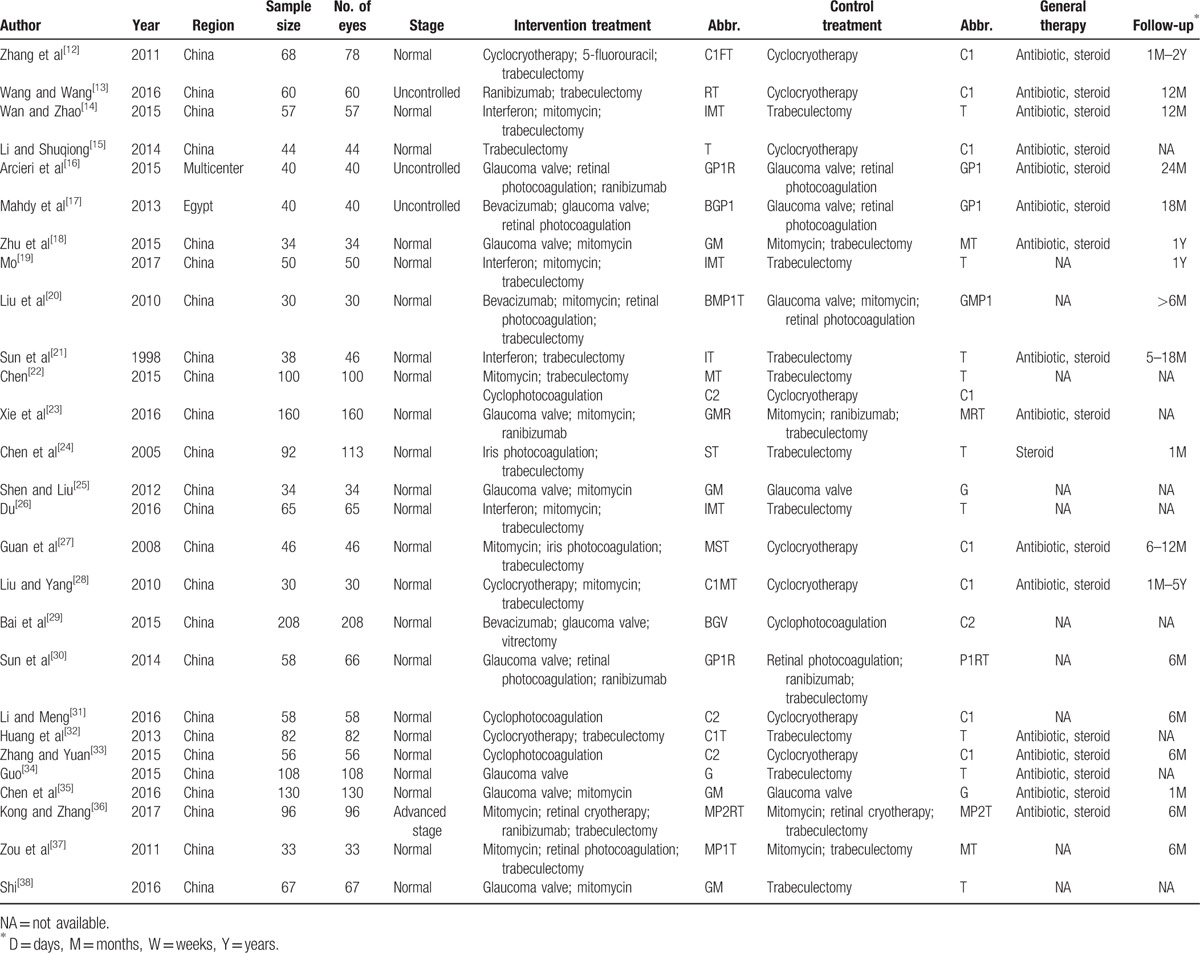
The included studies were published between 1998 and 2017. The type of NVG patients was not a special definition in most included studies. Three studies indicated uncontrolled NVG patients, and 1 study indicated advanced stage NVG patients.[13,16,17] In our study, the types of surgical treatments included cyclocryotherapy (C1), cyclophotocoagulation (C2), glaucoma valve implantation (G), retinal photocoagulation (P1), retinal cryotherapy (P2), iris photocoagulation (S), trabeculectomy (T), and vitrectomy (V). The types of agents used included bevacizumab (B), 5-fluorouracil (F), interferon (I), mitomycin (M), and ranibizumab (R). Antibiotics and steroids, such as tobramycin and dexamethasone, were generally used after surgery. The follow-up period was 1 month to 5 years; however, several studies did not specify the length of follow-up (Table 1). All included studies had a prospective RCT design, and most randomizations were not rigorous. However, the assessed outcomes were relatively objective; thus, the overall quality of the included studies was not ideal but was acceptable (Supplementary Fig. 1).
For the network meta-analysis of success rate outcomes, we analyzed 16 therapeutic regimens. Nine strategies were directly compared with trabeculectomy (T), and 7 strategies were directly compared with cyclocryotherapy (C1). In this analysis, the nodes were weighted according to the number of studies evaluated for each treatment, and the edges were weighted according to the precision of the direct estimate for each pairwise comparison. Therefore, trabeculectomy (T) was the most frequently investigated intervention, and the result of comparison between bevacizumab plus glaucoma valve and vitrectomy (BGV) versus cyclophotocoagulation (C2) was mostly precise in this network analysis (Fig. 1). An inconsistency plot was produced to assume the loop-specific heterogeneity estimate, and the exp(IF) of the glaucoma valve plus mitomycin (GM)—trabeculectomy plus mitomycin (MT)—trabeculectomy (T) loop was significant larger than zero (IF = 3.76; 95% CI, 0.88–6.65) (Supplementary Fig. 2). In addition, a global inconsistency analysis showed significant inconsistency among the studies (P = .0064). These inconsistencies may have resulted from differences in the criteria defining therapeutic success. We therefore used an inconsistency model to research pairwise comparisons. The results of the network meta-analysis are presented as a league table of all possible pairwise comparisons estimated in the network meta-analysis (Table 2). Furthermore, we ranked the comparative effects of all regimens; mitomycin and interferon plus trabeculectomy (IMT) (94.9%) were the most likely to improve success rate, followed by glaucoma valve (G) (86.9%) and iris photocoagulation plus trabeculectomy (ST) (81.9%) (Fig. 2). Other SUCRA of the regimens are shown in Table 2. Additionally, the comparison-adjusted funnel plot used to assess publication bias and determine the presence of small-study effects did not suggest the presence of any publication bias (Fig. 3).
Figure 1.
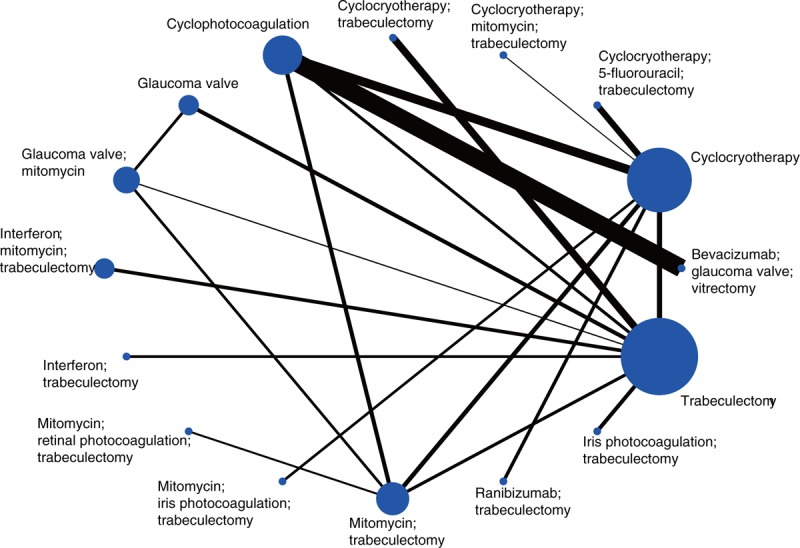
Network of comparisons for treatment success rate in the analysis.
Table 2.
The league table of the inconsistency model network meta-analysis for the treatment success rate estimates therapeutic strategies according to their relative effects.
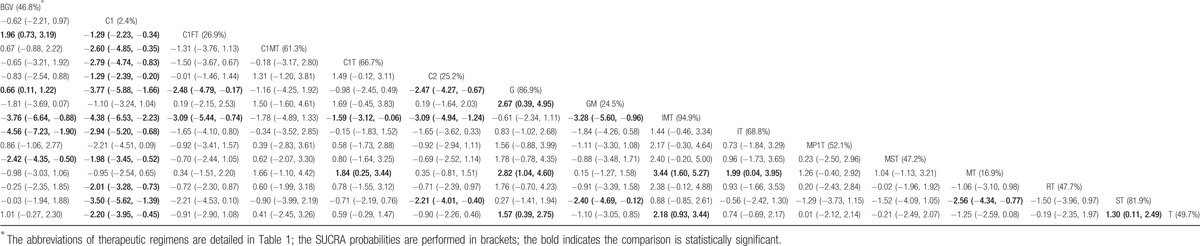
Figure 2.
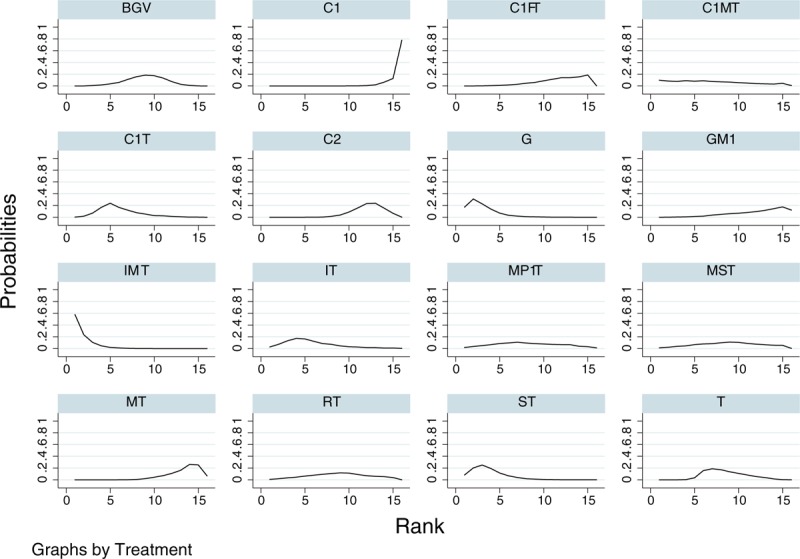
Cumulative ranking plots based on the estimated SUCRA probabilities for treatment success rate. The abbreviations for each therapeutic strategy are described in Table 1.
Figure 3.
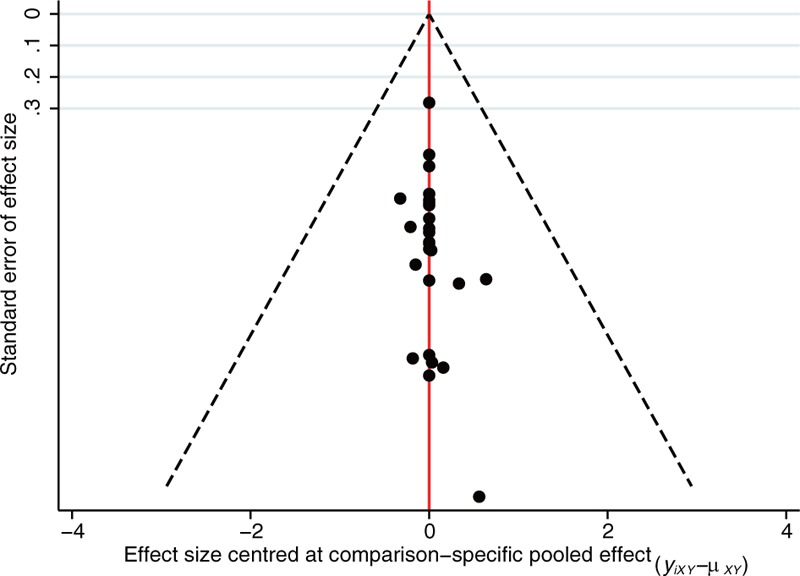
Comparison-adjusted funnel plot for assessing the results.
In addition, 10 regimens were not included in the network meta-analysis, reflecting a disconnection, and a traditional meta-analysis showed that bevacizumab plus glaucoma valve and retinal photocoagulation (BGP1) are superior to glaucoma valve plus retinal photocoagulation (GP1) (OR, 19.00; 95% CI, 2.12–170.39; P = .009); ranibizumab plus glaucoma valve and retinal photocoagulation (GP1R) are superior to ranibizumab plus retinal photocoagulation and trabeculectomy (P1RT) (OR, 4.13; 95% CI, 1.27–13.37; P = .018); and mitomycin and ranibizumab plus retinal cryotherapy and trabeculectomy (MP2RT) are superior to mitomycin plus retinal cryotherapy and trabeculectomy (MP2T) (OR, 4.91; 95% CI, 1.29–18.80; P = .02) (Fig. 4). However, the results of the above traditional meta-analysis had a large standard error with low robustness.
Figure 4.
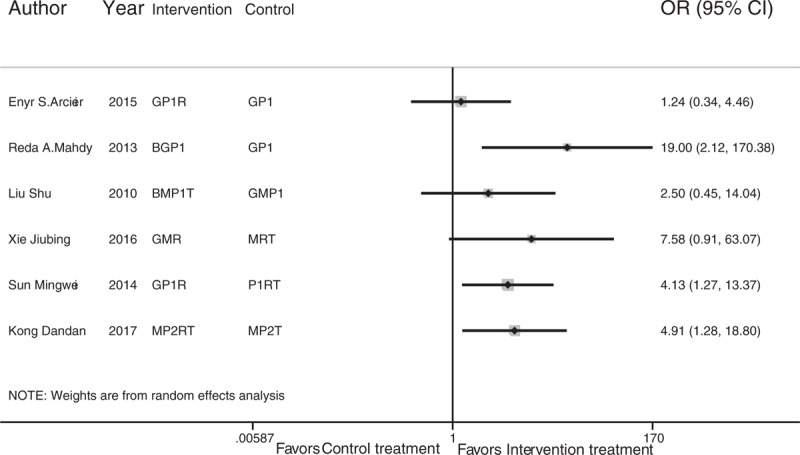
Traditional meta-analysis of treatment success rate among regimens that were not entered into the network meta-analysis. The abbreviations for each therapeutic strategy are described in Table 1.
For the multilevel mixed-effect logistic regression analysis, the results showed that glaucoma valve (OR, 9.90; 95% CI, 3.66–26.79; P < .001), bevacizumab (OR, 7.93; 95% CI, 2.31–27.30; P = .001), interferon (OR, 4.01; 95% CI, 1.64–9.80; P = .002), cyclophotocoagulation (OR, 3.64; 95% CI, 1.39–9.87; P = .011), trabeculectomy (OR, 3.41; 95% CI, 1.43–8.16; P = .006), iris photocoagulation (OR, 3.12; 95% CI, 1.26–7.67; P = .013), ranibizumab (OR, 2.61; 95% CI, 1.46–4.67; P = .001), and mitomycin (OR, 1.75; 95% CI, 1.09–2.81; P = .02) yielded a higher treatment success rate for NVG patients. Retinal photocoagulation (OR, 0.30; 95% CI, 0.15–0.61; P = .001) and vitrectomy (OR, 0.08; 95% CI, 0.02–0.37; P = .001) reduced the patient treatment success rate (Fig. 5).
Figure 5.
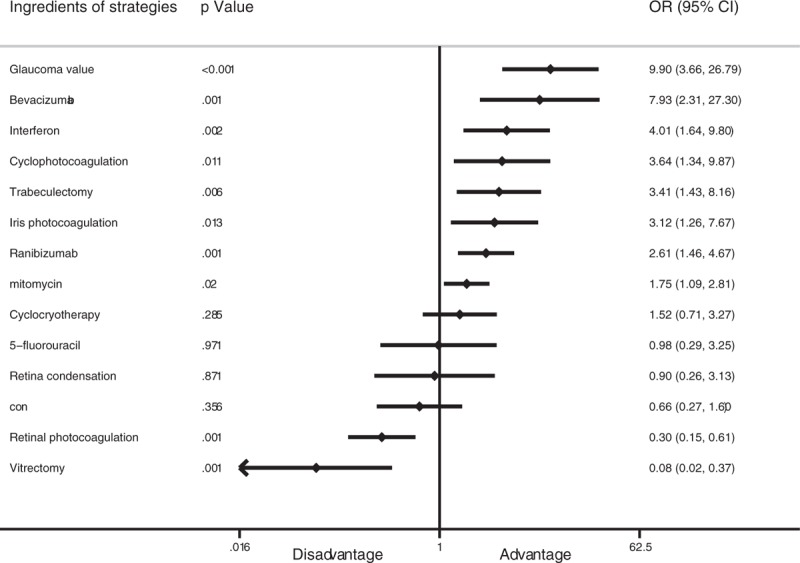
Forest plot of contributions of the different therapeutic strategies to treatment success rate based on the multilevel mixed-effects logistic regression.
4. Discussion
In the present study, we comprehensively analyzed several therapeutic strategies for NVG patients. We considered all regimens applied in the treatment process except antibiotics, steroids, and IOP-lowering agents. A network meta-analysis and a multilevel mixed-effect logistic regression were used to analyze the regimens and the ingredients of the regimens, respectively. Using the network analysis, interferon and mitomycin plus trabeculectomy (94.9%), glaucoma valve implantation (86.9%), and iris photocoagulation plus trabeculectomy (81.9%) were found to be the most likely to improve treatment success rate in NVG patients based on an inconsistency model. Ten regimens were not included in the network analysis, and the results from a traditional meta-analysis exhibited a large standard error and a lack of robustness. Multilevel logistic regression analysis showed that glaucoma valve, bevacizumab, interferon, cyclophotocoagulation, trabeculectomy, iris photocoagulation, ranibizumab, and mitomycin had advantages in improving patient treatment success rate. However, the application of retinal photocoagulation and vitrectomy reduced patient treatment success rate.
This study is the first to comprehensively analyze different NVG therapeutic strategies using network analysis. Compared with traditional meta-analysis, our analysis had more complete and abundant results. During the analysis, we found both global and local inconsistency; therefore, our main results were based on an inconsistency model. Local inconsistency analysis revealed that the main source was the glaucoma valve implantation plus mitomycin—trabeculectomy plus mitomycin—trabeculectomy loop. In addition, the difference between direct and indirect comparisons indicated glaucoma valve implantation plus mitomycin versus trabeculectomy plus mitomycin (Coef, 4.33; 95% CI, 2.00–6.65; P < .001) and trabeculectomy plus mitomycin versus trabeculectomy (Coef, 3.28; 95% CI, 0.80–5.77; P = .01). The inconsistency might be caused by the small sample size and the large standard error. Furthermore, slight differences between outcome criteria and nonblindness study design might have biased the results. We also used a consistency model and a Bayesian hierarchical model to analyze the results, which indicated that interferon and mitomycin plus trabeculectomy, glaucoma valve implantation plus mitomycin, and iris photocoagulation plus trabeculectomy were the most likely to improve treatment success rate in NVG patients. The above results showed that the effects of interferon and mitomycin plus trabeculectomy and iris photocoagulation plus trabeculectomy are robust, and glaucoma valve implantation plus mitomycin and glaucoma valve implantation remain controversial. Thus, further studies are still needed, particularly well-designed RCTs. Moreover, detailed descriptions of NVG stage and standardized surgical process are necessary to further reduce differences among studies.
The combination of interferon and mitomycin plus trabeculectomy in the treatment of NVG patients yielded a higher success rate. Three studies have described the clinical application of that strategy compared with trabeculectomy.[14,19,26] The operation process was conventional trabeculectomy with the removal of trabecular tissue and the surrounding iris. Sterile cotton containing 0.4 mg/mL mitomycin was used to cover the scleral flap bed and surface, which were then washed with saline. Interferon was injected into the conjunctiva near the filtering bleb from immediately during the operation to 14 days after the operation. In these 3 included studies, the regimen achieved an approximately 96% treatment success rate, and the mean IOP measured during follow-up ranged from 16.32 to 17.1 mm Hg. Therefore, this strategy is worth testing in the future with well-designed RCTs. The results of glaucoma valve implantation and glaucoma valve implantation plus mitomycin varied between the inconsistency and consistency models. These 2 regimens were carried out to reduce IOP with a glaucoma drainage device with or without mitomycin-containing cotton. In a direct comparison, the procedure with the mitomycin-containing cotton was better than the procedure without (OR, 6.66; 95% CI, 1.67–26.61; P = .007). Iris photocoagulation plus trabeculectomy also had an ideal treatment success rate. Laser photocoagulation was performed on the iris to inhibit neovascularization and to create microvessel occlusion and coagulation before trabeculectomy. Photocoagulation prevented hyphema during the perioperative period and created favorable conditions for the operation. However, this result depended on a single RCT, reducing the robustness of this result.
In the logistic regression analysis, several surgeries and drugs had a significant impact on the treatment success rate. Glaucoma valve implantation and bevacizumab were the best surgery and drug with the highest ORs, respectively. VEGF played a key role in the process of angiogenesis in NVG and was expressed in retinal inner nuclear layer cells and spread to the vitreous and anterior chamber angle.[39] Bevacizumab is a humanized antibody that blocks neovascularization by inhibiting VEGF. Bevacizumab combined with surgical treatment improved the treatment success rate in terms of short-term and long-term effects and in preventing postoperative recurrence. In our analysis, there were 2 types of glaucoma valve implantation. The classic AGV is a one-way pressure sensitive valve that is widely used in the clinic. Restriction of the AGV could prevent excessive drainage of the aqueous humor and significantly reduce postoperative complications. Another type of valve is the Ex-PRESS glaucoma valve, which channels aqueous humor through a fluid dynamic structure lumen to a half-thickness scleral flap to create a subconjunctival drainage device.[40] Due to their similar principles, these valves and other unspecified valves were classified as glaucoma valves in our analysis. In the network analysis, only regimens involving glaucoma valve, glaucoma valve plus mitomycin, and glaucoma valve plus bevacizumab and vitrectomy were included, resulting in the neglect of other glaucoma valve-related studies. In a traditional meta-analysis, glaucoma valve was better than trabeculectomy when combined with ranibizumab and retinal photocoagulation, according to a single study.
In conclusion, a regimen including mitomycin, interferon, and trabeculectomy was the most likely to improve the treatment success rate in NVG patients. The application of a glaucoma valve and bevacizumab were most beneficial for improving patient treatment success rate in terms of surgery and agent, respectively.
4.1. Limitations
Our study had several limitations. First, the results of the network meta-analysis contained global and local inconsistencies that might have affected accuracy. The inconsistencies might be caused by the small sample size, the large standard error, and differences in the criteria defining therapeutic success. Second, in the results of the traditional meta-analysis, large standard errors rendered the results imprecise and poorly robust. Third, we did not perform the Grading of Recommendations Assessment and Development and Evaluation analysis because the included studies did not include design blindness.
Author contributions
Conceptualization: Z. Dong.
Data curation: J. Gong, Z. Dong.
Formal analysis: J. Gong.
Investigation: J. Gong, R. Liao.
Methodology: J. Gong, R. Liao.
Project administration: R. Liao.
Resources: R. Liao.
Software: S. Xu.
Visualization: S. Xu.
Writing – original draft: Z. Dong.
Writing – review & editing: S. Xu.
Supplementary Material
Footnotes
Abbreviations: AGV = Ahmed glaucoma valve, CI = confidence interval, IF = inconsistency factor, IOP = intraocular pressure, NVG = neovascular glaucoma, OR = odds ratio, PEDF = pigment epithelium-derived growth factor, PRISMA = Preferred Reporting Items for Systematic Reviews, RCTs = randomized controlled trials, SUCRA = surface under the cumulative ranking curve, VEGF = vascular endothelial growth factor.
Treatment-related abbreviations are listed in Table 1.
Funding: This research was supported by the Doctoral Program of the First Hospital of Anhui Medical University.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Nadal J, Carreras E, Kudsieh B, et al. Neovascular glaucoma treatment with extraction of anterior chamber fibrovascular tissue. JAMA Ophthalmol 2013;131:1083–5. [DOI] [PubMed] [Google Scholar]
- [2].Anchala AR, Pasquale LR. Neovascular glaucoma: a historical perspective on modulating angiogenesis. Semin Ophthalmol 2009;24:106–12. [DOI] [PubMed] [Google Scholar]
- [3].Kim M, Lee C, Payne R, et al. Angiogenesis in glaucoma filtration surgery and neovascular glaucoma: a review. Surv Ophthalmol 2015;60:524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol 2013;28:165–72. [DOI] [PubMed] [Google Scholar]
- [5].Martinez-Carpio PA, Bonafonte-Marquez E, Heredia-Garcia CD, et al. [Efficacy and safety of intravitreal injection of bevacizumab in the treatment of neovascular glaucoma: systematic review]. Arch Soc Esp Oftalmol 2008;83:579–88. [DOI] [PubMed] [Google Scholar]
- [6].Simha A, Braganza A, Abraham L, et al. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev 2013;10:CD007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou M, Xu X, Zhang X, et al. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: a systematic review and meta-analysis. J Glaucoma 2016;25:551–7. [DOI] [PubMed] [Google Scholar]
- [8].Hwang HB, Han JW, Yim HB, et al. Beneficial effects of adjuvant intravitreal bevacizumab injection on outcomes of Ahmed glaucoma valve implantation in patients with neovascular glaucoma: systematic literature review. J Ocul Pharmacol Ther 2015;31:198–203. [DOI] [PubMed] [Google Scholar]
- [9].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greco T, Edefonti V, Biondi-Zoccai G, et al. A multilevel approach to network meta-analysis within a frequentist framework. Contemp Clin Trials 2015;42:51–9. [DOI] [PubMed] [Google Scholar]
- [11].Vermunt JK. Mixed-effects logistic regression models for indirectly observed discrete outcome variables. Multivariate Behav Res 2005;40:281–301. [DOI] [PubMed] [Google Scholar]
- [12].Zhang X, Li Y, Liu Z. Clinical observation of ciliary body cryotherapy, trabeculectomy and 5-FU in treatment of neovascular glaucoma. Int Eye Sci 2011;11:162–3. [In Chinese]. [Google Scholar]
- [13].Wang F, Wang L. Safety of anti-VEGF drugs with trabeculectomy for neovascular glaucoma. Int Eye Sci 2016;16:837–40. [In Chinese]. [Google Scholar]
- [14].Wan D, Zhao Q. Clinical observation of trabeculectomy with mitomycin and interferon therapy on neovascular glaucoma. Int Eye Sci 2015;15:146–8. [In Chinese]. [Google Scholar]
- [15].Li X, Hu S. Therapeutic effect of modified trabeculectomy in treatment of neovascular glaucoma. Int Eye Sci 2014;14:745–6. [In Chinese]. [Google Scholar]
- [16].Arcieri ES, Paula JS, Jorge R, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2-year follow-up. Acta Ophthalmol 2015;93:e1–6. [DOI] [PubMed] [Google Scholar]
- [17].Mahdy RA, Nada WM, Fawzy KM, et al. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. J Glaucoma 2013;22:768–72. [DOI] [PubMed] [Google Scholar]
- [18].Zhu Y, Li J, Xu S. Therapeutic effects of Ex-PRESS glaucoma filtration device implantation in neovascular glaucoma. Int J Ophthalmol 2015;15:534–6. [In Chinese]. [Google Scholar]
- [19].Mo J. Trabeculectomy combined mitomycin and interferon in the treatment of neovascular glaucoma. Jilin Med J 2017;38:367–8. [In Chinese]. [Google Scholar]
- [20].Liu S, Ma N, Wang Shouyan, et al. Clinical analysis of intravitreal injection of Bevacizumab combined with panretinal photocoagulation and compound trabeculectomy for neovascular glaucoma. Recent Adv Ophthalmol 2010;30:1054–9. [In Chinese]. [Google Scholar]
- [21].Sun J, Wang Q, Ma Y, et al. The effect of Alpha Interferon in neovascular glaucoma filtration surgery. Chin J Ophthalmol Otolaryngol 1998;3:29–30. 36. [In Chinese]. [Google Scholar]
- [22].Chen S. Clinical effect observation of different treatments for neovascular glaucoma. Pract Clin J Integr Tradit Chin West Med 2015;15:69–70. [In Chinese]. [Google Scholar]
- [23].Xie J, Kou Y, Liu R, et al. EX-Press implantation combined with intravitreal injection of ranibizumab for neovascular glaucoma. Recent Adv Ophthalmol 2016;36:61–4. [In Chinese]. [Google Scholar]
- [24].Chen B, Cheng W, He Y. Application of argon laser photocoagulation on iris surface new blood vessels in the surgical treatment of neovascular glaucoma. Mod J Integr Tradit Chin West Med 2005;14:316–7. [In Chinese]. [Google Scholar]
- [25].Shen J, Liu X. The curative effect of Ahmed glaucoma valve combined MMC in neovascular glaucoma treatment. Guide China Med 2012;10:263–4. [In Chinese]. [Google Scholar]
- [26].Du H. Clinical effect of trabeculectomy combined with mitomycin and interferon therapy on neovascular glaucoma. Shenzhen J Integr Tradit Chin West Med 2016;26:9–11. [In Chinese]. [Google Scholar]
- [27].Guan T, Zhang L, Wu H. Curative effect of laser coagulation of iris neovascularization combined with complex trabeculectomy for ueovascular glaucoma. Chin J Ocular Trauma Occup Eye Dis 2008;30:23–5. [In Chinese]. [Google Scholar]
- [28].Liu Z, Yang P. Combination treatment with cyclocryotherapy, trabeculectomy and mitomycin C for neovascular glaucoma. Chin J Ocular Trauma Occup Eye Dis 2010;32:837–8. [In Chinese]. [Google Scholar]
- [29].Bai Y, Li J, Tian L, et al. Comparison of the effects of different surgical protocols on the levels of IL-6, PEDF and VEGF in serum and aqueous humor of patients with neovascular glaucoma. Chin J Clin Rational Drug Use 2015;8:161–2. [In Chinese]. [Google Scholar]
- [30].Sun M, Liu X, Zhao C, et al. Observation of the effect of Ex-PRESS glaucoma drainage device implantation in the treatment of neovascular glaucoma. China J Ctrl Endem Dis 2014;29:245–6. [In Chinese]. [Google Scholar]
- [31].Li Y, Meng Q. Effective analysis of surgical treatment for neovascular glaucoma. China Prac Med 2016;11:95–6. [Google Scholar]
- [32].Huang Y, Cai G, Lei S. Clinical observation on the treatment of neovascular glaucoma by trabeculectomy combined with scleral buckling and ciliary body cryotherapy. Jilin Med J 2013;34:4220–1. [In Chinese]. [Google Scholar]
- [33].Zhang L, Yuan Q. Effect of surgical treatment on neovascular glaucoma. Chin J Modrn Drug Appl 2015;9:177–8. [In Chinese]. [Google Scholar]
- [34].Guo N. Clinical application and observation of Ahmed glaucoma drainage valve implantation for neovascular glaucoma. Chin Foreign Med Res 2015;13:45–6. [In Chinese]. [Google Scholar]
- [35].Chen J, Sun P, Gao X. Evaluation of subconjunctival injection of mitomycin C after Ahmed valve implantation treating neovascular glaucoma. China Med 2016;11:1400–3. [In Chinese]. [Google Scholar]
- [36].Kong D, Zhang Y. Effects of intravitreal and subconjunctival injection of ranibizumab combined with trabecular filtering surgery in advanced stage neovascular glaucoma. J Prevent Med Chin People's Liber Army 2017;35:174–7. [In Chinese]. [Google Scholar]
- [37].Zou H, Li J, Wang C, et al. Panretinal photocoagulation and compound trabeculectomy for neovascular glaucoma. World Health Digest 2011;8:12–3. [In Chinese]. [Google Scholar]
- [38].Shi H. Clinical efficacy of Ahmed glaucoma drainage valve in the treatment of neovascular glaucoma. J Today Health 2016;15:91.[In Chinese]. [Google Scholar]
- [39].Brar VS, Sharma RK, Murthy RK, et al. Bevacizumab neutralizes the protective effect of vascular endothelial growth factor on retinal ganglion cells. Mol Vis 2010;16:1848–53. [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang M, Li B, Sun Y. EX-PRESS and Ahmed glaucoma valve in treatment of refractory glaucoma. Acta Ophthalmol 2016;94:e382–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


