Abstract
Purpose
To determine the intra-visit and inter-visit reproducibility of optical coherence tomography angiography (OCTA) measurements of macular vessel density in eyes with and without retinal diseases.
Methods
Fifteen healthy volunteers and 22 patients with retinal diseases underwent repeated OCTA (Angiovue Imaging System, Optovue Inc.) scans after pupil dilation on two separate visit days. For each visit day, the eyes were scanned twice. Vessel density defined as the proportion of vessel area with flowing blood over the total measurement area was calculated using Angiovue software. Intra-visit and inter-visit reproducibility were summarized as coefficients of variation (CV) and intra-class correlation coefficients (ICC) calculated from variance component models.
Results
The CVs representing the intra-visit reproducibility of the superficial macular vessel density measurements for different quadrants on 3 × 3 mm scans varied from 2.1–4.9% and 3.4–6.8% for healthy and diseased eyes respectively, and for the inter-visit it was 2.9–5.1% and 4.0–6.8% respectively. The CVs were lower in healthy eyes than in diseased eyes, lower for intra-visit than for inter-visit, lower on 3 × 3 mm scans than on 6 × 6 mm scans, and lower for paracentral subfields than for central subfield.
Conclusion
The evidence presented here demonstrates good reproducibility of OCTA for measurement of superficial macula vessel density in both healthy eyes and eyes with diabetic retinopathy without diabetic macular edema.
Keywords: Optical coherence tomography angiography, reproducibility, macular blood flow, retinal superficial capillary
An accurate and reliable method of quantifying retinal vascular perfusion could play an important role in the diagnosis and management of various retinal vascular diseases, including diabetic retinopathy, retinal artery or vein occlusions and neovascular age-related macular degeneration. Fluorescein angiography (FA), and indocyanine green angiography (ICGA) have been clinically used to evaluate the retinal and choroidal vasculature in vivo. However, both of these imaging methods are invasive and associated with a variety of complications including painful dye extravasation, inadvertent intra-arterial injections, pruritus, nausea, temporary skin discoloration, and rarely but potentially catastrophic, anaphylactic reactions. 1,2 In addition, dye based angiography is not depth resolved, which means that the retinal and choroidal vasculatures cannot be resolved.
Optical coherence tomography angiography (OCTA) is a novel noninvasive imaging technology which uses motion contrast to produce angiographic data across the retina and choroid.3–11 The OCTA images provide high resolution of the capillary details and enable the ability to resolve superficial and deep retinal vascular networks separately.11 Recent studies have shown that OCTA can be a useful tool in qualitative and quantitative assessment of retinal, choroidal or optic nerve vessels in diabetic retinopathy, neovascular age related macular degeneration, macular telangiectasia and glaucoma.12–18 However, there is a paucity of data addressing the reproducibility of this technique. The current study sought to determine the intra-visit and inter-visit reproducibility of OCTA measurements of macular vessels in eyes with and without retinal diseases.
Methods
The study was approved by the institutional review board of University of California San Diego and complied with the Health Insurance Portability and Accountability Act of 1996, and the protocol adheres to the tenets of the Declaration of Helsinki. Written informed consent was obtained for each patient prior to enrollment into the study.
The commercially available Avanti spectral-domain optical coherence tomography (SD OCT) device (Optovue Inc, Fremont, California, USA) and the contained AngioVue OCTA system were used for imaging. This system used a split-spectrum amplitude decorrelation angiography (SSADA) software algorithm and acquired 70,000 A-scans per second to compose OCTA volumes consisting of 304 × 304 A-scans. Orthogonal registration and merging of 2 consecutive scans were used to obtain OCTA macula volume scans of a central 3 × 3 mm or 6 × 6 mm macula area of both eyes for each subject. 19,20
Pupils of all the participants were dilated with 2.5% phenylephrine and 1.0% tropicamide to ≥ 6 mm in diameter. During imaging, the subject was instructed to fixate on an internal fixation target. Initial camera alignment, illumination and focus were done in infrared (IR) mode and with assistance from the “Auto all” function of the machine. Only one randomly selected eye was scanned twice during the first and second testing visits. At each testing visit, the subject was asked to retract from the chin rest to have 1 or 2 minutes break after the first set of scans, and then reposition for the second set of scans to assess the intra-visit reproducibility. The fellow eye was scanned only once each session. The second visit took place approximately 2 weeks later, when the same scan protocol as the first visit was followed.
OCTA image quality review was performed for all scans. Eighty-three out of 351(23.6%) scans with segmentation errors or with poor image quality due to motion, blur, and floaters were excluded. Scans with a Signal Strength Index (SSI) > 46 were included. The SSI is a quantitative measure for image quality ranging from 1 (poor) to 100 (good). The threshold value 46 was chosen during the quality review before vessel density measurement. For included scans, the OCTA images of the superficial and deep capillary networks were generated separately using the automated software algorithm of the machine (ReVue, version 2014.2.0.65; Optovue Inc, Fremont, California, USA). Based on these default settings, the superficial network extends from 3 μm below the internal limiting membrane to 15 μm below the inner plexiform layer (IPL). The deep capillary network extends from 15 to 70 μm below the IPL. The vessel density of the superficial and deep capillary network were measured and assessed separately with the embedded AngioVue software. The vessel density was defined as the proportion of vessel area with blood flow over the total area measured (figure 1 and 2). Due to the projection artifacts from the superficial vessels, the accuracy of automated vessel density measurements in deep capillary network is limited, and was therefore excluded in the current study.
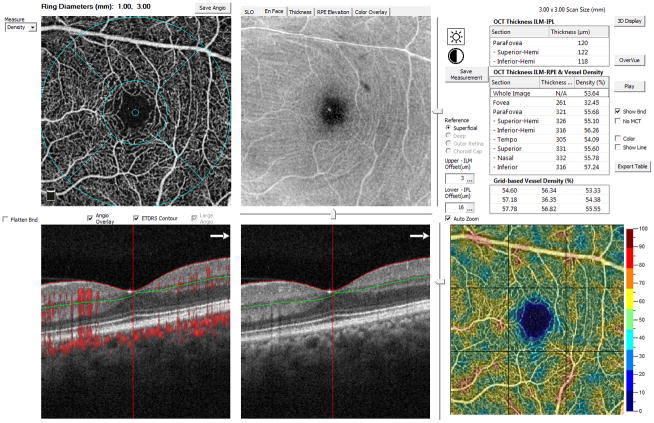
Figure 1.
Representative measurement of superficial macular vessel density of a normal eye with Optical Coherence Tomography Angiography on a 3 × 3 mm scan. The left upper panel shows the angiography images of the superficial layer and the measuring circles and subfields. On the left and the middle lower panel, the horizontal red and green lines mark the default superficial layer, and the red dots on the left lower panel mark the tomographic retinal vessels and the choroidal capillaries. The middle upper panel shows the en face OCT image of the superficial retina layer. The right upper panel shows the measurement values of the retinal thickness and the vessel densities of different subfields. The right lower panel shows the 9-grid based measurements with the value showed in the table above the images.
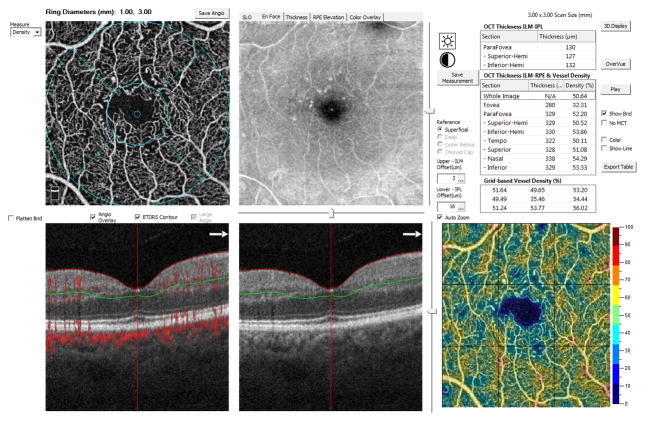
Figure 2.
Representative measurement of superficial macular vessel density of an eye with diabetic retinopathy with Optical Coherence Tomography Angiography on a 3 × 3 mm scan.
Statistical analyses were conducted using the R® statistical software. Descriptive statistics included mean, standard deviation (SD), median, range, and percentages were presented where appropriate. Intra-visit and inter-visit reproducibility were summarized as coefficients of variation (CV) and intra-class correlation coefficients (ICCs). It is important to be cognizant that these two measurements, whilst estimating the same thing, have different properties; while the CV attempts to scale the variability for the measurement size to reduce the effect of measurement scaling in estimating reproducibility, ICCs measure the reproducibility of the measurement relative to true measurement differences between subjects (i.e. if differences between subjects is low, the ICC will be reduced, but CV would be unaffected). Variance component models (a type of random effects model) were utilized to calculate the intra-visit and inter-visit variability of healthy and patient groups separately. Models were fitted with the vessel density measurement as a response with random effects for patient and visit to account for the inter patient and between-visit standard deviations (SD) respectively. This effectively separated the total variance into 3 parts: with variance components due to visit, between-subject variability, and a residual variance component from “random” variability that cannot be ascribed to the former two components. The square root of the residual variance, or the residual SD was defined to be the intra-visit SD, which represents the variability that would result from a single patient taking multiple tests in the same visit. The square root of the sum of the intra-visit variance and the between-visit variance was defined to be the inter-visit SD. Coefficients of variation (CV) were calculated by dividing the intra-visit and inter-visit SDs by the mean vessel density measurement; these statistics attempt to scale the variability according to the measurement size. Intra-class Correlation Coefficients (ICC) were also calculated as a summary of the intra-visit and inter-visit variability expressing the proportion of variance attributed to real differences (or disease status) between subjects. The ICCs were calculated by estimating the proportion of the total SD in measurements explained by actual measurement differences (i.e. the ratio of the inter-eye SDs and total SD). Large ICCs (close to 1) indicate that there are relatively small fluctuations between repeat measurements within an individual eye compared to variability between eyes. P-values represent results for 2-sided tests, with values less than 0.05 considered statistically significant.
Results
Thirty-seven eyes of 37 persons were enrolled in the study, including 15 normal control eyes, 2 eyes with dry AMD, 2 eyes with central serous chorioretinopathy, 16 eyes with diabetic retinopathy without diabetic macular edema, 1 eye with myopic CNV and 1 eye with macular telangiectasia type II. Control normal eyes were recruited in persons without previous disease history and with best corrected visual acuity ≥ 20/20, intraocular pressure ≥ 10 mmHg and ≤ 21 mmHg, without any abnormality found on dilated fundus examination and OCT scanning. No eyes included in the study received treatment, such as intravitreal injection or laser photocoagulation, during the study period. The mean age of the participants was 54 years (range: 37–68 years), and 57% of them were men. The demographic characteristics of the participants stratified by clinical diagnosis were summarized in table 1.
Table 1.
Demographic characteristic of the study participants.
| Scan pattern | Control group | Retinal disease group | ||
|---|---|---|---|---|
| 3.0×3.0 | Number of eyes | 15 | 18 | |
| Number of scans | 66 | 65 | ||
| Age (years, Lower and upper quartiles) | 49 (37 to 54) | 57 (53 to 66) | ||
| Gender (male: female) | 5:10 | 12:6 | ||
| Clinical diagnosis | Dry AMD | N/A | 2 | |
| Diabetic Retinopathy | 12 | |||
| Myopic CNV | 1 | |||
| CSCR | 2 | |||
| Mac Tel | 1 | |||
| 6.0×6.0 | Number of eyes | 15 | 22 | |
| Number of Scans | 61 | 76 | ||
| Age (years) | 49 (37 to 54) | 58 (53 to 68) | ||
| Gender (Male: Female) | 5:10 | 16:6 | ||
| Clinical diagnosis | Dry AMD | N/A | 2 | |
| Diabetic Retinopathy | 16 | |||
| Myopic CNV | 1 | |||
| CSCR | 2 | |||
| Mac Tel | 1 | |||
AMD: age-related macular degeneration
CNV: choroidal neovascularization
CSCR: central serous chorioretinopathy
N/A: not applicable
On 3 × 3 mm scans, the mean vessel densities in healthy eyes in the paracentral subfields and the whole area were significantly higher than the corresponding value of eyes with retinal diseases (see table 1). On 6 × 6 mm scans, the mean vessel density of the superficial network in the normal control group was significantly higher than that of eyes with retinal diseases in all subfields (Table 2).
Table 2.
Macular vessel density in healthy eyes and eyes with retinal diseases
| Normal eyes | Eyes with retinal diseases | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scan Size | Sector | N (scans) | Mean vessel density (%) | 95% Confidence interval of density (%) | N (scans) | Mean vessel density (%) | 95% Confidence interval of density (%) | |||
| 3×3 mm | Whole macular area | 66 | 53.8 | 53.3 | 54.3 | 65 | 50.4 | 49.4 | 51.4 | 0.004 |
| Central Fovea | 66 | 32.1 | 31.2 | 33.1 | 65 | 30.3 | 29.1 | 31.4 | 0.06 | |
| Para-foveal circle | 66 | 55.4 | 55.0 | 55.9 | 65 | 52.2 | 51.1 | 53.3 | 0.01 | |
| Para-fovea_superior hemifield | 66 | 55.3 | 54.8 | 55.9 | 65 | 52.0 | 50.8 | 53.3 | 0.02 | |
| Para-fovea_inferior hemifield | 66 | 55.6 | 55.1 | 56.0 | 65 | 52.4 | 51.3 | 53.5 | 0.009 | |
| Para-fovea_temporal sector | 66 | 54.1 | 53.6 | 54.7 | 65 | 51.3 | 50.1 | 52.4 | 0.03 | |
| Para-fovea_superior sector | 66 | 56.3 | 55.7 | 57.0 | 65 | 52.7 | 51.3 | 54.0 | 0.02 | |
| Para-fovea_nasal sector | 66 | 54.8 | 54.2 | 55.4 | 65 | 51.6 | 50.5 | 52.7 | 0.008 | |
| Para-fovea_inferior sector | 66 | 56.6 | 56.1 | 57.0 | 65 | 53.2 | 52.0 | 54.4 | 0.01 | |
| 6×6 mm | Whole macular area | 61 | 54.2 | 53.5 | 55.0 | 76 | 49.2 | 48.3 | 50.0 | <0.001 |
| Central Fovea | 61 | 36.5 | 35.4 | 37.6 | 76 | 33.0 | 31.8 | 34.3 | 0.01 | |
| Para-foveal circle | 61 | 55.6 | 54.6 | 56.6 | 76 | 50.5 | 49.3 | 51.6 | <0.001 | |
| Para-fovea_superior hemifield | 61 | 55.6 | 54.6 | 56.6 | 76 | 50.4 | 49.1 | 51.7 | <0.001 | |
| Para-fovea_inferior hemifield | 61 | 55.6 | 54.5 | 56.6 | 76 | 50.5 | 49.5 | 51.6 | <0.001 | |
| Para-fovea_temporal sector | 61 | 55.1 | 54.1 | 56.1 | 76 | 51.2 | 49.9 | 52.4 | 0.004 | |
| Para-fovea_superior sector | 61 | 55.9 | 54.8 | 57.1 | 76 | 50.4 | 49.1 | 51.6 | <0.001 | |
| Para-fovea_nasal sector | 61 | 56.0 | 55.0 | 56.9 | 76 | 49.9 | 48.7 | 51.2 | <0.001 | |
| Para-fovea_inferior sector | 61 | 55.4 | 54.2 | 56.6 | 76 | 50.4 | 49.3 | 51.5 | <0.001 | |
The CVs representing the intra-visit reproducibility of the superficial macular vessel density measurements for different quadrants on 3 × 3 mm scans varied from 2.1–4.9% and 3.4–6.8% for healthy and diseased eyes respectively, and for the inter-visit it was 2.9–5.1% and 4.0–6.8% respectively, showing a good intra-visit and inter-visit reproducibility (Table 3).
Table 3.
Intra-visit and inter-visit coefficient of variation for measurement of vessel density in healthy eyes and eyes with retinal diseases
| Intra-visit coefficient of variation (%) | Inter-visit coefficient of variation | ||||||
|---|---|---|---|---|---|---|---|
| Scan Size | Sector | Normal eyes (%) | Diseased eyes (%) | P value | Normal eyes (%) | Diseased eyes (%) | P value |
| 3×3 mm | Whole macular area | 2.6 | 3.7 | 0.01 | 3.2 | 4.1 | 0.06 |
| Central Fovea | 4.9 | 6.8 | 0.01 | 5.1 | 6.8 | 0.03 | |
| Para-foveal circle | 2.6 | 3.7 | 0.01 | 3.3 | 4.0 | 0.13 | |
| Para-fovea_superior hemifield | 3.1 | 3.3 | 0.48 | 4.0 | 4.0 | 0.93 | |
| Para-fovea_inferior hemifield | 2.4 | 4.3 | <0.001 | 2.9 | 4.3 | 0.004 | |
| Para-fovea_temporal sector | 3.1 | 4.7 | 0.002 | 3.2 | 4.9 | 0.002 | |
| Para-fovea_superior sector | 3.5 | 3.6 | 0.79 | 4.5 | 4.0 | 0.28 | |
| Para-fovea_nasal sector | 3.4 | 3.4 | 0.97 | 3.9 | 5.2 | 0.02 | |
| Para-fovea_inferior sector | 2.1 | 4.4 | <0.001 | 3.4 | 4.4 | 0.05 | |
| 6×6 mm | Whole macular area | 2.6 | 4.0 | 0.001 | 3.8 | 5.1 | 0.01 |
| Central Fovea | 5.5 | 7.4 | 0.02 | 6.4 | 10.4 | <0.001 | |
| Para-foveal circle | 3.8 | 4.9 | 0.05 | 6.5 | 6.4 | 0.93 | |
| Para-fovea_superior hemifield | 4.3 | 4.9 | 0.28 | 6.6 | 6.3 | 0.74 | |
| Para-fovea_inferior hemifield | 3.6 | 5.2 | 0.004 | 6.6 | 6.8 | 0.81 | |
| Para-fovea_temporal sector | 3.9 | 4.6 | 0.18 | 6.9 | 6.8 | 0.96 | |
| Para-fovea_superior sector | 4.9 | 5.8 | 0.16 | 7.2 | 6.6 | 0.55 | |
| Para-fovea_nasal sector | 4.0 | 6.8 | <0.001 | 5.8 | 7.8 | 0.02 | |
| Para-fovea_inferior sector | 4.6 | 5.6 | 0.12 | 7.5 | 7.4 | 0.91 | |
In general, the CVs were lower in healthy eyes than in diseased eyes, showing a slightly better reproducibility in healthy eye compared to diseased eyes. Simultaneously, the CVs for intra-visit were always lower than for inter-visit, showing a slightly better intra-visit repeatability than inter-visit repeatability. In addition, the CVs on 3 × 3 mm scans tend to be slightly lower than 6 × 6 mm scans, showing a better repeatability of 3 × 3 mm scans than 6 × 6 mm scans (Table 3).
For different subfield measurement, the central subfields in both healthy and diseased eyes had reduced reproducibility in terms of CV than the paracentral subfields. The intra-visit CVs of the superficial macular vessel density measurement were 4.9% and 2.1–3.5% for central subfield and paracentral subfields respectively in healthy eyes on 3 × 3 mm scans, which were better (lower) than the corresponding data of 6.8% and 3.4–4.7% respectively in eyes with retinal diseases. Similarly, the inter-visit CVs of the measurement were better (lower) in healthy eye than in eyes with retinal diseases (Table 3).
The intra-visit ICC for measurement of the whole macular area for normal controls and diseased eyes on 3mm × 3mm scans was 0.3 and 0.8, and on 6mm × 6mm scans it was 0.7 and 0.7 respectively. The inter-visit ICC for the whole macular measurement for normal and diseased eyes was 0.3 and 0.8 on 3mm × 3mm scans and 0.5 and 0.6 on 6mm × 6mm scans respectively. The ICCs tended to be substantially higher for eyes with retinal diseases than healthy eyes, both for the whole macular area measurement and for each subfields, likely reflecting greater variability in the range of macular vessel densities among eyes with retinal diseases. In addition, 3mm × 3mm scans tended to yield higher ICC measurements than corresponding 6mm × 6mm scans, particularly for diseased eyes. (Table 4)
Table 4.
Intra-visit and inter-visit intraclass correlation for measurement of vessel density in healthy eyes and eyes with retinal diseases
| Intra-visit intraclass correlation | Inter-visit intraclass correlation | ||||||
|---|---|---|---|---|---|---|---|
| Scan Size | Sector | Normal eyes (%) | Diseased eyes (%) | P value | Normal eyes (%) | Diseased eyes (%) | P value |
| 3×3 mm | Whole macular area | 0.3 | 0.8 | <0.001 | 0.3 | 0.8 | <0.001 |
| Central Fovea | 0.9 | 0.8 | 0.36 | 0.8 | 0.8 | 0.44 | |
| Para-foveal circle | 0.2 | 0.8 | <0.001 | 0.1 | 0.8 | <0.001 | |
| Para-fovea_superior hemifield | 0.1 | 0.9 | <0.001 | 0.1 | 0.8 | <0.001 | |
| Para-fovea_inferior hemifield | 0.3 | 0.7 | <0.001 | 0.2 | 0.7 | <0.001 | |
| Para-fovea_temporal sector | 0.4 | 0.7 | <0.001 | 0.4 | 0.7 | <0.001 | |
| Para-fovea_superior sector | 0.1 | 0.9 | <0.001 | 0.1 | 0.8 | <0.001 | |
| Para-fovea_nasal sector | 0.3 | 0.8 | <0.001 | 0.2 | 0.7 | <0.001 | |
| Para-fovea_inferior sector | 0.2 | 0.8 | <0.001 | 0.1 | 0.8 | <0.001 | |
| 6×6 mm | Whole macular area | 0.7 | 0.7 | 0.42 | 0.5 | 0.6 | 0.21 |
| Central Fovea | 0.8 | 0.8 | 0.37 | 0.7 | 0.6 | 0.08 | |
| Para-foveal circle | 0.3 | 0.7 | <0.001 | 0.1 | 0.6 | <0.001 | |
| Para-fovea_superior hemifield | 0.2 | 0.8 | <0.001 | 0.1 | 0.7 | <0.001 | |
| Para-fovea_inferior hemifield | 0.4 | 0.6 | 0.003 | 0.2 | 0.5 | <0.001 | |
| Para-fovea_temporal sector | 0.0 | 0.8 | <0.001 | 0.0 | 0.6 | <0.001 | |
| Para-fovea_superior sector | 0.4 | 0.7 | <0.001 | 0.2 | 0.7 | <0.001 | |
| Para-fovea_nasal sector | 0.4 | 0.6 | 0.003 | 0.2 | 0.6 | <0.001 | |
| Para-fovea_inferior sector | 0.4 | 0.6 | 0.02 | 0.2 | 0.4 | 0.002 | |
Discussion
OCTA as a novel noninvasive imaging technology has enormous potential for assessing retinal vessels both in research and clinical settings. Assessment of its reproducibility is essential to determine its reliability for its application in clinical management and for research. The current study showed good intra-visit and inter-visit reproducibility for measurement of superficial macula vessel density, with most of the CVs lower than 5%. In addition, the reproducibility tended to be better in healthy subjects compared to patients with retinal diseases, better in paracentral area compared to central foveal area and better on 3mm × 3mm scans compared to 6mm × 6mm scans. The results also showed the superficial vessel density in eyes with retinal diseases (mostly non-proliferative diabetic retinopathy with mild or no macular edema) were lower compared to normal eyes.
The worse reproducibility for measurement of superficial vessel density in central foveal area compared to paracentral area might be related with the central foveal avascular zone (FAZ). Due to FAZ, the vessels only exist in periphery of the central measuring circle (figure 1). Any mild change of the measuring circle center location will induce substantial variation on the vessel density value, which was defined as the proportion of vessel area with flowing blood over the total area measured. In addition, the FAZ size and/or shape, which varied individually, also influence the vessel density measurement for the central fovea region. On the other hand, the measurement of paracentral subfields would not be affected by the FAZ. And the FAZ has a less effect on the measurement of the whole macular area, for which the denominator is bigger than the central subfield. This result suggests that when assessing the macular vessel density, the measurement of paracentral area or the whole macular area is more reliable.
Quantitatively measuring the macular microcirculation is clinically important, as even mild microcirculation changes may lead to pathological damage.21 Small vessel changes have been demonstrated in multiple retinal vascular diseases, including, diabetic retinopathy, macular telangiectasia and radiation retinopathy.22–24 Several methods have been used for evaluating retinal circulation, such as fluorescein angiography, Retinal Vessel Analyzer, Bidirectional laser Doppler velocimetry, Laser Doppler flowmetry and laser speckle flowgraphy, the blue-field simulation technique and color Doppler imaging.25 Each of these methods had its own limitations. For example, fluorescein angiography is limited by invasiveness, absence of quantitative measurements, and resolution of flow limited to large vessels. The Retinal Vessel Analyzer is mainly used for retinal vessel diameter measurement by analyzing the brightness profile of the vessel. The Bidirectional Laser Doppler Velocimetry can measure the blood flow velocity, however, it is time costing and not very accurate in small branch vessels.25
The current study demonstrates that OCTA provides good reproducibility and is a reliable method for assessing macular microcirculation in superficial capillaries both in healthy eyes and eyes with retinal diseases. Good reproducibility and repeatability of foveal avascular zone measurements in superficial network layer in healthy subjects by OCTA has been recently reported.26,27 Carpineto et al. assessed 60 healthy eyes and reported the coefficients of variations of 1.83% (95% CI 1.51% to 2.20%) and 1.86% (95% CI 1.33% to 2.43%) for the first and second observers, respectively.26 Shahlaee et al. measured 17 healthy subjects and found inter-observer agreement was high for all superficial FAZ measurements (ICC ≥0.90) but did not meet the lowest acceptable grader agreement for the deep vascular network (ICC <0.85). 27 Here, we showed that the intra- and inter-visit reproducibility of measuring the vessel density in the paracentral areas is better relative to the central foveal area.
There are several limitations of current study. Firstly the study was limited by only assessing the reproducibility of measurement of superficial network vessel density without analyzing the deep layer network. However, although the OCTA can image separately the superficial and deep capillary plexuses, the quantitative measurement of deep retinal vascular networks is limited by projection artifacts.28 A recent study shows that in 68% of cases, the image of the superficial network variably superimposed on the deep capillary plexuses, interfering with its analysis.29 Therefore, when doing quantitative analysis, focusing on the superficial network is technically more accurate. The second limitation of the current study is the relative small sample size and the difference in age between healthy controls and patients with retinal diseases.
The evidence presented here demonstrates good reproducibility of OCTA for measurement of superficial macula vessel density in both healthy eyes and eyes with retinal diseases. In addition, reproducibility tends to be better in healthy subjects compared to retinal diseases patients, better in parafoveal area compared to central foveal area, and intra-visit reproducibility tended to be better than inter-visit reproducibility.
Footnotes
Financial disclosure
The study was supported in part by UCSD Vision Research Center Core Grant P30EY022589, an unrestricted grant from Research to Prevent Blindness, NY (WRF); ICO-Retina Research Foundation Helmerich Fellowship, Beijing Talents Fund (2015000021223ZK22) and National Natural Science Foundation of China (No. 81400422) (QSY)
Qi Sheng You, William R. Freeman, Patricia Isabel C. Manalastas, and Luke J. Saunders have nothing to disclose.
Linda Zangwill: Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc. Quark (F); Carl Zeiss Meditec Inc, Optovue Inc. (R)
Robert N. Weinreb: Alcon, Allergan, Bausch+Lomb, Forsight, Topcon (C); Heidelberg Engineering, Carl Zeiss Meditec, Genentech, Konan, National Eye Institute, Neurovision, Optovue, Quark, Tomey, Topcon (F); Carl Zeiss Meditec (R)
Eric Nudleman: Allergan (C), Visunex Medical Systems (C)
References
- 1.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, Zang E. Fluorescein angiography complication survey. Ophthalmology. 1986 May;93(5):611–7. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 2.Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995 Jun;6(3):25–32. doi: 10.1097/00055735-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express. 2006 Aug 21;14(17):7821–40. doi: 10.1364/oe.14.007821. [DOI] [PubMed] [Google Scholar]
- 4.Fingler J, Schwartz D, Yang C, Fraser SE. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt Express. 2007 Oct 1;15(20):12636–53. doi: 10.1364/oe.15.012636. [DOI] [PubMed] [Google Scholar]
- 5.Mariampillai A, Standish BA, Moriyama EH, Khurana M, Munce NR, Leung MK, Jiang J, Cable A, Wilson BC, Vitkin IA, Yang VX. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008 Jul 1;33(13):1530–2. doi: 10.1364/ol.33.001530. [DOI] [PubMed] [Google Scholar]
- 6.An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express. 2008 Jul 21;16(15):11438–52. doi: 10.1364/oe.16.011438. [DOI] [PubMed] [Google Scholar]
- 7.Makita S, Jaillon F, Yamanari M, Miura M, Yasuno Y. Comprehensive in vivo micro-vascular imaging of the human eye by dual-beam-scan Doppler optical coherence angiography. Opt Express. 2011 Jan 17;19(2):1271–83. doi: 10.1364/OE.19.001271. [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa K, Sasaki K, Makita S, Hong YJ, Yasuno Y. Three-dimensional retinal and choroidal capillary imaging by power Doppler optical coherence angiography with adaptive optics. Opt Express. 2012 Sep 24;20(20):22796–812. doi: 10.1364/OE.20.022796. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012 Feb 13;20(4):4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, Flaxel CJ, Lauer AK, Wilson DJ, Hornegger J, Fujimoto JG, Huang D. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015 May 5;112(18):E2395–402. doi: 10.1073/pnas.1500185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaide RF, Fujimoto JG, Waheed NK. Optical Coherence Tomography Angiography. Retina. 2015 Nov;35(11):2161–2. doi: 10.1097/IAE.0000000000000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014 Jul;121(7):1435–44. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG, Huang D. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014 Jul;121(7):1322–32. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY OF TIME COURSE OF CHOROIDAL NEOVASCULARIZATION IN RESPONSE TO ANTI-ANGIOGENIC TREATMENT. Retina. 2015 Nov;35(11):2260–4. doi: 10.1097/IAE.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015 Jan;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 16.Bradley PD, Sim DA, Keane PA, Cardoso J, Agrawal R, Tufail A, Egan CA. The Evaluation of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016 Feb 1;57(2):626–631. doi: 10.1167/iovs.15-18034. [DOI] [PubMed] [Google Scholar]
- 17.Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016 Jan;21:1–7. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chidambara L, Gadde SG, Yadav NK, Jayadev C, Bhanushali D, Appaji AM, Akkali M, Khurana A, Shetty R. Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br J Ophthalmol. 2016 Jan 28; doi: 10.1136/bjophthalmol-2015-307941. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012 Jun 1;3(6):1182–99. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus MF, Liu JJ, Schottenhamml J, Chen CL, Budai A, Branchini L, Ko T, Ishikawa H, Wollstein G, Schuman J, Duker JS, Fujimoto JG, Hornegger J. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed Opt Express. 2014 Jul 11;5(8):2591–613. doi: 10.1364/BOE.5.002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 22.Sakata K, Funatsu H, Harino S, Noma H, Hori S. Relationship between macular microcirculation and progression of diabetic macular edema. Ophthalmology. 2006;113:1385–1391. doi: 10.1016/j.ophtha.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Chin EK, Kim DY, Hunter AR, et al. Staging of macular telangiectasia: power-Doppler optical coherence tomography and macular pigment optical density. Invest Ophthalmol Vis Sci. 2013;54:4459–4470. doi: 10.1167/iovs.12-11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veverka KK, AbouChehade JE, Iezzi R, Jr, Pulido JS. NONINVASIVE GRADING OF RADIATION RETINOPATHY: The Use of Optical Coherence Tomography Angiography. Retina. 2015 Nov;35(11):2400–10. doi: 10.1097/IAE.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 25.Pournaras CJ, Riva CE. Retinal blood flow evaluation. Ophthalmologica. 2013;229(2):61–74. doi: 10.1159/000338186. [DOI] [PubMed] [Google Scholar]
- 26.Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2015 Sep 16; doi: 10.1136/bjophthalmol-2015-307330. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of Foveal Avascular Zone Dimensions and its Reliability in Healthy Eyes Using Optical Coherence Tomography Angiography. Am J Ophthalmol. 2016 Jan;161:50–55. doi: 10.1016/j.ajo.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Spaide RF, Fujimoto JG, Waheed NK. IMAGE ARTIFACTS IN OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina. 2015 Nov;35(11):2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnin S, Mané V, Couturier A, Julien M, Paques M, Tadayoni R, Gaudric A. NEW INSIGHT INTO THE MACULAR DEEP VASCULAR PLEXUS IMAGED BY OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina. 2015 Nov;35(11):2347–52. doi: 10.1097/IAE.0000000000000839. [DOI] [PubMed] [Google Scholar]