Summary
Chrysomya megacephala is a saprophagous fly whose larvae can compost manure and yield biomass and bio‐fertilizer simultaneously. However, there are concerns for the safety of the composting system, that is risk of diseases spread by way of manure pathogens, residue of harmful metals and emission of greenhouse gases. Microbiota analysis and heavy metal speciation by European Communities Bureau of Reference were evaluated in raw, C. megacephala‐composted and natural stacked swine manure to survey pathogenic bacterial changes and mobility of lead and cadmium in manure after C. megacephala feeding; the emission rate of CH4 and N2O from manure during C. megacephala composting and natural stacking was also measured. C. megacephala composting altered manure microbiota, reduced the risk of pathogenic bacteria and maintained the stability, and microbiota changes might be associated with heavy metal fractions, especially in Pseudomonas and Prevotella. In addition, C. megacephala‐composting significantly reduced the emission rate of CH4 and N2O in comparing with natural stacking situation and the first two days should be the crucial period for CH4 and N2O emission measurement for manure treatment by C. megacephala. Moreover, OTU26 and Betaproteobacteria were changed after C. megacephala composting which might play a role in emission of CH4 and N2O, respectively.
Introduction
According to the Food and Agriculture Organization, the production of livestock increases annually at a rapid rate worldwide (http://www.fao.org/home/en/). It was estimated that over 110 billion livestock will be cultivated and slaughtered worldwide by 2050 and the production of pig/swine had reached nearly 1 billion head in 2013 which ranked top fourth among livestock (Ilea, 2009; Zhu and Hiltunen, 2016). It provides the main sources of animal protein in China along with large amounts of manure to be processed (Zheng et al., 2014). Therefore, it has long become a problem for developing countries to manage swine manure quickly while combining safety, environmental protection and high efficiency (He et al., 2016).
Saprophagous insects could consume swine manure and provide high added‐value products, such as feedstuff, biodiesel and fertilizer (Cickova et al., 2015). Chrysomya megacephala is a new saprophagous blowfly for transforming kitchen wastes and manure from livestock farms. Larvae of C. megacephala are high energy and might be used for providing protein, fat and biodiesel (Li et al., 2012). An ideal processing flow was suggested for integration with swine farm; manure transformation of C. megacephala would provide win–win profits for both the economy and the environment (Yang and Liu, 2014). However, urgent precautions have been proposed to evaluate potential health risks during the manure management (Committee, 2015), such as the disease‐causing microorganisms, potential heavy metal contamination and greenhouse gas (GHG) emissions.
The pathogenicity of manure has raised concerns for a long time. How bacterial communities, especially for potential pathogenic bacteria, change during composting has always been of interest. Recently, metagenomic 16S rRNA sequencing is a powerful method to explore diversity and structure of microbial communities (Logares et al., 2014) which could be applied in diverse ecological and biological niches (Finney et al., 2015). In addition, many bacteria are associated with heavy metal absorption, management and greenhouse gas emissions. For example, Pseudomonas putida KT2440 could tolerate heavy metals and metalloids (Cánovas et al., 2003), and the poultry industry is responsible for methane emission to a certain extent, such as rumen digestion in ruminant (Snelling and Wallace, 2017).
Heavy metals in the manure have generated concern because they cannot be decomposed (Song et al., 2014; Lv et al., 2016). Direct land application or composted application of heavy metal contaminative manure might lead further pollution to soil. Bioactivity and mobility of heavy metals could be evaluated by sequential extraction methods (Ptistišek et al., 2001). The BCR (European Communities Bureau of Reference) method is one of the most commonly used methods for heavy metal speciation (Kede et al., 2014; Razek, 2014). Heavy metals are more difficult to be extracted which indicates that they are more settled, less available and have a lower risk to organisms.
Moreover, GHG emissions should be taken into account for a clean manure management system to avoid a secondary pollution. The GHG, carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) were key factors for global warming (Solomon et al., 2010). According to the FAO livestock holding for only 9% of global CO2 emission, however, it represents approximately 35% of CH4 and 60% of N2O, respectively. Moreover, these two non‐carbon dioxide greenhouse gases (NCGGs) have 23 and 300 times than the global warming potential of CO2, respectively (FAO, 2014). The manure management would release CH4 and N2O during digestion and decomposition, and the production rate could be adjusted by manure storage and handling methods (Paik et al., 2016).
Therefore, to evaluate the three possible environment risks during C. megacephala manure composting: microbiota changes, the activity of lead (Pb) and cadmium (Cd), the emission rate of methane (CH4) and nitrous oxide (N2O) during manure handling process was evaluated to assure how C. megacephala feeding would influence the pathogenic microbiota of manure; the stability of Pb and Cd and the emission rate of CH4 and N2O were compared with those of natural stacked manure treatment. These results would promote the utilization of insects to consume organic wastes and provide valuable, available and potential strategies for risk reduction of mobile heavy metals and harmful microbes.
Results
C. megacephala composting Altered Manure Microbiota
On average, approximately 33 000 raw reads per sample were obtained and approximately 32 000 clean reads were obtained with a good read utilization ratio of about 96% per sample. All clean reads were connected to 280 562 tags with a fine connect ratio over 99%. In total, 1162 OTUs were obtained (Table 1, Table S1). First, after C. megacephala composting, the count of dominating microorganism changed; 290 OTUs were shared commonly among RSM, NSM and CMSM, and 153, 91 and 228 OTUs were found specifically in RSM, NSM and CMSM, respectively (Fig. 1A, Table S2). In total, 35 known genera were assigned and their relative taxonomic abundance was estimated by a histogram (Fig. 1B). Microbiota of CMSM varied greatly compared with that of RSM according to apparent colour patterns. After deeply digging, the relative abundance of genera Acholeplasma, Alcaligenes, Fibrobacter, Flavobacterium, Ignatzschineria, Pseudidiomarina and Pseudomonas increased dramatically in CMSM compared with RSM. Among the increased genera, Pseudomonas was the most abundant (Fig. 1C). However, bacterial species of Pseudomonas were not well identified in the different abundance analysis at the species level. On the other hand, the relative abundance of genera Acinetobacter, CF231, Lactobacillus, Megasphaera, Oscillospira, Phascolarctobacterium, Prevotella, Streptococcus and Treponema decreased sharply. The relative abundance of Prevotella in the RSM decreased from approximately 35% down close to zero (Fig. 1D). Second, after C. megacephala composting, species composition of manure altered. Alpha diversity in bacterial communities of RSM, NSM and CMSM was calculated by five methods (Fig. 2). Observed species (P = 0.02732), Chao (P = 0.03899) and ace (P = 0.02732) indicated that a significant difference was observed among the three groups. However, Shannon's diversity (P = 0.05091) and Simpson diversity (P = 0.06081) indicated that no significant difference was observed. Generally, C. megacephala played a role in bacterial community changes during swine manure composting. Beta diversity distance showed similar results, which are listed in Table S3. Common Salmonella sp. are faecal indicator bacteria (Mantha et al., 2017) and were significantly reduced after C. megacephala‐treated swine manure (Fig. 3).
Table 1.
Sample information and number of sequences obtained
| Sample name | Raw reads | Clean reads | Utilization ratio of reads (%) | Tags | Connect ratio (%) | OTUs |
|---|---|---|---|---|---|---|
| RSM.1 | 33 831 | 31 558 | 93.28 | 31 332 | 99.28 | 614 |
| RSM.2 | 33 718 | 31 494 | 93.4 | 31 278 | 99.31 | 633 |
| RSM.3 | 34 250 | 31 614 | 92.3 | 31 422 | 99.39 | 616 |
| NSM.1 | 33 574 | 31 454 | 93.69 | 31 176 | 99.12 | 546 |
| NSM.2 | 33 254 | 31 702 | 95.33 | 31 459 | 99.23 | 573 |
| NSM.3 | 33 696 | 31 461 | 93.37 | 31 208 | 99.20 | 537 |
| CMSM.1 | 32 455 | 31 314 | 96.48 | 31 083 | 99.26 | 388 |
| CMSM.2 | 32 278 | 30 993 | 96.02 | 30 786 | 99.33 | 444 |
| CMSM.3 | 32 206 | 31 057 | 96.43 | 30 818 | 99.23 | 470 |
Figure 1.
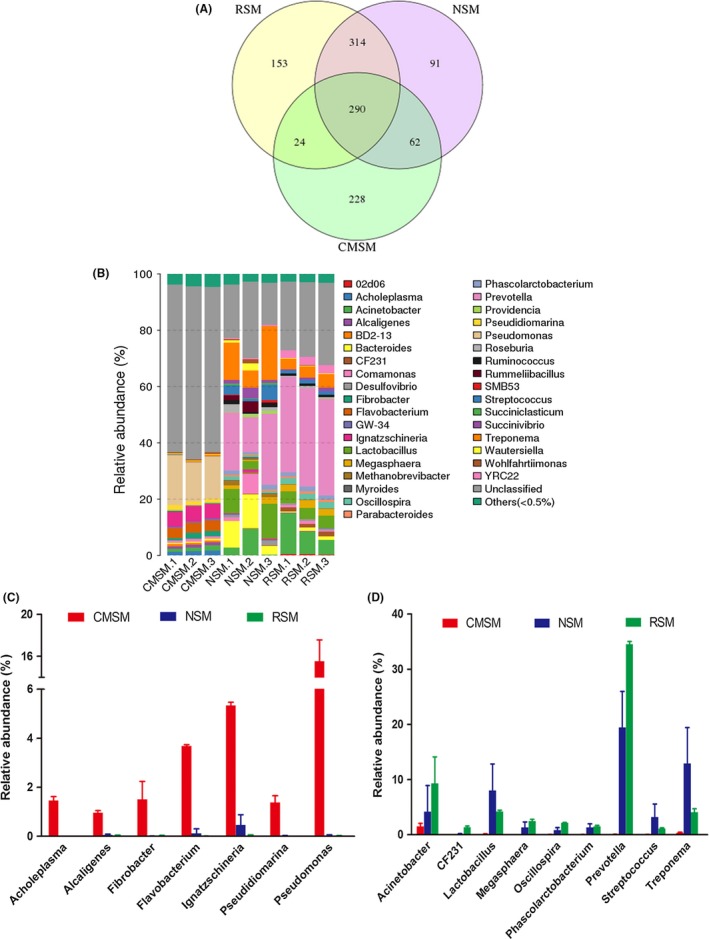
Comparison of swine manure microbiota. A. Venn diagram of shared OTUs of RSM, NSM and CMSM with 97% similarity.
B. Microbial composition of RSM, NSM and CMSM at the genus level. The top 37 abundant genera are shown by relative abundance of each bacterial genus within a group. C. The increased genera of CMSM compared with RSM. D. The decreased genera of CMSM compared with RSM. Each bar is the mean with SD from three replicates.
Figure 2.
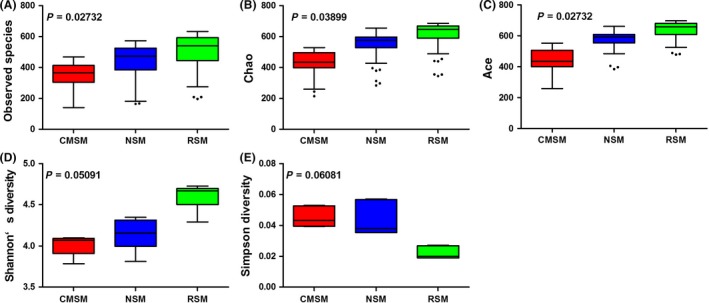
Alpha diversity analysis of RSM, NSM and CMSM. A. Observed species. B. Chao. C. Ace. D. Shannon's diversity. E. Simpson diversity. The five lines of boxplot from bottom to top are the minimum value, the first quartile, median, the third quartile and the maximum value.
Figure 3.
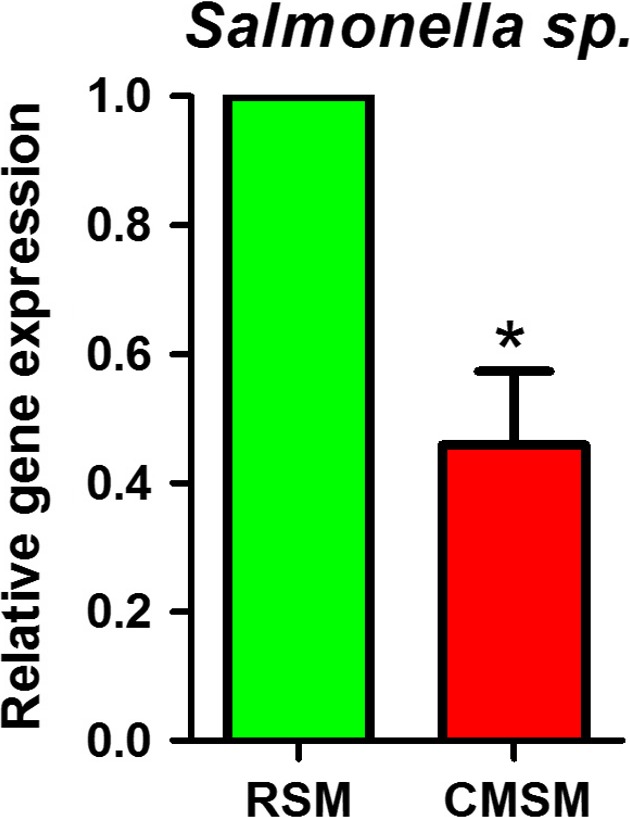
Salmonella sp. quantification by PCR. Independent samples test is performed and the asterisk (*) indicates that a significant difference is detected (P < 0.05).
C. megacephala composting maintained heavy metal stability
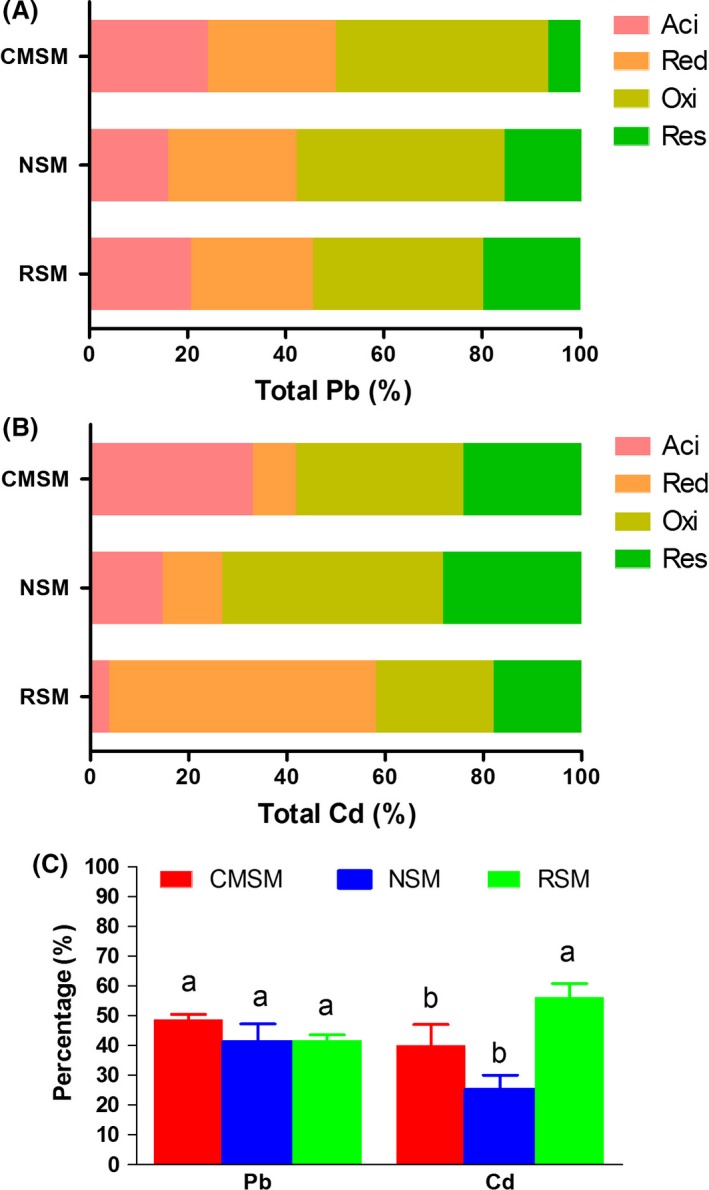
Fractions of metals did not vary significantly after C. megacephala composting. Pb and Cd speciation of RSM, NSM and CMSM was shown as four fractions, and the mobility factor was calculated accordingly. Speciation of Cd changed more than that of Pb (Fig. 4A and B). Although no significant difference was detected in the mobility of Pb, the active fractions of Pb increased to a certain extent, while the mobility of Cd decreased during C. megacephala composting and natural stack conditions (Fig. 4C).
Figure 4.
Heavy metal speciation comparison of swine manure.
A. Speciation of Pb in RSM, NSM and CMSM. Each bar indicates an average value. Four steps were conducted for the sequential extraction of acid‐soluble fraction‐bound carbonates (Aci), reducible fraction‐bound Fe and Mn oxides (Red), oxidizable fraction‐bound organic matter and sulphides (Oxi) and residual fraction strongly associated with the crystalline structures of the minerals (Res), respectively. B. Speciation of Cd in RSM, NSM and CMSM. Each bar indicates an average value. Abbreviation of Cd speciation is the same as Pb. C. Comparisons of the mobility factor of Pb and Cd in RSM, NSM and CMSM. Each bar indicates a value (mean with SD). The mobility factor (MF) was calculated as the percentage of heavy metal in Aci+Red fractions to the total contents to evaluate the potential mobility of the heavy metals. The LSD test was performed, and different letters indicate that a significant difference is detected (P < 0.05).
A PCA plot showed that Pb and Cd speciation of NSM and CMSM was similar. Pb and Cd fractions of swine manure were divided into different groups. Cd made a greater contribution than Pb did in the first two axes. C. megacephala composting affected the ResCd and OxiCd the most (Fig. 5). The correlation analysis of Pseudomonas, Prevotella and heavy metal speciation was viewed by a matrix graph (Fig. 6). A significant correlation was detected in Pseudomonas and ResPb (r = −0.74478, P = 0.02132), Pseudomonas and AciCd (r = 0.87815, P = 0.00184), Pseudomonas and OxiCd (r = 0.77825, P = 0.01351), Prevotella and ResPb (r = 0.81667, P = 0.00722), Prevotella and AciCd (r = −0.69457, P = 0.03786), and Prevotella and OxiCd (r = −0.66667, P = 0.04987). It seems that the correlations between heavy metal speciation and Pseudomonas and Prevotella were different based on opposite colour view. Moreover, heavy metal fractions ResPb, AciCd and OxiCd are consistent with the results of Fig. 6 and Fig. S2. The genus Pseudomonas was not refined to the species level in this study, while the genus Prevotella refined to Prevotella copri and Prevotella stercorea. The correlation matrix graph of heavy metal speciation and Prevotella copri/Prevotella stercorea are shown in Table S4, 5. A significant correlation was detected in Prevotella copri and ResPb (r = 0.88333, P = 0.00159), Prevotella copri and AciCd (r = −0.81172, P = 0.00789), Prevotella stercorea and ResPb (r = 0.93234, P = 2.48E‐04), Prevotella stercorea and AciCd (r = −0.92774, P = 3.11E‐04), and Prevotella stercorea and OxiCd (r = −0.74587, P = 0.02103). Except for Prevotella copri and Prevotella stercorea, different abundance of other bacterial species is shown in Table S6.
Figure 5.
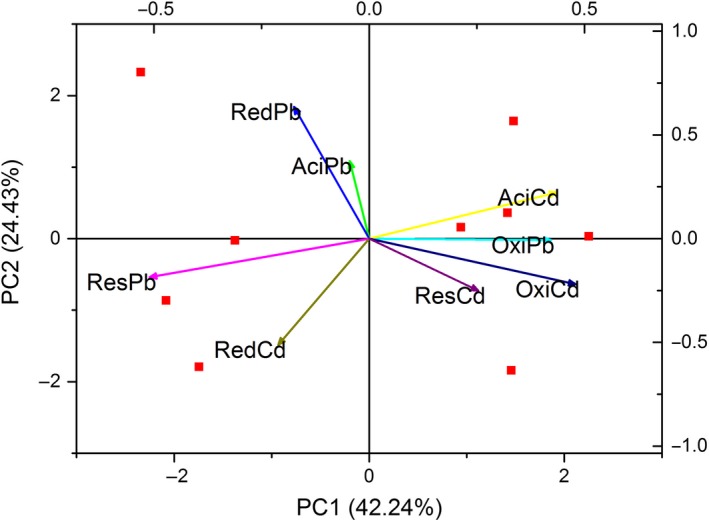
Principal component analysis of swine manure in heavy metal speciation.
Figure 6.
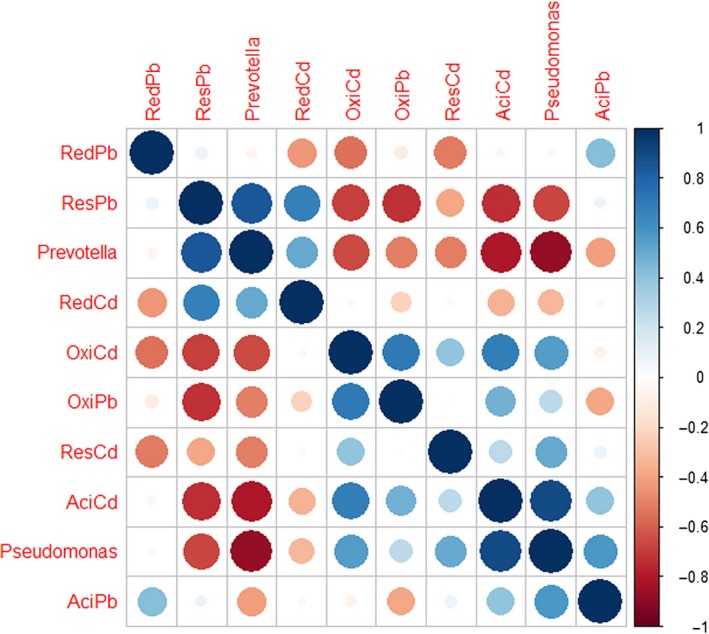
Correlation coefficient matrix of genera Prevotella, Pseudomonas and heavy metal speciation.
C. megacephala composting facilitated GHG emission management
The emission of CH4 from CMSM and NSM is shown in Fig. 7A. The first 2 days of CH4 emissions from CMSM and NSM had a remarkable rise. After that, the CH4 emission rate showed different decreasing trends. The emission of CH4 from CMSM on day (D) 3 dropped considerably, then kept a decreasing trend and almost reached zero on D6. However, the emission of CH4 from NSM kept on a relative high level with a slightly decreasing trend. Moreover, on D6 of NSM, it still had an almost equal rate on D1 with the original manure. The N2O emission of CMSM and NSM is shown in Fig. 7B. The emission of N2O from both NSM and CMSM happened on D1. Moreover, the emission rate of N2O from NSM was almost two times than that of CMSM. On later days, the emission rate from CMSM was undetected indicating that the N2O emissions might not happen. Meanwhile, the emission from NSM dropped down to almost zero on D2 and then fluctuated at a relative low rate. In summary, the emission rate of CH4 was much higher than that of N2O and the emission of CH4 and N2O was lower in CMSM than in NSM. The first 2 days were the emission peak. Taking the clean production of the C. megacephala composting system into consideration, the first 2 days of composting were a critical period to take steps.
Figure 7.
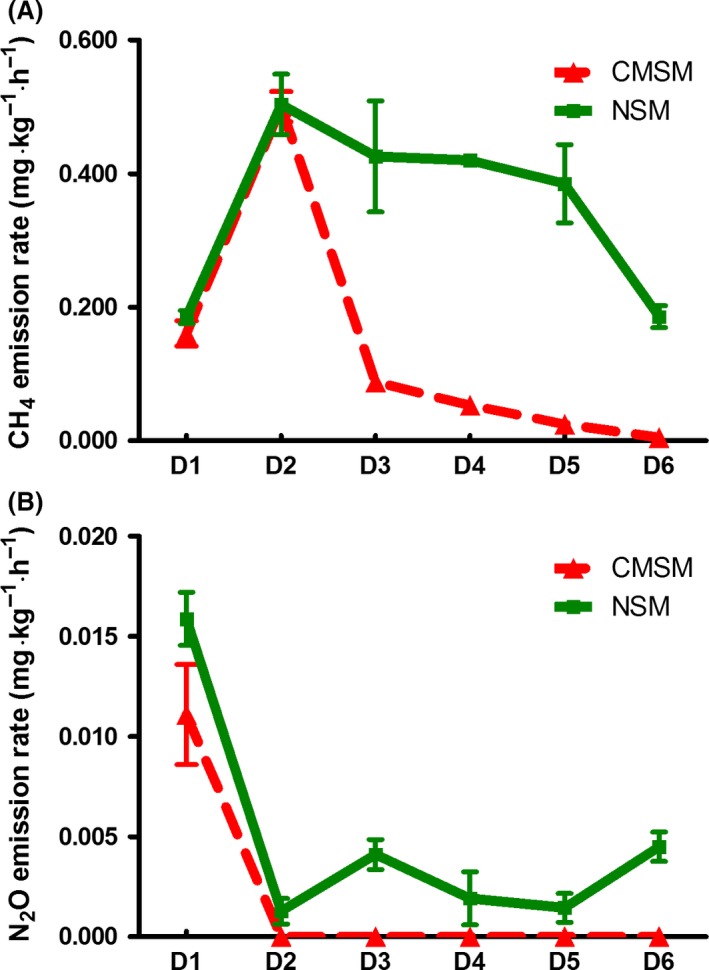
Emission rate of greenhouse gas from swine manure during C. megacephala and natural composting. A. Emission rate of CH 4; B. Emission rate of N2O.
GHG‐Associated manure microbiota
CH4 and N2O‐associated bacterial changes before and after C. megacephala composting are shown in Fig. 8. OTU26, OTU984 and OTU624 were clustered as methanobacteria which belong to Archaea and contribute to metabolize CH4. OTU26 counts the most times, and nearly none were detected after C. megacephala composting (Fig. 8A). Nitrification and denitrification are two major processes to contribute to N2O emissions which take effect under aerobic and anaerobic conditions, respectively (Rogers and Whitman, 1994). Ammonia oxidation is a critical process of nitrification, and ammonia oxidizing bacteria (AOB) are mostly from class Betaproteobacteria (Arp and Stein, 2003). The relative abundance of Betaproteobacteria was significantly increased after C. megacephala composting (Fig. 8B). Methanogens, Methanomassiliicoccaceae and Methanobrevibacter are three methanogenic bacteria (Duarte et al., 2017), and all of them were detected to have a significantly lower expression in CMSM than RSM which indicated that C. megacephala composting might reduce the methane emission by inhibiting methanogenic bacteria (Fig. 8C).
Figure 8.
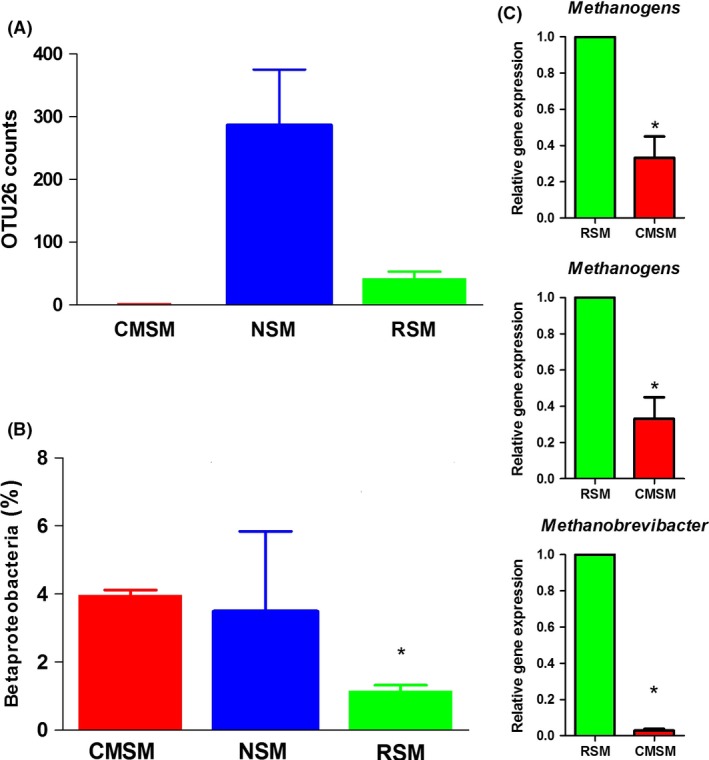
Changes in CH 4 and N2O‐associated bacteria. A. The counts of OTU26 in RSM, NSM and CMSM. B. The relative abundance of Betaproteobacteria in RSM, NSM and CMSM. C. Methanogenic bacterial quantification by relative gene expression. Independent samples test is performed and the asterisk (*) indicates that a significant difference is detected (P < 0.05).
Sequence deposit
The raw pyrosequencing and Illumina read data for all samples have been deposited to the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under accession number SRP072077.
Discussion
After C. megacephala composting, the bacterial composition of swine manure changed which might have a double effect on manure bacterial‐related disease. The diversity of CMSM was markedly lowered compared with that of RSM. Accordingly, on one side, 19 bacterial species of CMSM were found to be lower than that of RSM. Among them, 10 species reached a significant level. Most of the decreased species were gut microbes. Using genus Prevotella as an example, Prevotella copri and Prevotella stercorea were identified to decrease to near zero in CMSM compared to RSM (Fig. S1). Prevotella copri and Prevotella stercorea are animal gut microbes that were first isolated from human faeces (Hayashi et al., 2007). Prevotella copri and Prevotella stercorea were related to rheumatoid arthritis and carcinoma in adenoma, respectively (Scher et al., 2013; Moreno, 2015; Kasai et al., 2016). On the other side, 15 species were identified to be more abundant in CMSM than in RSM. Among them, only Acholeplasma laidlawii, Alcaligenes faecalis and Flavobacterium gelidilacus reached a significant level. Acholeplasma laidlawii is a unique mycoplasma species that was widely distributed and related to environmental adaptation, ‘parasite‐host’ system and virulence realization (Lazarev et al., 2011; Chernov et al., 2012). Alcaligenes faecalis even cause peritonitis occasionally (Kahveci et al., 2011). The identified Alcaligenes faecalis strain MOR02 from Galleria mellonella larva is dangerous and pathogenic to larva as a result of toxic protein secretion and nematode cooperation (Quiroz‐Castañeda et al., 2015). Increased Alcaligenes faecalis in CMSM might originate from excretion or from dead bodies of C. megacephala. Notably, C. megacephala were well grown in swine manure. It could be speculated that C. megacephala is not sensitive or that it possesses corresponding strategies to fight back. Flavobacterium gelidilacus was isolated from microbial mats in Antarctic lakes and might have a similar function in consuming the high molecular mass fraction of dissolved organic matter (Stefanie et al., 2003). Moreover, Flavobacterium gelidilacus was not detected in RSM; Flavobacterium gelidilacus in NSM was also identified to be much less than in CMSM. Therefore, the feeding and movement of C. megacephala larvae might contribute in this respect, and role of Flavobacterium gelidilacus is worth further confirmation. Salmonella sp. was significantly reduced after C. megacephala larvae consuming. Salmonella sp. are faecal indicators in wastewater (Mantha et al., 2017), which suggested that C. megacephala composting might reduce the risk in Salmonella Enteritidis, a standout foodborne disease worldwide (Borges et al., 2017).
Based on the correlation analysis of Pseudomonas, Prevotella and heavy metal speciation (Fig. 6), it seems that the correlations between heavy metal speciation and Pseudomonas and Prevotella were different. Moreover, bacteria related to heavy metal speciation during C. megacephala composting were identified for potential application. Bacteria of genus Pseudomonas were of interest because of their high resistance to heavy metals and other toxicants (Pardo et al., 2003). Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas stutzeri were reported to have a high resistance to Pb and Zn (Ceylan and Aysel, 2012). Pseudomonas fluorescens strain ZY2 isolated from swine wastewater had a cross‐resistance to Pb and some antibiotics (Zhou et al., 2015). Pseudomonas putida combined with humic acid had a higher Cd sorption; Cd was preferentially bonded to the bacteria in the ternary clay mineral‐humic acid‐bacteria higher affinity composite (Du et al., 2016). Bacteria of Pseudomonas also performed well in dealing with other heavy metals. For example, Pseudomonas sp. JF122, Pseudomonas gessardii strain LZ‐E and Pseudomonas taiwanensis would be a potential applicant for chromium (Cr) remediation (Huang et al., 2016; Majumder et al., 2016; Zhou and Chen, 2016). Prevotella is a genus of Gram‐negative bacteria, which is closely related to various infections as introduced above (Könönen et al., 1998). No studies have shown that Prevotella contributes to heavy metal speciation changes during composting. It might be possible that the decrease in Prevotella relative abundance might be derived from the increase in other bacteria, for example Pseudomonas. There was no definite conclusion on the associations between bacteria and heavy metal speciation.
People are concerned about the safety of manure bio‐fertilizer because of possible metal accumulation and transferring. For example, short‐term fertilization with pig manure‐based compost increased the content of Pb and Cd in soil (Tian et al., 2015). Moreover, the Cd content in plants was positively correlated with Cd‐contaminated soils, indicating that risks would occur with Cd transfer (Nookabkaew et al., 2016). Heavy metals which are extracted difficultly from substances indicate that they are unlikely to be transferred into environment and might reduce toxic risks in entering organisms. The more difficult to be extracted, more difficult to be transferred and less mobile and lower risky for a heavy metal. According to Fig. 4, Cd in CMSM seems to be easier to migrate from swine manure into plants than Pb because the percentage of ResCd was lower than that of Pb. Similar results were found in the contaminated soils that Cd was more labile than Pb (Ptistišek et al., 2001; Kede et al., 2014). However, we were pleased to see that C. megacephala maintained the stability of containing metals in manure which are acceptable in environment. What we have to control when using C. megacephala to transform manure is the original content of heavy metals because some manure might contain a higher value of heavy metals than permitted (Zhang et al., 2012).
We concluded that the first 2 days would be the crucial processing period for GHG emission management which might result from the decrease in methanogenic bacteria. Furthermore, another merit of C. megacephala swine manure composting is that it lasted for a short time. In the long term of swine manure composting, the emission peak of CH4 occurred immediately after swine manure was piled up, and the N2O occurred around the middle stage of the composting period and thus had a long gas control term as well (Fukumoto et al., 2003). Centralized control measures of C. megacephala swine manure composting could reduce the maintenance of device and energy consumption. The full‐scale bio‐filter with rock wool mixture that used in livestock manure composting might also be used in the construction of C. megacephala swine manure composting system (Yasuda et al., 2009). Another merit of C. megacephala swine manure composting is that it uses relatively less area of soil with shelf production system (Yang and Liu, 2014). Earthworms could also reduce the GHS emission during composting (Lim et al., 2016). However, in China, earthworms composting has a relatively longer period and larger land use than C. megacephala composting because earthworms composting is a continuous system and C. megacephala composting is batch model (Zhang et al., 2013).
Pilot‐scale biodegradation of swine manure via C. megacephala larvae for biodiesel production has been reported (Yang and Liu, 2014). The manure processing system in this study also used 6 kg as a treatment tank with more eggs to ensure that the manure was fully transformed. We also used manure from the same farm contained no sawdust with a moisture around 70%. The system was downsized in this study; however, the measured parameters should have reference values as reaction material proportion was not changed. Therefore, relative environmental parameters can help develop environmental protection measures in this pilot scale, especially in microbiological safety, heavy metal and greenhouse gas emission control.
In conclusion, C. megacephala composting altered manure microbiota, reduced the risk of pathogenic bacteria and maintained the stability, and microbiota changes might be associated with heavy metal fractions, especially in Pseudomonas and Prevotella. In addition, during C. megacephala composting of manure significantly reduced the emission rate of CH4 and N2O in comparing with natural stacking situation and the first 2 days should be the crucial period for CH4 and N2O emission measurement during C. megacephala composting. Moreover, OTU26 and Betaproteobacteria were changed after C. megacephala composting which might play a role in emission of CH4 and N2O, respectively.
Experimental procedures
Experiment design and swine manure sampling
Chrysomya megacephala was provided by Hubei International Cooperation Base for Waste Conversion by Insects, and swine manure was taken from the swine breeding farm of the Huazhong Agricultural University (HZAU). Adults of C. megacephala were reared in mesh cages (35 × 35 × 35 cm) with water and sugar, and the cages were kept in a rearing room at 25 ± 3°C under a 13:11‐h light: dark photoperiod. Eggs were first collected in a swine manure gauze bag by putting into cages for 4 h, and then, eggs were separated from the gauze. The manure was well mixed by electric blender as raw swine manure (RSM) and then applied for different treatments: C. megacephala‐transformed manure (CMSM) and natural stacked manure (NSM). On average, 6 kg of manure was applied for each treatment in plastic tanks. For CMSM treatment, eggs were loaded on manure in the proportion of 1.5 g eggs per kilogram manure. Before and after the conversion process, RSM, CMSM and NSM were sampled in 1.5‐ml sterile centrifuge tube and stored at −80°C for DNA extraction. Five tubes of different manure types were applied for DNA extraction and combined as one sample to ensure the samples to be representative. RSM, CMSM and NSM manure samples were also sampled, oven dried at 70°C and sieved for heavy metal speciation. During the conversion process, static closed chambers were also applied for measuring the emission rate of CH4 and N2O. Each experiment was conducted three times.
DNA extraction, PCR, pyrosequencing and bioinformatics analysis
Genomic DNA of manure samples was extracted by a TIANamp Stool DNA Kit (TIANGEN Biotech Beijing, China: DP328). After concentration and integrity testing, the genomic DNA for individual sample was normalized to 30 ng per PCR. The sequencing for bacterial variable V4 regions of the 16S rDNA gene was performed by BGI Tech (BGI Tech Solutions Co., Ltd., Wuhan, China) on the Illumina MiSeq platform with the 515f/806r primer set (515f: 5′‐GTG CCA GCM GCC GCG GTA A‐3′, 806r: 5′‐XXX XXX GGA CTA CHV GGG TWT CTA AT‐3′). Three replicates were conducted. Raw sequences of all samples were processed accordingly to obtain clean data by filtering out reads with sequencing adapters, N base, poly base and low quality (Fadrosh et al., 2014). Clean data of swine manure samples were applied for tag generation by FLASH and operational taxonomic units (OTUs) cluster analysis by USEARCH (Magoč and Salzberg, 2011; Edgar, 2013) to survey bacterial communities in natural composting and C. megacephala composting conditions. Then, OTUs classification, alignment of the representative sequence of each OTU, chimera removal, taxonomic assignment and alpha and beta diversity analyses were performed with QIIME (macQIIME 1.7) (Caporaso et al., 2010).
Quantitative PCR validation
SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) was used for quantitative PCR (Cat#: RR820A; Takara, Beijing, China). The reaction system was prepared as SYBR 5 μl; H2O 3.6 μl; primer F/R 0.2 μl; DNA template 1 μl (40 ng μl−1). The PCR was performed on QuantStudio 6 Flex Real‐Time PCR System (Life Technologies, Waltham, USA) following 40 cycles of 95°C 30 s; 95°C 5 s; 60°C 30 s. Primers of Methanogens, Methanomassiliicoccaceae, Methanobrevibacter, Salmonella sp. and 16S are listed in Table S7. The expression level was calculated by method with 16S as a reference gene.
Speciation and mobility assessment of heavy metal
The BCR method was applied to the swine manure (Cuong and Obbard, 2006). Four steps were conducted for the sequential extraction of acid‐soluble fraction‐bound carbonates (Aci), reducible fraction‐bound Fe and Mn oxides (Red), oxidizable fraction‐bound organic matter and sulphides (Oxi) and residual fraction strongly associated with the crystalline structures of the minerals (Res), respectively (Ptistišek et al., 2001; Cuong and Obbard, 2006). The content of lead (Pb) and cadmium (Cd) was measured with Agilent Technologies 240FS AA (USA). Moreover, the mobility factor (MF) was calculated as the percentage of heavy metal in Aci+Red fractions to the total contents to evaluate the potential mobility of the heavy metals (Lv et al., 2016). A permutation test of BCR speciation was conducted by SAS (USA). Principal component analysis (PCA) and correlation analysis were conducted with Origin 9.0. Figures were partly selected from R‐based reports of BGI Tech and partly made by GraphPad Prism 5.0 (GraphPad Software, La Jolla, USA).
GHG emission rate
The content of CH4 and N2O was measured with the gas chromatography (GC, Shimadzu GC‐14B) according to the relative peak area of the standard gas (Li et al., 2013). The fluxes of CH4 and N2O were calculated as described by the following equation:
where F denotes the emission of CH4 and N2O as mg kg−1 h−1; ρ denotes the CH4 (0.717 kg m−3) or N2O (1.978 kg m−3) density; dC∕dt indicates the accumulation rate of CH4 and N2O; V is the volume of the chamber on top; T is the number of mean temperature inside the chamber. The density of the standard gas used in this study for CH4 and N2O was 1.79 and 0.353 mg l−1, respectively. The preceding calculation process was referred from Zhang et al. (Zhang et al., 2015).
Conflict of interests
The authors declare that they have no conflict of interests.
Supporting information
Fig. S1 Relative abundance of Prevotella copri and Prevotella stercorea in RSM, NSM and CMSM.
Fig. S2 Correlation coefficient matrix of heavy metal speciation
Table S1 Taxonomy of OTUs.
Table S2 Detailed OTUs of RSM, NSM and CMSM in Venn diagram.
Table S3 Beta diversity distance of RSM, NSM and CMSM.
Table S4. Correlation matrix graph of heavy metal speciation and Prevotella copri (Pcop).
Table S5. Correlation matrix graph of heavy metal speciation and Prevotella stercorea (Pste).
Table S6. Different abundance of bacteria species in RSM, NSM and CMSM.
Table S7. Primers used for quantitative PCR.
Acknowledgements
This study was funded by EU‐funded project PROteINSECT (Grant No. 034082), the Fundamental Research Funds for the Central Universities (Grant No. 2014PY059) and HZAU‐funded Doctoral research project (Grant No. 2014bs01). We appreciated the help by BGI Tech (Wuhan, China) in the microbiota sequencing and further data analysis. We thanked Yao Zhao, Wenjuan Zhang and Wei Han for their assistance during experiments. We also thanked Mr. Jinping Wang and Tianqi Liu for their assistance in greenhouse gas measurement.
Microbial Biotechnology (2018) 11(3), 498–509
Funding Information
This study was funded by EU‐funded project PROteINSECT (Grant No.034082), the Fundamental Research Funds for the Central Universities (Grant No.2014PY059) and HZAU‐funded Doctoral research project (Grant No. 2014bs01).
References
- Arp, D.J. , and Stein, L.Y. (2003) Metabolism of inorganic N compounds by ammonia‐oxidizing bacteria. Crit Rev Biochem Mol 38: 471–495. [DOI] [PubMed] [Google Scholar]
- Borges, K.A. , Furian, T.Q. , de Souza, S.N. , Menezes, R. , Salle, C.T.P. , de Souza Moraes, H.L. , et al (2017) Phenotypic and molecular characterization of Salmonella enteritidis SE86 isolated from poultry and salmonellosis outbreaks. Foodborne Pathog Dis 14: 12. [DOI] [PubMed] [Google Scholar]
- Cánovas, D. , Cases, I. , and De, L.V. (2003) Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ Microbiol 5: 1242–1256. [DOI] [PubMed] [Google Scholar]
- Caporaso, J.G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F.D. , Costello, E.K. , et al (2010) QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov, V.M. , Chernova, O.A. , Mouzykantov, A.A. , Baranova, N.B. , Gorshkov, O.V. , Trushin, M.V. , et al (2012) Extracellular membrane vesicles and phytopathogenicity of Acholeplasma laidlawii PG8. The Scientific World J 2012: 315474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cickova, H. , Newton, G.L. , Lacy, R.C. , and Kozanek, M. (2015) The use of fly larvae for organic waste treatment. Waste Manage 35: 68–80. [DOI] [PubMed] [Google Scholar]
- Committee, E.S. (2015) Risk profile related to production and consumption of insects as food and feed. Efsa J 13: 4257. [Google Scholar]
- Cuong, D.T. , and Obbard, J.P. (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR‐sequential extraction procedure. Appl Geochem 21: 1335–1346. [Google Scholar]
- Du, H.H. , Chen, W.L. , Cai, P. , Rong, X.M. , Dai, K. , Peacock, C.L. , and Huang, Q.Y. (2016) Cd(II) sorption on montmorillonite‐humic acid‐bacteria composites. Sci Rep 6: 19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, A.C. , Durmic, Z. , Vercoe, P.E. , and Chaves, A.V. (2017) Dose‐response effects of dietary pequi oil on fermentation characteristics and microbial population using a rumen simulation technique (Rusitec). Anaerobe 48: 59–65. [DOI] [PubMed] [Google Scholar]
- Edgar, R.C. (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10: 996–998. [DOI] [PubMed] [Google Scholar]
- Fadrosh, D.W. , Ma, B. , Gajer, P. , Sengamalay, N. , Ott, S. , Brotman, R.M. , and Ravel, J. (2014) An improved dual‐indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2014) The Role of Livestock in Climate Change. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Finney, C.A. , Kamhawi, S. , and Wasmuth, J.D. (2015) Does the arthropod microbiota impact the establishment of vector‐borne diseases in Mammalian hosts? PLoS Pathog 11: e1004646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, Y. , Osada, T. , Hanajima, D. , and Haga, K. (2003) Patterns and quantities of NH3, N2O and CH4 emissions during swine manure composting without forced aeration‐effect of compost pile scale. Bioresource Technol 89: 109–114. [DOI] [PubMed] [Google Scholar]
- Hayashi, H. , Shibata, K. , Sakamoto, M. , Tomita, S. , and Benno, Y. (2007) Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 57: 941–946. [DOI] [PubMed] [Google Scholar]
- He, Z. , Pagliari, P.H. , and Waldrip, H.M. (2016) Applied and environmental chemistry of animal manure: a review. Pedosphere 26: 779–816. [Google Scholar]
- Huang, H. , Wu, K. , Khan, A. , Jiang, Y. , Ling, Z. , Liu, P. , et al (2016) A novel Pseudomonas gessardii strain LZ‐E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresource Technol 207: 370–378. [DOI] [PubMed] [Google Scholar]
- Ilea, R.C. (2009) Intensive livestock farming: global trends, increased environmental concerns, and ethical solutions. J Agr Environ Ethics 22: 153–167. [Google Scholar]
- Kahveci, A. , Asicioglu, E. , Tigen, E. , Ari, E. , Arikan, H. , Odabasi, Z. , and Ozener, C. (2011) Unusual causes of peritonitis in a peritoneal dialysis patient: Alcaligenes faecalis and Pantoea agglomerans . ANN Clin Microb 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, C. , Sugimoto, K. , Moritani, I. , Tanaka, J. , Oya, Y. , Inoue, H. , et al (2016) Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: terminal restriction fragment length polymorphism and next‐generation sequencing analyses. Oncol Rep 35: 325–333. [DOI] [PubMed] [Google Scholar]
- Kede, M.L. , Correia, F.V. , Conceição, P.F. , Junior, S.F. , Marques, M. , Moreira, J.C. , and Pérez, D.V. (2014) Evaluation of mobility, bioavailability and toxicity of Pb and Cd in contaminated soil using TCLP, BCR and earthworms. Int J Environ Res Public Health 11: 11528–11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könönen, E. , Eerola, E. , Frandsen, E.V. , Jalava, J. , Mättö, J. , Salmenlinna, S. , and Jousimies‐Somer, H. (1998) Phylogenetic characterization and proposal of a new pigmented species to the genus Prevotella: Prevotella pallens sp. nov. Int J Syst Evol Microbiol 48: 47–51. [DOI] [PubMed] [Google Scholar]
- Lazarev, V.N. , Levitskii, S.A. , Basovskii, Y.I. , Chukin, M.M. , Akopian, T.A. , Vereshchagin, V.V. , et al (2011) Complete genome and proteome of Acholeplasma laidlawii . J Bacteriol 193: 4943–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Yang, D. , Huang, M. , Hu, X. , Shen, J. , Zhao, Z. , and Chen, J. (2012) Chrysomya megacephala (Fabricius) larvae: a new biodiesel resource. Appl Energ 94: 349–354. [Google Scholar]
- Li, C. , Zhang, Z. , Guo, L. , Cai, M. , and Cao, C. (2013) Emissions of CH4 and CO2 from double rice cropping systems under varying tillage and seeding methods. Atmos Environ 80: 438–444. [Google Scholar]
- Lim, S.L. , Lee, L.H. and Wu, T.Y. (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111 Part A, 262–278. [Google Scholar]
- Logares, R. , Sunagawa, S. , Salazar, G. , Cornejo‐Castillo, F.M. , Ferrera, I. , Sarmento, H. , et al (2014) Metagenomic 16S rDNA Illumina tags are a powerful alternative to amplicon sequencing to explore diversity and structure of microbial communities. Environ Microbiol 16: 2659–2671. [DOI] [PubMed] [Google Scholar]
- Lv, B. , Xing, M. , and Yang, J. (2016) Speciation and transformation of heavy metals during vermicomposting of animal manure. Bioresource Technol 209: 397–401. [DOI] [PubMed] [Google Scholar]
- Magoč, T. , and Salzberg, S.L. (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27: 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder, S. , Raghuvanshi, S. , and Gupta, S. (2016) Application of a hybrid biofilter column for the removal of Cr(VI) from aqueous solution using an indigenous bacterial strain Pseudomonas taiwanensis . Bioremediat J 20: 10–23. [Google Scholar]
- Mantha, S. , Anderson, A. , Acharya, S.P. , Harwood, V.J. , and Weidhaas, J. (2017) Transport and attenuation of Salmonella enterica, fecal indicator bacteria and a poultry litter marker gene are correlated in soil columns. Sci Total Environ 598: 204–212. [DOI] [PubMed] [Google Scholar]
- Moreno, J. (2015) Prevotella copri and the microbial pathogenesis of rheumatoid arthritis. Reumatología Clínica 11: 61–63. [DOI] [PubMed] [Google Scholar]
- Nookabkaew, S. , Rangkadilok, N. , Prachoom, N. , and Satayavivad, J. (2016) Concentrations of trace elements in organic fertilizers and animal manures and feeds and cadmium contamination in herbal tea (Gynostemma pentaphyllum Makino). J Agric Food Chem 4: 3119–3126. [DOI] [PubMed] [Google Scholar]
- Ceylan, O. , Aysel, U. (2012) Bio‐monitoring of heavy metal resistance in non‐aeruginosa Pseudomonas and Pseudomonas related genus. J Biolog Environ Sci 16, 233–242. [Google Scholar]
- Paik, C. , Chung, Y. , Kim, H. , and Kim, Y.J. (2016) Comparison of emission estimates for non‐CO2 greenhouse gases from livestock and poultry in Korea from 1990 to 2010. Anim Sci J 87: 612–623. [DOI] [PubMed] [Google Scholar]
- Pardo, R. , Herguedas, M. , Barrado, E. , and Vega, M. (2003) Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas Putida . Anal Bioanal Chem 376: 26–32. [DOI] [PubMed] [Google Scholar]
- Ptistišek, N. , Milačič, R. , and Veber, M. (2001) Use of the BCR three‐step sequential extraction procedure for the study of the partitioning of Cd, Pb and Zn in various soil samples. J Soil Sediment 1: 25–29. [Google Scholar]
- Quiroz‐Castañeda, R.E. , Mendoza‐Mejía, A. , Obregón‐Barboza, V. , Martínez‐Ocampo, F. , Hernández‐Mendoza, A. , Martínez‐Garduño, F. , et al (2015) Identification of a NEW Alcaligenes faecalis strain MOR02 and assessment of its toxicity and pathogenicity to insects. Biomed Res Int 2015: 570243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razek, A.A.A.E. (2014) The mobility and speciation of lead and cadmium in Bahr El Baqar region, Egypt. J Environ Chem Eng 2: 685–691. [Google Scholar]
- Rogers, J.E. , and Whitman, W.B. (1994) Microbial production and consumption of greenhouse gases: methane, nitrogen oxides, and halomethanes. J Environ Qual 23: 211–212. [Google Scholar]
- Scher, J.U. , Sczesnak, A. , Longman, R.S. , Segata, N. , Ubeda, C. , Bielski, C. , et al (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling, T.J. , and Wallace, R.J. (2017) The rumen microbial metaproteome as revealed by SDS‐PAGE. BMC Microbiol 17: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, S. , Daniel, J.S. , Sanford, T.J. , Murphy, D.M. , Plattner, G.K. , Knutti, R. , and Friedlingstein, P. (2010) Persistence of climate changes due to a range of greenhouse gases. PNAS 107: 18354–18359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. , Liu, M. , Wu, D. , Qi, L. , Ye, C. , Jiao, J. , and Hu, F. (2014) Heavy metal and nutrient changes during vermicomposting animal manure spiked with mushroom residues. Waste Manage 34: 1977–1983. [DOI] [PubMed] [Google Scholar]
- Stefanie, V.T. , Joris, M. , and Jean, S. (2003) Flavobacterium gelidilacus sp. nov., isolated from microbial mats in Antarctic lakes. Int J Syst Evol Micr 53: 1241–1245. [DOI] [PubMed] [Google Scholar]
- Tian, W. , Zhang, Z. , Hu, X. , Tian, R. , Zhang, J. , Xiao, X. , and Xi, Y. (2015) Short‐term changes in total heavy metal concentration and bacterial community composition after replicated and heavy application of pig manure‐based compost in an organic vegetable production system. Biol Fert Soils 51: 593–603. [Google Scholar]
- Yang, S. , and Liu, Z. (2014) Pilot‐scale biodegradation of swine manure via Chrysomya megacephala (Fabricius) for biodiesel production. Appl Energ 113: 385–391. [Google Scholar]
- Yasuda, T. , Kuroda, K. , Fukumoto, Y. , Hanajima, D. , and Suzuki, K. (2009) Evaluation of full‐scale biofilter with rockwool mixture treating ammonia gas from livestock manure composting. Bioresource Technol 100: 1568–1572. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Li, Y. , Yang, M. , and Li, W. (2012) Content of heavy metals in animal feeds and manures from farms of different scales in Northeast China. Int J Environ Res Public Health 9: 2658–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.J. , Liu, M. , and Zhu, J. (2013) Organic waste treatment by earthworm vermicomposting and larvae bioconversion: review and perspective. Environ Sci 34: 1679–1686. [PubMed] [Google Scholar]
- Zhang, Z.S. , Guo, L.J. , Liu, T.Q. , Li, C.F. , and Cao, C.G. (2015) Effects of tillage practices and straw returning methods on greenhouse gas emissions and net ecosystem economic budget in rice‐wheat cropping systems in central China. Atmos Environ 122: 636–644. [Google Scholar]
- Zheng, C. , Bluemling, B. , Liu, Y. , Mol, A.P.J. , and Chen, J. (2014) Managing manure from China's pigs and poultry: the influence of ecological rationality. Ambio 43: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.‐J. , and Chen, T.‐H. (2016) Biodegradation of phenol with chromium (VI) reduction by the Pseudomonas sp strain JF122. Desalin Water Treat 57: 3544–3551. [Google Scholar]
- Zhou, Y. , Xu, Y.B. , Xu, J.X. , Zhang, X.H. , Xu, S.H. , and Du, Q.P. (2015) combined toxic effects of heavy metals and antibiotics on a Pseudomonas fluorescens strain ZY2 isolated from swine wastewater. Int J Mol Sci 16: 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L.D. , and Hiltunen, E. (2016) Application of livestock waste compost to cultivate microalgae for bioproducts production: a feasible framework. Renew Sust Energ Rev 54: 1285–1290. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Relative abundance of Prevotella copri and Prevotella stercorea in RSM, NSM and CMSM.
Fig. S2 Correlation coefficient matrix of heavy metal speciation
Table S1 Taxonomy of OTUs.
Table S2 Detailed OTUs of RSM, NSM and CMSM in Venn diagram.
Table S3 Beta diversity distance of RSM, NSM and CMSM.
Table S4. Correlation matrix graph of heavy metal speciation and Prevotella copri (Pcop).
Table S5. Correlation matrix graph of heavy metal speciation and Prevotella stercorea (Pste).
Table S6. Different abundance of bacteria species in RSM, NSM and CMSM.
Table S7. Primers used for quantitative PCR.


