Summary
Background
Epistaxis and gastrointestinal (GI) tract hemorrhages are common symptoms of aged hereditary hemorrhagic telangiectasia (HHT) patients that result in anemia. Clinical as well as animal studies have suggested that vascular endothelial growth factor (VEGF) neutralizing antibodies lessen hemorrhage associated with adult onset arteriovenous malformations (AVMs).
Objectives
The goal of this study is to evaluate potential therapeutic effects of oral delivery of four anti-angiogenic tyrosine-kinase inhibitors (TKIs) in the development of adult onset AVMs in a murine model of HHT.
Methods
Adult activin receptor-like kinase 1 (Alk1)-inducible knockout (iKO) model was utilized to evaluate the effect of oral administration of sorafenib, sunitinib, erlotinib, and a pazopanib analog (GW771806), on hemoglobin level, GI hemorrhages, and formation of wound-induced skin AVMs.
Results and Conclusions
Sorafenib and GW771806 significantly improved, yet erlotinib worsened anemia and GI-bleeding in the Alk1-iKO model. However, none of these TKIs appeared to be effective for inhibiting the development of wound-induced skin AVMs. Taken together, these results suggest that oral delivery of anti-angiogenic TKIs are selectively more effective on GI bleeding than mucocutaneous AVMs, and it may provide an experimental basis for selective therapeutic options depending on the symptoms of HHT.
Keywords: Activin Receptors, Anemia, Angiogenesis Inhibitors, Arteriovenous Malformation, Telangiectasia, Hereditary Hemorrhagic
Introduction
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Rendu-Weber syndrome, is an autosomal dominant vascular disorder with mutations in endoglin (ENG), activin receptor-like kinase 1 (ACVRL1; ALK1) or SMAD4 [1–3]. It affects about 1 in 5,000~8,000 individuals worldwide. Arteriovenous malformation (AVM), an abnormal connection between arteries and veins, produces vulnerable lesions that may rupture and/or alter blood flow, resulting in the dramatic clinical symptoms of cerebral hemorrhage, pulmonary shunts and/ or congestive heart failure. A hallmark of the clinical symptoms of HHT includes epistaxis, mucocutaneous telangiectases, and hemorrhages of internal organs such as the gastrointestinal (GI) tract. Prevalence and severity of epistaxis and mucocutaneous telangiectasia get higher with age as more than 90 % of HHT patients over the age of 60 experience epistaxis [4]. Epistaxis and GI bleeding often result in anemia, iron deficiency, and the need for blood transfusions, leading to a substantial compromise in the quality of life. New treatment modalities are essential, as the current therapeutic options for treating these HHT symptoms are limited.
Tamoxifen-inducible global (R26CreER;Alk12f/2f) or endothelial cell-specific (Scl-CreER;Alk12f/2f) Alk1-deletion recapitulated HHT phenotypes, including GI and lung hemorrhages with associated anemia [5, 6]. However, the vascular anomalies in Alk1 or Eng-deficient adult skin and brains formed only in the presence of additional insults such as excisional wounds or proangiogenic stimulation by vascular endothelial growth factor (VEGF) [5, 7, 8]. Conversely, VEGF blockades could alleviate wound-induced AVMs and GI bleeding in the Alk1-inducible knockout (iKO) model [7].
Increased levels of VEGF have been detected in the plasma [9] and serum [10] of HHT patients. It was found that there was a correlation of these levels with the intensity of VEGF staining and microvessel density in their respective nasal mucosa [9]. Intravenous, submucosal, and topical bevacizumab (a monoclonal antibody against VEGF-A) treatments have shown to be effective in reducing nasal bleeds, quantified by the improvement in an epistaxis severity index [11–14]. However, recent randomized clinical trials of short-term use of nasal spray of bevacizumab revealed no significant benefit for epistaxis [15, 16]. Tyrosine-kinase inhibitors (TKIs) target receptor tyrosine kinases such as VEGF receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and epidermal growth factor receptor (EGFR). These drugs have been used as anti-angiogenic and anti-cancer drugs for renal cell carcinoma (sunitinib, sorafenib and pazopanib), gastro-intestinal stromal tumor (sunitinib), unresectable hepatocellular carcinoma (sorafenib), soft tissue sarcoma (pazopanib), and metastatic non-small cell lung cancer, pancreatic cancer (erlotinib) [17–22]. Essentially, blockade of angiogenesis in the setting of a solid tissue tumor can limit the growth of the tumor itself.
We assessed the therapeutic potential of anti-angiogenic pharmaceutical drugs including anti-angiogenic small molecules (erlotinib, sorafenib, sunitinib and a pazopanib analog) for GI bleeding and mucocutaneous AVMs using an HHT mouse model. The results from this preclinical animal model may provide insights into drug selection for clinical studies for HHT.
Materials
Animals
All in vivo procedures were conducted in accordance with animal use guidelines established by the University of Florida Institutional Animal Care and Use Committee. Establishment of the Alk12f allele in laboratory mice was described previously [23]. R26CreER mice were purchased from the Jackson Laboratory. R26CreER/+; Alk12f/2f mice used in this study were on a mixed (129Sv/C57BL6) background.
Skin Wound Generation and Drug Treatment
Full-thickness skin wounds were created in the mid-dorsum of mice with a sterile disposable biopsy punch (3 mm diameter). Tamoxifen was injected intraperitoneally at 0.1 mg/ g of body weight (b.w.) on the day of wounding (Day 0). 40 mg/kg b.w. of sorafenib (TSZ Chem), sunitinib (TSZ Chem), and GW771806 (GSK, comparable with pazopanib), and 20 mg/kg b.w. of erlotinib (Biotang) were orally administrated twice daily. One set of common control group (5% DMSO) was used for sorafenib, sunitinib, and erlotinib, while a different set of control group (a vehicle treatment) was used for GW771806. To test whether topical applications of TKIs could be effective for inhibiting wound-induced AVM formation, we utilized dorsal skin fold window chamber model. Sixty µl of GW771806 (480 ng, 8 mg/ml) or vehicle was directly injected into the window chamber once a day
Latex Dye Injection and Image Processing
Detailed procedure for latex dye perfusion and image processing is the same as previously described [5, 7].
Hemoglobin Concentration and GI Hemorrhage Index
Hemoglobin (Hb) concentrations in the blood collected from mouse tails were measured by a hemoglobin photometer (Hemopoint H2, STANBIO Laboratory).
Hyperspectral image acquisition and processing
Surgical procedure for the window chamber installation, image acquisition, and processing was described previously [6, 7].
Statistics
Data were represented as mean ± standard deviation (SD). One-way ANOVA with post-Tukey multiple comparison tests (GraphPad Prism 4.03) was used to analyze Hb and GI bleeding results. Student’s t-test was also used to determine a statistical significance between two groups with at least 3 pairs. A value of p<0.05 was considered statistically significant (* p<0.05, ** p<0.01 and *** p<0.001).
Results and Discussions
Oral administration of sorafenib or GW771806 improves low hemoglobin caused by Alk1-depletion
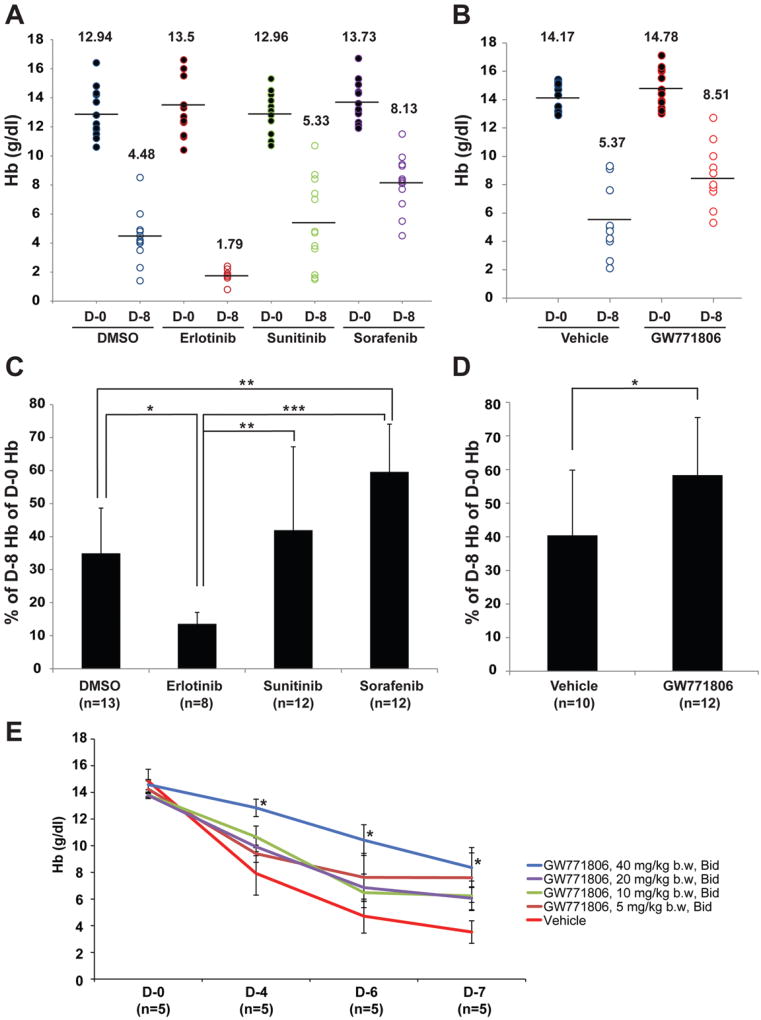
We evaluated the efficacy of oral treatment of FDA registered anti-angiogenic TKIs (erlotinib, sunitinib, sorafenib) as well as a pazopanib-like agent (GW771806) on HHT symptoms using the Alk1-iKO mouse model (R26CreER/+;Alk12f/2f). Tamoxifen administration produces in this model an ALK1-deficiency, which results in GI bleeding, anemia, and wound-induced skin AVMs in 6–8 days. Since one of the most common and severe systemic HHT symptoms is anemia, we examined hemoglobin (Hb) levels. Hb levels before tamoxifen treatment (day 0) of control groups varied in the range of 11 and 17mg/dl (Fig.1A and 1B). The means of the DMSO and vehicle control groups were 12.94 g/dl (n=13) and 14.17 g/dl (n=10), respectively. These mean values dropped to 4.48 g/dl and 5.37 g/dl, respectively, 8 days after tamoxifen treatment. The TKI-treated groups also had the similar trend of reduced Hb levels after Alk1-deletion, but the magnitude of reduction was different among groups. Percentage reduction of Hb levels during 8 days in the sorafenib (n=12; 40.38%; Fig. 1C) and the GW771806 (n=12; 41.65%; Fig. 1D)-treated groups was significantly less than that observed in the control groups (DMSO 65.04% [Fig. 1C], vehicle 59.59% [Fig. 1D]). The sunitinib-treated group (n=12; 58.01%) also exhibited the similar trend toward improvement of Hb levels compared to the controls (Fig. 1C) but did not reach significance. On the other hand, the erlotinib group (n=8) revealed lower Hb levels than the DMSO-treated group (Fig. 1C). Erlotinib targets epidermal growth factor receptor. Recent studies demonstrated that erlotinib induces barrier dysfunction of small intestine epithelial cells and enhances ER-stress-mediated intestinal epithelial injury [24]. We speculate that the adverse effect of erlotinib could be due to combined effects of erlotinb on both intestinal epithelial cells and angiogenesis.
Figure 1.
Oral administration of anti-angiogenic TKIs is effective for ameliorating anemia phenotype. A and B. Hb levels of Alk1-iKO mice on the first day (D-0) and 8 days after (D-8) tamoxifen treatment. Effects of three drugs (erlotinib, sunitinib, and sorafenib) compared with DMSO (A) and effect of a pazopanib analog with its control vehicle (B) were shown. Each circle indicates the Hb level of an individual mouse. The mean of the each group is shown as a number and a bar. C and D. Decreased Hb levels 8 days after TM treatment are shown as percentage of Hb of D-8 compared with that of D-0. Percent decreases of each individual mouse were used for statistical analysis. Decrease of Hb levels in sorafenib and GW771806 groups was significantly less while that in erlotinib group was more than that in control treatment groups. E. Various doses of GW771806 (5, 10, 20, and 40 mg/kg, Bid) were orally administered twice a day. Mice treated with 40 mg/kg showed significant increase of Hb levels compared to vehicle controls. Bid (bis in die) indicates twice a day. * P<0.05, ** P<0.01 and *** P<0.001. Error bars in C, D and E indicate SD.
To test the potency of the drug to produce the effect, we examined one TKI, GW771806, at progressively lower dosing levels in addition to the previous 40mg dose. While only the highest dose (40 mg/kg bw) demonstrated significance, all treated groups showed a similar trend of improving hemoglobin levels compared with the vehicle treated group, perhaps suggesting a Type II error, or lack of significance due to small numbers.
Oral administration of sunitinib, sorafenib or GW771806 ameliorates GI bleeding caused by Alk1-depletion
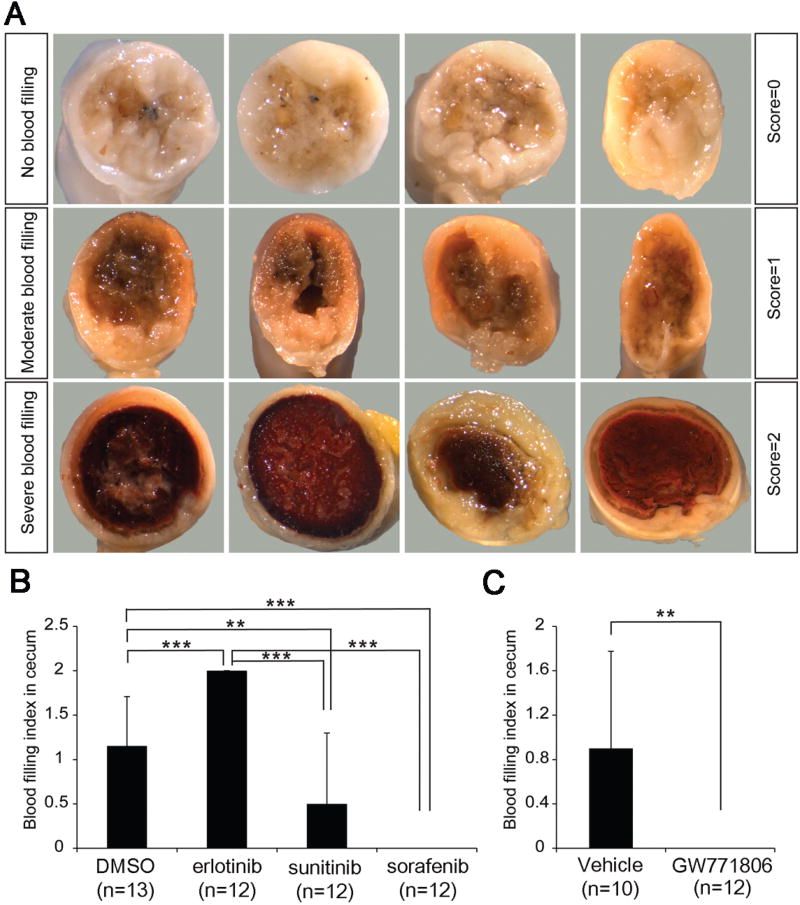
GI bleeding seems to be a key contributor to the anemia symptoms in Alk1-iKO mice [5]. To examine the severity of GI bleeding, the entire GI tract from the stomach to colon was examined, and categorized into three groups according to the severity determined by darkness of brown color in the colon and cecum area: none (score=0), weak-moderate (score=1), moderate-strong (score=2) (Fig. 2A; Sup Fig. 1). As shown in Fig. 2B, the GI bleeding index results are well correlated with hemoglobin levels in TKI-treated mice. All mice in the erlotinib-treated group displayed more severe GI bleeding (average: 2, P<0.001, n=12) than the DMSO-treated mice (Fig.1A and 2B). In contrast, sorafenib (average: 0, P<0.001, n=12) and GW771806 (average: 0, P<0.01, n=12) reduced GI bleeding compared with that of DMSO (average: 1.15, n=13) and vehicle (average: 0.9, n=10), respectively (Fig.2B and 2C). The sunitinib-treated group also showed significantly less GI bleeding (average: 0.5, P<0.05, n=12; Fig. 2B).
Figure 2.
Anti-angiogenic TKI administration effectively reduces GI bleeding in the Alk1-iKO model. A. Cross sectional view of ceca showing signs of bleeding (dark brown). GI bleeding phenotypes are categorized according to the severity of cecal hemorrhages. No, moderate, and severe blood filled ceca were scored 0, 1, and 2, respectively. Four representative images in each scale were shown regardless of the treatment conditions. B and C. GI bleeding index indicates that sorafenib, sunitinib or GW771806 significantly alleviated but erlotinib exacerbated GI hemorrhages compared with controls. ** P<0.01 and *** P<0.001. Error bars in B and C indicate SD.
Oral administration of anti-angiogenic TKIs was unable to suppress the formation of wound-induced skin AVMs
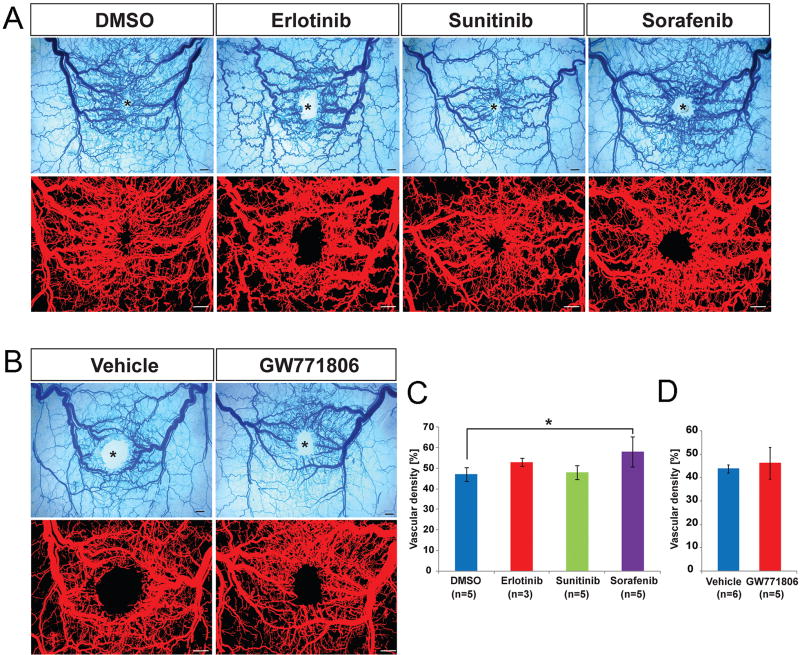
Unlike the GI tract, AVMs in subdermal vasculature develop in the setting of a secondary insult such as wounding in the Alk1-iKO model [5]. We have previously shown that VEGF could mimic the wound effect and VEGF neutralizing antibodies could inhibit wound-induced skin AVMs in the Alk1-iKO model [7]. Since sorafenib, pazopanib and sunitinib are known as strong inhibitors of VEGF receptors, we examined whether the oral administration of TKIs would have a similar effect with VEGF antibodies in inhibiting the development of wound-induced AVMs. Unexpectedly treatment with the four TKIs did not inhibit wound-induced skin AVMs (Fig. 3). It appeared that sorafenib treatment slightly increased the vascular density (Fig. 3C). This result might be related to the report showing that sorafenib treatment (30 mg/kg daily) led to intratumoral recruitment of F4/80- and CD11b-positive macrophages which secret cytokines for promoting proangiogenic microenvironment [25].
Figure 3.
Anti-angiogenic TKIs does not inhibit the formation of wound-induced skin AVMs. A and B. Top panels show latex dye-perfused images of vasculature surrounding the wound given in the dorsal skin of Alk1-iKO mice 8 days after tamoxifen treatment. Blood vessels containing the latex dye are processed for quantification (bottom panels). The wound sites are indicated by asterisks. The scale bars indicate 1 mm. C and D. Quantification of vascular area containing latex per a given area. Note that AVMs develop in both control and drug-treated groups at equivalent levels and the vascular density of erlotinib-, sunitinib- or GW771806-treated mice is comparable with that of DMSO or vehicle controls. Vascular density of sorafenib treatment was shown to be increased slightly. Error bars represent SDs. * P<0.05.
Clinical as well as animal studies have suggested that angiogenic stimulation is a critical environmental factor for the development of AVMs in HHT [5, 7], and that anti-angiogenic blockades are effective therapies for prevalent HHT symptoms, such as epistaxis, GI bleeding and liver AVMs [7, 11, 13, 14, 26]. In this study, we evaluated the efficacy of the potent anti-angiogenic TKIs using the Alk1-iKO mice to diversify therapeutic options for HHT. Sorafenib and GW771806 administration significantly improved Hb and GI bleeding, whereas they were not effective in preventing wound-induced skin AVMs. Although sunitinib treatment did not reach a statistically significant level, it also showed a tendency of improving the Hb level, and ameliorated GI bleeding. In contrast, oral administration of erlotinib, an EGFR inhibitor, worsened anemia and GI bleeding. The dosage used in this study, 20 mg/kg b.w. (total 40 mg/kg b.w., daily), is well below the dosage of erlotinib used in previous studies (100 mg/kg b.w., daily), in which no significant intestinal toxicity was observed [26]. Therefore, this deleterious influence is not due to over-dose. Our results may indicate that erlotinib should be used with caution for HHT patients.
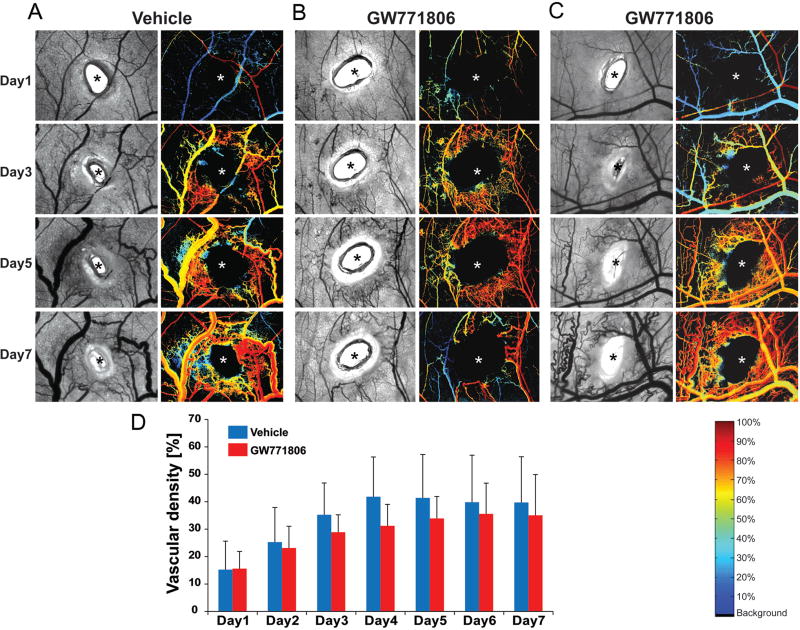
TKIs in this study were not effective for wound-induced skin AVMs. This recommends underdosing, potentially from poor drug access in the skin tissue for such drugs. To test whether topical applications of TKIs could be effective for inhibiting wound-induced AVM formation, we utilized dorsal skin fold window chamber model. In this model, the progress of wound-induced AVM development can be observed daily with hyperspectral imaging system which detects the oxygen saturation content in the blood vessels, and the drug can be directly applied to the wounded skin area [7, 27]. All vehicle injected Alk1-iKO mice develop AVMs as expected (Fig 4A). A varied response to GW771806 treatment was observed. While one of the GW771806 treated mice showed remarkable reduction of AVM formation (Fig. 4B), another mouse in the treated group had no treatment effect (Fig. 4C), and the rest had a mid-level influence. Overall there was a trend of reduction in AVM formation represented by vascular density, but it did not reach significance (Fig. 4D). Since the window chamber is not sealed from neighboring skin, the drug could be diffused away from the chamber, and thus once-a-day treatment may not be sufficient to sustain the effect on the wound site. It would warrant further testing with the increased sample size, and different dosage, frequency and the mode of topical application of these drugs for skin AVMs.
Figure 4.
Effect of topical application of pazopanib on vascular remodeling during development of wound-induced AVMs. A–C. Representative brightfield (left) and corresponding hyperspectral images (right) of the wounded area of the skin in a dorsal window chamber of Alk1-iKO mice treated with vehicle (A) or GW771806 (B and C). Images show days 1, 3, 5, and 7 after TM administration, wounding, and drug treatment. All images are shown at the same magnification. Typical tortuous vasculature surrounding the wound (indicated by asterisks) was shown in vehicle treated mice (A). Hb (O2) saturation map shows arterial blood flow (shown red) in venous vessels (blue or yellow) in vehicle -treated mice, indicating AV shunts. Reduced vessels density, tortuosity, and sustained AV shunts were observed in one of GW771806-treated mice (B), while another drug-treated mouse showed a similar vascular remodeling compared with the control (C). The color bar in the bottom right of panel C indicates Hb saturation levels. D. Quantification and statistical analysis of vascular density in wound area of vehicle and GW771806-treated groups (vehicle, n=4; GW771806, n=5). No significant difference was detected between control and .GW771806-treated groups.
Supplementary Material
Essentials.
Anti-angiogenic drugs are indicated as therapies for hereditary hemorrhagic telangiectasia.
We interrogated the response to four anti-angiogenic drugs for anemia and intestinal bleeding.
Sorafenib and a pazopanib analog significantly improved while erlotinib worsened anemia.
Some oral anti-angiogenic drugs were effective in reducing intestinal bleeding.
Acknowledgments
We thank Peter Adamson (GlaxoSmithKline) for providing GW771806 used in this study.
Sources of Funding
This work was supported by NIH grant HL128525 and by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A080588-23) to SPO, Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2016R1D1A1B03934950) to SWC, and predoctoral fellowship from the American Heart Association (AHA) to YHK.
Abbreviations
- ALK1
Activin receptor-like kinase 1
- AVM
arteriovenous malformation
- ENG
endoglin
- HHT
Hereditary Hemorrhagic Telangiectasia
- TKI
tyrosine-kinase inhibitors
- iKO
inducible knockout
- GI
gastrointestinal
- Hb
hemoglobin
- VEGF
vascular endothelial growth factor
- PDGFR
platelet-derived growth factor receptor
- EFGR
epidermal growth factor receptor
Footnotes
Author Contributions:
S. Paul Oh: designed and supervised the entire experiments, edited manuscript, interpreted of data; Dennis Sprecher & Young Jae Lee: designed some experiments, edited manuscript; Yong Hwan Kim: performed experiments and statistical analysis, drafted manuscript; Mi-Jung Kim and Se-woon Choe: performed experiments.
References
- 1.Bideau A, Plauchu H, Brunet G, Robert J. Epidemiological investigation of Rendu-Osler disease in France: its geographical distribution and prevalence. Popul. 1989;44:3–22. [PubMed] [Google Scholar]
- 2.Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999;245:31–9. doi: 10.1046/j.1365-2796.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43:97–110. doi: 10.1136/jmg.2005.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24:203–19. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, Oh SH, Walter G, Raizada MK, Sorg BS, Oh SP. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119:3487–96. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido-Martin EM, Nguyen HL, Cunningham TA, Choe SW, Jiang Z, Arthur HM, Lee YJ, Oh SP. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models--brief report. Arterioscler Thromb Vasc Biol. 2014;34:2232–6. doi: 10.1161/ATVBAHA.114.303984. [DOI] [PubMed] [Google Scholar]
- 7.Han C, Choe SW, Kim YH, Acharya AP, Keselowsky BG, Sorg BS, Lee YJ, Oh SP. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis. 2014;17:823–30. doi: 10.1007/s10456-014-9436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–62. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadick H, Naim R, Sadick M, Hormann K, Riedel F. Plasma level and tissue expression of angiogenic factors in patients with hereditary hemorrhagic telangiectasia. Int J Mol Med. 2005;15:591–6. [PubMed] [Google Scholar]
- 10.Cirulli A, Liso A, D'Ovidio F, Mestice A, Pasculli G, Gallitelli M, Rizzi R, Specchia G, Sabba C. Vascular endothelial growth factor serum levels are elevated in patients with hereditary hemorrhagic telangiectasia. Acta Haematol. 2003;110:29–32. doi: 10.1159/000072411. [DOI] [PubMed] [Google Scholar]
- 11.Flieger D, Hainke S, Fischbach W. Dramatic improvement in hereditary hemorrhagic telangiectasia after treatment with the vascular endothelial growth factor (VEGF) antagonist bevacizumab. Ann Hematol. 2006;85:631–2. doi: 10.1007/s00277-006-0147-8. [DOI] [PubMed] [Google Scholar]
- 12.Davidson TM, Olitsky SE, Wei JL. Hereditary hemorrhagic telangiectasia/avastin. Laryngoscope. 2010;120:432–5. doi: 10.1002/lary.20757. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell A, Adams LA, MacQuillan G, Tibballs J, vanden Driesen R, Delriviere L. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl. 2008;14:210–3. doi: 10.1002/lt.21417. [DOI] [PubMed] [Google Scholar]
- 14.Oosting S, Nagengast W, de Vries E. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;361:931. doi: 10.1056/NEJMc091271. author reply-2. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead KJ, Sautter NB, McWilliams JP, Chakinala MM, Merlo CA, Johnson MH, James M, Everett EM, Clancy MS, Faughnan ME, Oh SP, Olitsky SE, Pyeritz RE, Gossage JR. Effect of Topical Intranasal Therapy on Epistaxis Frequency in Patients With Hereditary Hemorrhagic Telangiectasia: A Randomized Clinical Trial. JAMA. 2016;316:943–51. doi: 10.1001/jama.2016.11724. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis-Girod S, Ambrun A, Decullier E, Fargeton AE, Roux A, Breant V, Colombet B, Riviere S, Cartier C, Lacombe P, Chinet T, Blivet S, Blondel JH, Gilbert-Dussardier B, Dufour X, Michel J, Harle JR, Dessi P, Faure F. Effect of Bevacizumab Nasal Spray on Epistaxis Duration in Hereditary Hemorrhagic Telangectasia: A Randomized Clinical Trial. JAMA. 2016;316:934–42. doi: 10.1001/jama.2016.11387. [DOI] [PubMed] [Google Scholar]
- 17.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 18.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 19.Keisner SV, Shah SR. Pazopanib: the newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs. 2011;71:443–54. doi: 10.2165/11588960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 21.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther. 2007;29:1338–53. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 23.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–42. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan L, Hu L, Yang B, Fang X, Gao Z, Li W, Sun Y, Shen Y, Wu X, Shu Y, Gu Y, Xu Q. Erlotinib promotes endoplasmic reticulum stress-mediated injury in the intestinal epithelium. Toxicol Appl Pharmacol. 2014;278:45–52. doi: 10.1016/j.taap.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi KF, Yanagisawa M, Sekiguchi F, Tanaka Y. Antitumor activity of erlotinib in combination with capecitabine in human tumor xenograft models. Cancer Chemother Pharmacol. 2006;57:693–702. doi: 10.1007/s00280-005-0079-3. [DOI] [PubMed] [Google Scholar]
- 27.Sorg BS, Moeller BJ, Donovan O, Cao Y, Dewhirst MW. Hyperspectral imaging of hemoglobin saturation in tumor microvasculature and tumor hypoxia development. J Biomed Opt. 2005;10:44004. doi: 10.1117/1.2003369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.