Abstract
A one-step formic acid-catalyzed organosolv process using a low-boiling point acid–solvent system was studied for fractionation of sugarcane bagasse. Compared to H2SO4, the use of formic acid as a promoter resulted in higher efficiency and selectivity on removals of hemicellulose and lignin with increased enzymatic digestibility of the cellulose-enriched solid fraction. The optimal condition from central composite design analysis was determined as 40 min residence time at 159 °C using water/ethanol/ethyl acetate/formic acid in the respective ratios of 43:20:16:21%v/v. Under this condition, a 94.6% recovery of cellulose was obtained in the solid with 80.2% cellulose content while 91.4 and 80.4% of hemicellulose and lignin were removed to the aqueous–alcohol–acid and ethyl acetate phases, respectively. Enzymatic hydrolysis of the solid yielded 84.5% glucose recovery compared to available glucan in the raw material. Physicochemical analysis revealed intact cellulose fibers with decreased crystallinity while the hemicellulose was partially recovered as mono- and oligomeric sugars. High-purity organosolv lignin with < 1% sugar cross-contamination was obtained with no major structural modification according to Fourier-transform infrared spectroscopy. The work represents an alternative process for efficient fractionation of lignocellulosic biomass in biorefineries.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1244-9) contains supplementary material, which is available to authorized users.
Keywords: Biorefinery, Fractionation, Organosolv, Sugarcane bagasse, Solvent recovery
Introduction
The negative impacts of fossil resources have stimulated interest in alternative renewable feedstocks, such as lignocellulosic plant biomass for platform industries. (Zhang et al. 2016a, b). Lignocellulosic materials typically comprise three major biopolymers: (1) cellulose, a linear homopolymer of d-glucose linked by β-1,4-glycosidic linkages, organized into highly crystalline microfibers which are linked to (2) hemicellulose, an amorphous branched heteropolymer of pentoses, hexoses, and sugar acids, and (3) lignin, a heteropolymer of phenolic alcohols, which shields the polysaccharide fractions, giving strength to the plant cell wall (Fengel and Wegener 1984). This complex, multi-component structure is highly recalcitrant to external physical, chemical, and biological attacks.
Integrated biorefinery is the industrial process in which biopolymers are separated and converted to biofuels and a spectrum of commodity and specialty compounds. Several processes have been developed for the initial step in the biorefinery of lignocellulosic biomass, in which lignocellulosic components are fractionated. The organosolv process uses solvents to solubilize and separate lignocellulose components, and is versatile to different types of biomass. Several organic solvents, such as alcohols (e.g., methanol and ethanol), esters, and ketones have been studied for pretreatment and fractionation of various lignocellulosic materials. The efficiency of separating different biopolymers varies among solvents. Selection of solvent systems with desirable properties and selectivity is thus the basis in developing an organosolv process (Katahira et al. 2014; Boeriu et al. 2014).
The clean fractionation (CF) organosolv process uses a miscible ternary mixture of solvents comprising a short-chain alcohol, methylisobutylketone (MIBK) and water in the presence of sulfuric acid as a promoter. This process has been reported for one-step separation of lignocellulose components from a variety of feedstocks (Black et al. 1998; Bozell et al. 2011; Brudecki et al. 2013; Reisinger et al. 2014). In the CF process, the phase of immiscible solvent mixture is disturbed by the addition of water, which allows the separation of hemicellulose-derived products into the aqueous–alcohol fraction and lignin into the organic phase, while leaving the cellulose in the solid fraction. The removal of hemicellulose and lignin from the cellulose fraction increases enzymatic accessibility to the cellulose fibers, which increases the efficiency of enzymatic digestion (Sathitsuksanoh et al. 2012; Wildschut et al. 2013; Brudecki et al. 2013). The CF process has later been modified by the use of alternative solvent systems (Cybulska et al. 2013; Imman et al. 2015; Sun et al. 2015) and heterogenous promoters (Klamrassamee et al. 2013; Li et al. 2016).
Organic acids can play roles both as solvents and promoters in organosolv pretreatment processes (Sindhu et al. 2010; Qin et al. 2012; Snelders et al. 2014). Compared with strong mineral acids, organic acids such as formic, acetic, and oxalic acids have less environmental impact and are easily recovered owing to their lower boiling points. Among various organic acids, formic acid is a weak acid with a low boiling point similar to water, allowing its recycling by evaporation (Lam et al. 2001; Zhang et al. 2010b; Du et al. 2016). Formic acid has been tested for pretreatment and fractionation of various lignocellulosic materials (Zhang et al. 2010a; Yu et al. 2013; Snelders et al. 2014; Liu et al. 2016). In this work, a modified one-step CF process is reported based on a low-boiling point formic acid–organic solvent system. The effects of varying key reaction parameters and solvent composition on the performance of the fractionation process in terms of yield and selectivity were investigated using a central composite design approach. Physicochemical properties of the separated biopolymer-derived fractions relevant to further processing and valorization were studied. The work provided an improved approach for biomass fractionation in integrated biorefineries.
Materials and methods
Materials
Sugarcane bagasse was obtained from PTT Global Chemicals PCL, Rayong, Thailand. It was dried at 70 °C for 24 h and cut by Retsch ZM200 into particles 0.5–0.85 mm in diameter. According to the standard NREL analysis (Sluiter et al. 2011), the biomass contained 38.3 wt% cellulose, 20.7 wt% hemicelluloses, 24.6 wt% lignin, and 4.3 wt% ash. Cellic CTec2 cellulase was obtained from Novozymes A/S (Bagvaerd, Denmark). Analytical grade organic solvents and chemicals were purchased from major chemical suppliers, i.e., Sigma-Aldrich, Merck, and Fluka.
Biomass fractionation
The fractionation process in this study was modified from a previous report (Bozell et al. 2011). Reactions were performed in a 600-mL stainless steel high-pressure reactor equipped with a mixing system and a thermocouple for internal temperature measurement (Parr Reactor 4560, Parr instrument, Moline, IL, USA). The solvent ratio in the solvent mixture was set up according to the phase diagram to obtain a single-phase mixture (Lin et al. 2005). The standard reaction contained 10 g of sugarcane bagasse and 100 mL of the single-phase solvent mixture containing 70–90% by volume of the conventional solvent mixture [water/ethanol/ethyl acetate (50:25:25% as the starting ratio)] and 10–30% by volume of formic acid. Nitrogen was flowed into the reactor for purging and adjusting the initial pressure to 20 bar. The reaction was heated to the desired temperature (140–180 °C) for a specified residence time (20–60 min) in a temperature-controlled jacket with stirring at 100 rpm. The reaction was quenched in a water bath after heating under the desired conditions. The solid cellulose-enriched fraction was separated by filtration on filter paper (Whatman No. 4) using a Büchner funnel and washed with 25 mL of ethyl acetate and then 50 mL of water. The liquid fraction was combined with the rinsate and placed into a separatory funnel. Water was added (approximately 40 mL) to the aqueous–organic fraction until phase separation was achieved. The mixture was stirred and then placed at room temperature for 20 min for complete phase separation. The aqueous phase containing hemicelluloses and soluble products was recovered. The separated organic phase was dried at 105 °C to obtain lignin. The diagram showing the overall process with acid and solvent recoveries is shown in Fig. S1. The chemical composition of the solid fraction was determined according to the NREL method (Sluiter et al. 2011). Cellulose yield was defined as the percentage of cellulose recovered in the separated solid fraction compared with its initial content in the native biomass (Eq. 1). Cellulose purity was defined as the percentage of relative content of cellulose to other components in the separated solid fraction (Eq. 2). Hemicellulose solubilization efficiency was determined based on the remaining hemicellulose in the solid residues compared with their respective content in the native bagasse (Eq. 3). Lignin removal was determined based on the remaining lignin in solid pulp compared with the lignin content in raw material on a weight basis (Eq. 4). Lignin recovery was based on the weight of lignin recovered from the organic phase after evaporation of the organic solvent compared with the lignin content in the raw material (Eq. 5).
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
Enzymatic hydrolysis
Enzymatic hydrolysis reactions (1 mL) contained 5% (w/v) fractionated solid as a substrate in 50 mM sodium citrate buffer, pH 4.8 and 0.25% w/v sodium azide with 15 FPU/g Cellic Ctec2 (Novozymes A/S, Bagvaerd, Denmark). The reactions were incubated at 50 °C for 72 h with vertical rotation at 30 rpm. Hydrolysis experiments were performed in duplicate. Profiles of released sugars were analyzed on a high performance liquid chromatograph (LDC Model 4100, Shimadzu, Kyoto, Japan) equipped with a refractive index detector and an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) operating at 65 °C with 5 mM H2SO4 as the mobile phase at a flow rate of 0.5 mL/min. Biomass digestibility was defined as the amount of sugars obtained from the enzymatic hydrolysis reaction based on the fractionated biomass used as the substrate. Glucose recovery was calculated as the percentage of glucose recovered from the theoretical available glucose in the native biomass (cellulose × 1.11) on a weight basis (Eq. 6):
| 6 |
Central composite design
The effects of selected reaction components and conditions on fractionation efficiency and selectivity were studied and optimized using central composite design (CCD) with four independent variables and five coded levels (− 2, − 1, 0, 1, 2). The CCD comprised 25 combinations with temperature (140–180 °C), time (20–60 min), ratio of formic acid to the basic ternary solvent mixture (10–30% v/v), and %composition of ethyl acetate in the ternary mixture (15–35% v/v) as variables (Table 1). Four responses of the design experiments included cellulose yield, hemicellulose solubilization, lignin removal, and glucose recovery. Response predictions were analyzed by response surface regression and fitted to the second-order polynomial multiple regression equation involving main effects and interactive effects for each variable (Eq. 7). Analysis of variance (ANOVA) was used to evaluate statistical significance of the model. P values less than 0.05 were considered significant. No correction for multiple testing was used. The fitting quality of the polynomial model equation was expressed as the co-efficient of determination R2. STATISTIC 8.0 (Statsoft, Tulsa, OK, USA) was used for all statistical and graphical analyses.
| 7 |
where Y is the predicted response; n is the number of factors; xi and xj are the coded variables; b0 is the offset term; bi, bii, and bij are the first-order, quadratic, and interaction effects, respectively; i and j are the index numbers for factor; and ei is the residual error.
Table 1.
Central composite design with four independent variables
| Run no. | Temperature (°C) | Time (min) | HCOOH (%v/v) | EA (%v/v) |
|---|---|---|---|---|
| 1 | 150 | 50 | 15 | 20 |
| 2 | 150 | 30 | 15 | 30 |
| 3 | 150 | 30 | 25 | 20 |
| 4 | 150 | 50 | 25 | 30 |
| 5 | 170 | 30 | 15 | 20 |
| 6 | 170 | 50 | 15 | 30 |
| 7 | 170 | 50 | 25 | 20 |
| 8 | 170 | 30 | 25 | 30 |
| 9 | 160 | 40 | 20 | 25 |
| 10 | 150 | 30 | 15 | 20 |
| 11 | 150 | 50 | 15 | 30 |
| 12 | 150 | 50 | 25 | 20 |
| 13 | 150 | 30 | 25 | 30 |
| 14 | 170 | 50 | 15 | 20 |
| 15 | 170 | 30 | 15 | 30 |
| 16 | 170 | 30 | 25 | 20 |
| 17 | 170 | 50 | 25 | 30 |
| 18 | 140 | 40 | 20 | 25 |
| 19 | 180 | 40 | 20 | 25 |
| 20 | 160 | 40 | 10 | 25 |
| 21 | 160 | 40 | 30 | 25 |
| 22 | 160 | 40 | 20 | 15 |
| 23 | 160 | 40 | 20 | 35 |
| 24 | 160 | 20 | 20 | 25 |
| 25 | 160 | 60 | 20 | 25 |
Analysis of solid fraction
The microstructure of bagasse samples and remaining solid obtained from all optimal conditions was analyzed by scanning electron microscopy (JSM-6301F, JEOL, Japan) with electron beam energy of 20 kV. The samples were dried and coated with gold for SEM analysis. The degree of crystallinity of the untreated and pretreated bagasse samples was measured using an X-ray diffraction (XRD) instrument (JDX-3530, JEOL, Japan) with Cu Kα radiation source at 40 kV and 30 mA. Samples were scanned at a speed of 1°/min, ranging from 2θ = 5°–40°, and a step size of 0.004° at room temperature. Crystallinity index (CrI) was calculated according to the following equation (Segal et al. 1959).
where I002 is the scattered intensity at the main peak of cellulose, which typically lies around the 002 plane, and Iam is the scattered intensity of amorphous portion evaluated as the minimum intensity diffraction between the main and secondary (101 and 002) planes.
Analysis of aqueous fraction
Soluble product profiles in the aqueous fraction were analyzed on a high performance liquid chromatograph (LDC Model 4100, Shimadzu, Kyoto, Japan) equipped with a refractive index and a UV–Vis detector and an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) operating at 65 °C with 5 mM H2SO4 as the mobile phase at a flow rate of 0.5 mL/min. The amount of oligosaccharides was determined according to the NREL method (Sluiter et al. 2008, NREL/TP-510-42623).
Lignin characterization
Klason lignin
Lignin composition recovered from the organic phase was determined based on the Klason lignin content according to the method of laboratory analytical procedure provided by the National Renewable Energy Laboratory (NREL) (Sluiter et al. 2011).
Molecular weight determination
The molecular weight of the extracted lignin was determined by size exclusion chromatography. The samples were solubilized in tetrahydrofuran (THF) at a concentration of 0.2 mg/mL and analyzed on a high performance liquid chromatograph (HPLC) (WATER e2695, Waters, MA, USA) equipped with an Agilent PLgel 10 um MIXED-B column. The mobile phase was THF with the flow rate of 0.5 mL/min. The temperature of column was maintained at 30 °C. Sodium polystyrene sulphonate standards with molecular weights 1530, 4950, 16,600, and 34,700 g/mol were used to prepare a standard calibration curve. Weight average molecular weight (Mw) and number average molecular weight (Mn) were determined from the standard curve.
Fourier-transform infrared spectroscopy (FT-IR)
Chemical characteristics of the extracted lignin were determined by FT-IR on a Perkin-Elmer System 2000 (PerkinElmer, Waltham, MA, USA). The samples were prepared by the KBr pellet method. The measurement resolution was set at 4 cm−1 with a mirror velocity of 0.6329 cm/s. Infrared spectra were collected in the range 4000–400 cm−1 with 64 co-added scans. Peaks for lignin were compared with the standards of functional groups.
Results and discussion
Comparison of acid promoters in the fractionation process
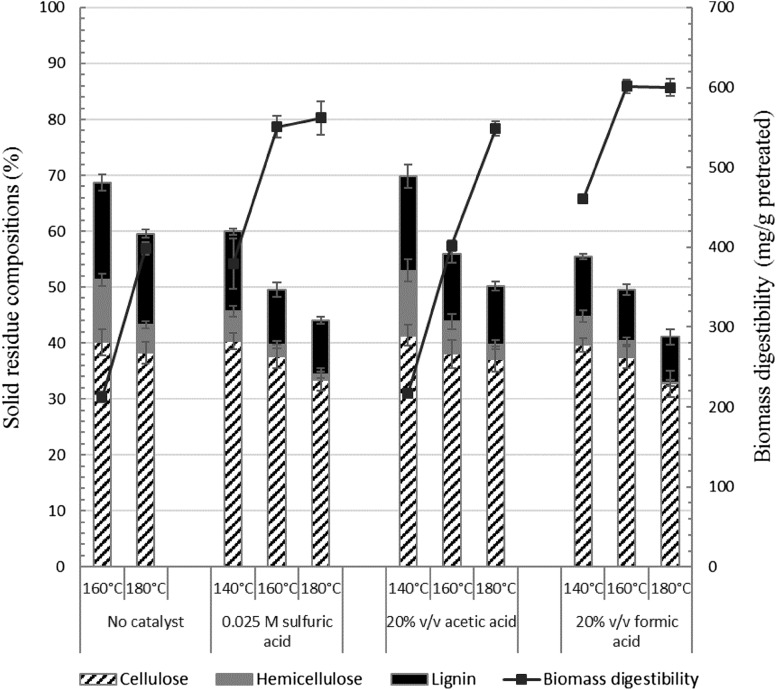
The use of organic acids as promoters in the CF-based fractionation process was studied. The efficiency of formic acid (pKa 3.77) and acetic acid (pKa 4.76) was compared with H2SO4 (0.025 M), a conventional strong acid used in the CF process. The final concentration of formic and acetic acids was 20% v/v, equivalent to 0.025 and 0.008 M H3O+, respectively. Different acid promoters were tested and compared under fixed conditions of reaction temperature, solid loading and solvent systems based on previous reports (Klamrassamee et al. 2013; Imman et al. 2015). The fractionation process using acid promoters enhanced the solubilization of hemicellulose and lignin fractions from the native bagasse to varying degrees compared with the control reaction with no acid promoters, which showed markedly lower solubilization of the hemicellulose and lignin from the raw material (Fig. 1). The use of formic acid led to greater release of hemicellulose and lignin from the biomass compared with acetic acid and H2SO4, particularly at 140 °C. Increasing the temperature up to 180 °C led to lower weight recovery of the residual solid, together with increasing removals of hemicellulose and lignin, leading to respective increase in cellulose purity in the solid fraction.
Fig. 1.
Comparison of acid promoters on fractionation of sugarcane bagasse based on solid residue composition (left y-axis) and biomass digestibility (right y-axis). The reactions contained 10 g sugarcane bagasse in 100 mL of the single-phase solvent mixture containing water/ethanol/ethyl acetate (62.5/25/12.5%v/v) with 0.025 M H2SO4 or 20% acetic acid or formic acid with an initial pressure of 20 bar by nitrogen. Reactions were performed at 140–180 °C for 60 min before phase separation
The native biomass was recalcitrant to enzymatic hydrolysis, resulting in a low sugar yield (66.1 mg/g). In contrast, fractionated cellulose-enriched pulp showed a marked increase in polysaccharide digestibility (Fig. 1). The highest levels of biomass digestibility and glucose recovery were achieved at 160 °C in the reaction containing formic acid, whereas the glucose recovery tended to decrease with increasing temperature owing to the greater loss of cellulose from hydrolysis. We did not test reaction temperatures greater than 180 °C, since degradation of sugar products to inhibitory by-products in the liquid phase was significant at this temperature. The highest digestibility of 601.3 mg/g pretreated biomass and a glucose recovery of 68.1% from the native biomass were achieved using formic acid at 160 °C. These results thus demonstrate the superior performance of formic acid as a promoter compared with H2SO4 and acetic acid, in terms of fractionation efficiency and enzymatic digestibility of the cellulose-enriched fractions.
Influences of reaction variables
The effects of reaction variables (temperature, time, concentration of acid, and ethyl acetate content) previously reported to influence reaction selectivity and efficiency (Cybulska et al. 2013) were studied using CCD with four target responses considered:cellulose yield and selectivity, hemicellulose solubilization, lignin removal, and glucose recovery. These responses were calculated from the measured composition in the solid fraction and glucose recovery from the enzymatic hydrolysis step (Table 2; Table S1).
Table 2.
Effects of reaction factors on composition of solid fraction and target responses
| Run no. | Chemical composition of solids (% w/w)a | Cellulose yieldb (%) | Cellulose purityc (%) | Hemicellulose solubilizationb (%) | Lignin removalb (%) | Glucose recoveryb (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | AIL | ASL | Ash | ||||||
| 1 | 75.9 | 4.7 | 14.1 | 0.8 | 2.2 | 99.7 | 75.9 | 88.6 | 70.3 | 84.2 |
| 2 | 69.8 | 10.9 | 12.1 | 0.8 | 3.1 | 99.1 | 71.7 | 71.0 | 73.5 | 73.7 |
| 3 | 75.7 | 8.0 | 12.7 | 0.9 | 2.1 | 91.4 | 74.7 | 80.6 | 73.8 | 80.5 |
| 4 | 72.0 | 7.5 | 11.7 | 0.7 | 3.2 | 96.3 | 74.7 | 82.4 | 76.0 | 76.9 |
| 5 | 79.5 | 4.6 | 11.2 | 0.9 | 1.9 | 91.1 | 79.2 | 89.7 | 78.0 | 79.4 |
| 6 | 77.9 | 4.1 | 11.1 | 1.0 | 2.4 | 93.9 | 79.0 | 91.0 | 78.7 | 79.6 |
| 7 | 83.4 | 2.5 | 10.4 | 0.9 | 2.2 | 95.3 | 81.8 | 94.8 | 81.1 | 70.7 |
| 8 | 80.4 | 4.7 | 10.9 | 0.6 | 2.5 | 92.2 | 78.8 | 89.6 | 79.2 | 75.8 |
| 9 | 76.6 | 4.8 | 9.5 | 0.9 | 3.1 | 94.7 | 79.6 | 89.4 | 81.4 | 83.6 |
| 10 | 70.8 | 7.7 | 15.2 | 0.8 | 3.1 | 88.8 | 71.8 | 80.9 | 67.3 | 79.5 |
| 11 | 67.4 | 8.1 | 11.4 | 0.9 | 3.3 | 98.3 | 73.0 | 80.3 | 75.8 | 71.4 |
| 12 | 79.4 | 6.6 | 10.5 | 1.0 | 2.5 | 96.9 | 77.3 | 84.8 | 79.4 | 74.7 |
| 13 | 75.5 | 8.9 | 10.6 | 1.1 | 2.2 | 86.5 | 75.1 | 78.7 | 78.5 | 74.8 |
| 14 | 75.8 | 3.5 | 12.6 | 0.9 | 2.0 | 96.3 | 77.8 | 92.6 | 76.8 | 76.0 |
| 15 | 81.7 | 5.6 | 8.3 | 0.8 | 2.3 | 90.8 | 80.5 | 87.7 | 84.2 | 77.6 |
| 16 | 80.3 | 2.6 | 11.1 | 0.8 | 3.0 | 81.6 | 80.8 | 94.5 | 80.1 | 78.0 |
| 17 | 73.0 | 2.6 | 8.1 | 0.8 | 2.8 | 99.7 | 81.2 | 94.7 | 85.4 | 72.0 |
| 18 | 70.5 | 9.8 | 16.0 | 0.9 | 3.4 | 93.0 | 68.9 | 74.4 | 64.0 | 71.4 |
| 19 | 84.4 | 2.4 | 13.6 | 0.6 | 1.7 | 94.5 | 80.4 | 95.2 | 75.9 | 70.9 |
| 20 | 77.6 | 6.4 | 9.9 | 0.8 | 2.5 | 92.9 | 78.3 | 85.5 | 80.5 | 75.8 |
| 21 | 81.1 | 3.5 | 9.7 | 0.8 | 2.4 | 96.8 | 81.0 | 92.6 | 82.1 | 75.0 |
| 22 | 81.2 | 3.9 | 10.4 | 0.9 | 2.4 | 96.6 | 80.5 | 91.5 | 80.2 | 83.0 |
| 23 | 76.3 | 6.8 | 8.9 | 0.7 | 2.9 | 98.8 | 78.9 | 84.0 | 82.1 | 81.5 |
| 24 | 77.5 | 7.3 | 11.4 | 0.9 | 2.0 | 98.3 | 76.9 | 82.6 | 76.9 | 85.0 |
| 25 | 82.3 | 5.3 | 8.2 | 1.0 | 2.0 | 99.7 | 81.4 | 88.4 | 84.7 | 76.4 |
aBased on dry weight residue of the solid fraction
bBased on the respective content of each fraction in the native biomass
cBased on relative content of cellulose to other components on percentage basis
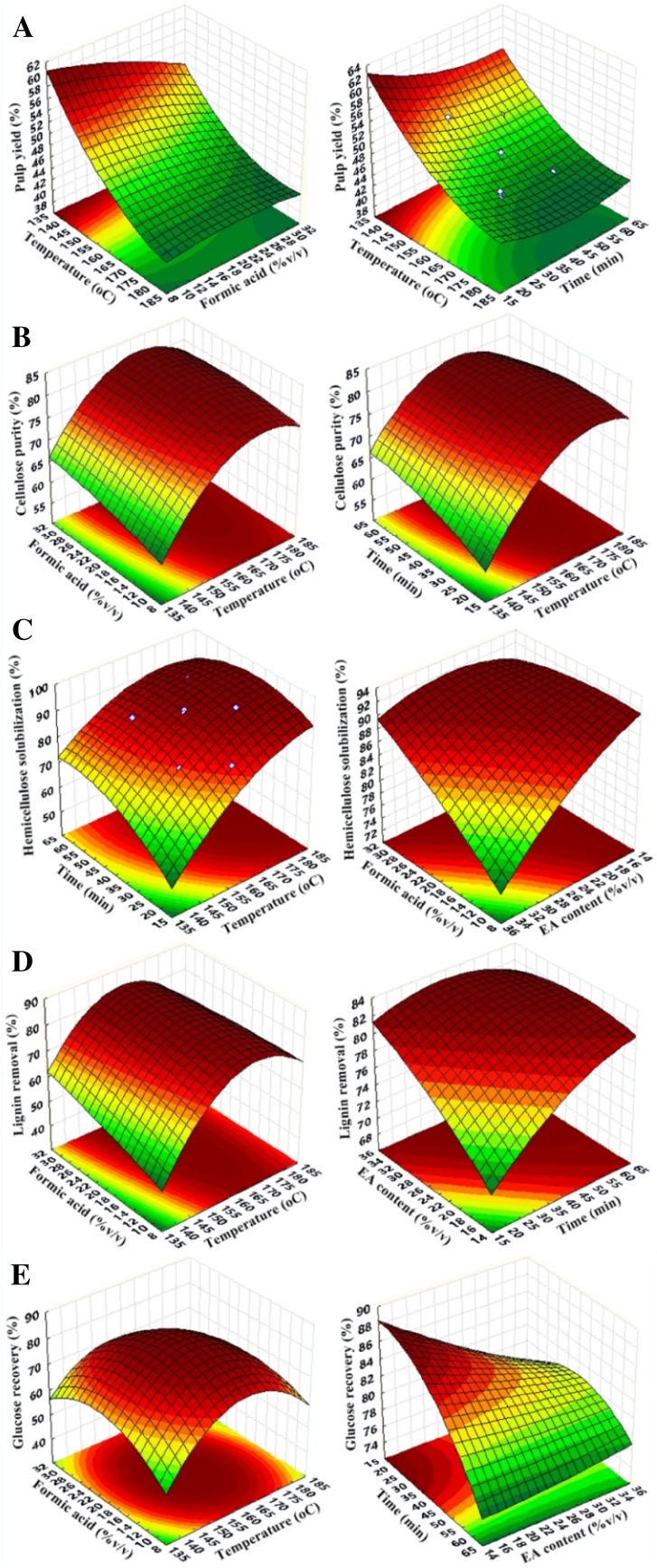
Cellulose yield and selectivity
Recovery of cellulose in the solid fraction depends on weight recovery of the solid pulp and its cellulose content. Overall, increasing temperature led to marked decreases in the pulp yield, whereas varying the time and formic acid content had a less marked effect (Fig. 2a). The solid recovery was in the range of 42.2–54.8% on a weight basis, equivalent to the cellulose yield of 81.6–99.7%. Increasing reaction severity, i.e., higher temperature and formic acid concentration resulted in lower pulp yield and higher cellulose decomposition. The cellulose purity in the solid fraction was in the range of 68.9–81.8% (Fig. 2b), with the highest purity in run 7 (Table 2).
Fig. 2.
Response surface showing the effects of independent variables on a pulp yield; b cellulose yield; c hemicellulose solubilization; d lignin removal; e glucose recovery. The experimental design comprised 25 combinations with temperature (140–180 °C), time (20–60 min), ratio of formic acid to the ternary solvent mixture (10–30% v/v), and % composition of ethyl acetate (EA) in the ternary mixture (15–35% v/v) as variables
Hemicellulose solubilization
The efficiency of hemicellulose solubilization ranged from 71.0 to 95.2% (w/w) and showed an inversed trend to pulp yield recovered (Fig. 2c). The maximal hemicellulose solubilization was found in run no. 19, which was carried out at the highest temperature (180 °C), formic acid concentration (30% v/v) and time (60 min). Increasing the ratio of ethyl acetate or decreasing the water content of the solvent mixture led to lower efficiency of hemicellulose removal (run no. 1 and 11 in Table 2). The negative effect of ethyl acetate on solubilization efficiency can be attributed to the lower polarity of this compound compared with water and ethanol in the solvent mixture.
Lignin removal
The degree on lignin removal was in the range of 64.0–85.4% (w/w) (Table 2). According to the response surface (Fig. 2d), increasing the temperature and the concentration of formic acid showed strong positive effect on lignin removal efficiency, whereas the influence of time and ethyl acetate content on lignin removal was less marked. The highest lignin removal was found in run no.17 under operation conditions of 170 °C, 50 min residence time, 25% formic acid and 30% ethyl acetate. Under this condition, a lignin recovery of 1.90 g from 10 g of the starting material was obtained, equivalent to 80.2% recovery of lignin in the organic phase (Table S1).
Glucose recovery
Recovery of glucose after enzymatic hydrolysis is dependent on the digestibility of fractionated solid and weight recovery of the solid pulp. The cellulose-enriched solid was highly susceptible to enzymatic hydrolysis, in which the level of released glucose varied from 560.9 to 773.6 mg/g pretreated biomass (Fig. 2e), which corresponded to a glucose recovery of 70.7–85.0% (w/w) of theoretical glucose content based on available cellulose in the native biomass. Increasing temperature, time and concentration of formic acid showed positive influence on enzymatic digestibility of the biomass, albeit with decreasing pulp yield. The highest glucose yield was observed in run no. 24 (Table 2).
Influences of single and interactive effects on the reaction performance and selectivity were evaluated using multiple regression analysis (Table 3). When considering linear terms, temperature, concentration of formic acid, and reaction time were significant for cellulose recovery, whereas ethyl acetate content was not significant. All four variables significantly influenced hemicellulose removal. Temperature and formic acid concentration were significant for lignin removal efficiency and only time was significant for glucose recovery. Among quadratic terms, temperature × temperature was significant for all responses and %formic acid × %formic acid was significant for glucose recovery. None of the interaction terms were significant.
Table 3.
Multiple regression analysis (ANOVA) results
| Source of variances | Response variable p valuea | |||
|---|---|---|---|---|
| Cellulose yield (%) | Hemicellulose solubilization (%) | Lignin removal (%) | Glucose recovery (%) | |
| Linear term | ||||
| Temperature | 0.000003 | 0.000000 | 0.000279 | 0.590060 |
| Time | 0.036552 | 0.000203 | 0.097701 | 0.043751 |
| %(v/v) HCOOH | 0.016887 | 0.003175 | 0.036311 | 0.176113 |
| %(v/v) EA | 0.276982 | 0.000278 | 0.058434 | 0.099728 |
| Quadratic term | ||||
| Temperature × temperature | 0.008273 | 0.039235 | 0.003761 | 0.002547 |
| Time × time | 0.500440 | 0.070857 | 0.709626 | 0.314022 |
| %(v/v) HCOOH × %(v/v) HCOOH | 0.697200 | 0.764444 | 0.823201 | 0.022992 |
| %(v/v) EA × %(v/v) EA | 0.717194 | 0.374946 | 0.783252 | 0.570960 |
| Interaction term | ||||
| Temperature × time | 0.248095 | 0.083260 | 0.486934 | 0.332537 |
| Temperature × %(v/v) HCOOH | 0.596588 | 0.342660 | 0.267935 | 0.226786 |
| Temperature × %(v/v) EA | 0.410899 | 0.071036 | 0.890252 | 0.061121 |
| Time × %(v/v) HCOOH | 0.598808 | 0.178539 | 0.309684 | 0.182300 |
| Time × %(v/v) EA | 0.448542 | 0.374507 | 0.480520 | 0.395664 |
| %(v/v) HCOOH × %(v/v) EA | 0.494594 | 0.097820 | 0.188069 | 0.288127 |
aSignificant P values are in bold
Optimization of reaction parameters
Model equations for all target responses are shown in Table 4. Based on the effect estimates for the final regression model, the reaction parameters optimized for maximization of individual responses are shown in Table 5. The highest theoretical cellulose recovery of 99.7% was predicted to occur at 140 °C, 40 min residence time, ethyl acetate content of 25% in the ternary mixture and 20% formic acid. The maximum efficiency of 93.5% hemicellulose solubilization was predicted at 168.5 °C, 46.7 min, 13.4% ethyl acetate in the ternary mixture, and 11.7% formic acid. Higher ethyl acetate and acid contents were needed for maximum removal of lignin (85.2%). The maximum glucose recovery of 86.2% was predicted under milder conditions, i.e., lower temperature and ethyl acetate ratio with a shorter residence time. The experimental results for all responses were within 2% of the predicted values, validating the accuracy of model equations.
Table 4.
Effect estimates for final regression model
| The predictive equation of polynomial model with independent variables in terms of coded value; A = temperature, B = time, C = concentration of formic acid, D = proportion of ethyl acetate | R 2 |
|---|---|
| Cellulose yield = 79.56100 + 5.64413A − 2.86408A2 + 1.44436B − 0.61022B2 + 1.71400C − 0.34961C2 − 0.68850D − 0.32527D2 − 0.89951AB − 0.40092AC + 0.62936AD + 0.39846BC − 0.57846BD − 0.51987CD | 0.9269 |
| Hemicellulose removal = 89.38847 + 10.73493A − 2.42983A2 + 3.99649B − 2.07151B2 + 2.71207C − 0.31563C2 − 3.83649D − 0.95184D2 − 1.65683AB + 0.85786AC + 1.73912AD − 1.24598BC + 0.80047BD + 1.57243CD | 0.9711 |
| Lignin removal = 81.44070 + 6.04246A − 6.06154A2 + 2.02353B − 0.61872B2 + 2.67628C − 0.37040C2 + 2.36608D − 0.45633D2 − 0.97931AB − 1.59158AC − 0.19203AD + 1.45194BC − 0.99416BD − 1.91678CD | 0.8833 |
| Released glucose = 771.37040 + 77.89330A − 70.17410A2 + 2.00700B − 28.53370B2 + 10.03920C − 34.43090C2 − 30.50870D − 17.06030D2 − 16.34580AB − 24.11710AC + 27.72490AD − 20.80220BC + 11.33870BD + 13.32820CD | 0.9045 |
| Glucose yield = 83.62166 − 0.62253A − 6.51073A2 − 2.58010B − 1.72853B2 − 1.62834C − 4.37388C2 − 2.02913D − 0.95523D2 − 1.39497AB − 1.76428AC + 2.88942AD − 1.96341BC + 1.21576BD + 1.53686CD | 0.8237 |
Table 5.
Predicted reaction parameters optimized for the highest yield of individual responses
| Responses | Critical values of variables | Predicted % theoretical maximum response | Experimental % theoretical maximum response | SD | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | HCOOH (%v/v) | EA (%v/v) | ||||
| Cellulose recovery (%) | 140.0 | 40.0 | 20 | 25 | 99.7 | 99.3 ± 0.5 | ± 0.7 |
| Hemicellulose solubilization (%) | 168.5 | 46.7 | 11.7 | 13.4 | 93.5 | 91.4 ± 1.2 | ± 1.5 |
| Lignin removal (%) | 164.0 | 45.3 | 21.3 | 31.8 | 85.2 | 85.3 ± 0.9 | ± 0.1 |
| Released glucose (mg glucose/g pretreated) | 164.7 | 38.1 | 19.6 | 22.0 | 784.9 | 790.5 ± 6.1 | ± 4.0 |
| Glucose recovery (%) | 154.8 | 25.8 | 18.6 | 10.1 | 86.2 | 87.2 ± 1.3 | ± 0.7 |
The optimal conditions according to the following criteria: glucose recovery > 80%; lignin removal > 80% and hemicellulose removal > 90% were sought, which would be of practical value in the CF process in order to obtain a high conversion of cellulose to sugars while still achieve high recoveries of hemicellulose-derived products and lignin in the aqueous and organic phases, respectively. According to the regression model analysis, the optimal conditions under these requirements were predicted at 159 °C for 40 min residence time using 21% v/v formic acid, and 79% v/v of the ternary mixture comprising 20% ethyl acetate, 55%v/v water, and 25% ethanol, equivalent to the water/ethanol/ethyl acetate/formic acid ratios of 43:20:16:21 in the final solvent. Under this condition, the predicted maximum glucose yield, hemicellulose and lignin removal efficiencies were 84.5, 91.4 and 80.4%, respectively. The experimentally determined values under this condition (82.9% glucose recovery, 89.6 and 80.9% hemicellulose and lignin removal efficiencies, respectively) were close to the predicted values, verifying the accuracy of the model equation. The total mass balance under the optimal condition is demonstrated in Fig. S2.
Characterization of fractionated products
The physical characteristics and chemical properties of the fractionated cellulose, hemicellulose, and lignin obtained from the modified CF process under the optimal condition were determined.
Cellulose-enriched solid fraction
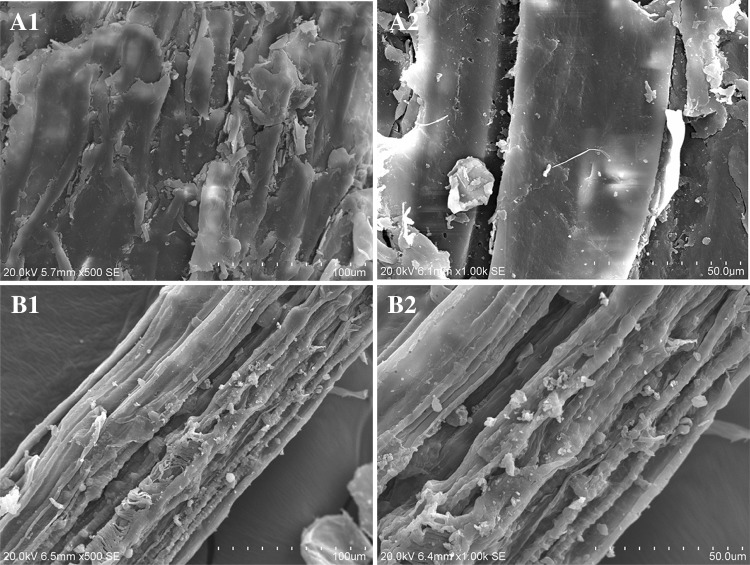
The physicochemical properties of the solid pulps were determined using SEM and XRD. The surface of native bagasse was covered by a waxy layer and embedded matrix components (Fig. 3A1, A2).The fractionation process led to disruption of the biomass surface and microstructure, as shown by the removal of covered waxy materials together with the associated hemicellulose and lignin, resulting in highly pure cellulose fibers as seen in the samples of fractionated solid (Fig. 3B1, B2). The observation of distinct cellulose fibers explains the greater digestibility of fractionated solid compared with the native biomass. Crystallinity of the solid fraction was examined using XRD. For all samples, broad peaks were demonstrated at approximately 16° and 22.5°. The crystallinity of fractionated cellulose solid showed decreased CrI (67.09%) compared with the native biomass (72.83%).
Fig. 3.
Scanning electron micrographs of native biomass (A1 and A2) and solid from fractionation under the optimal conditions (B1 and B2)
Hemicellulose-derived products in the aqueous phase
The aqueous fraction contained hemicellulose-derived products as the main components. Nearly half of the hemicellulose (48.5% of the available hemicellulose content in the starting material) was recovered as monomeric sugars (70.8 mg/g) and oligosaccharides (42.9 mg/g) based on a weight basis of the starting material under the optimal condition. According to the data in supplementary data Fig, S2, pentoses were found as the major sugars (86.84%), followed by hexoses (13.16%). Low levels of furan products from sugar dehydration were found (furfural, 9.75 mg/g biomass and 5-hydroxymethyl furfural, 2.4 mg/g biomass), suggesting less than 10.68% conversion of sugar products to furan compounds.
Lignin recovered from the organic phase
Efficient recovery of lignin removed from the biomass during the organosolv process is important, because the lignin from organosolv has a high purity and can be converted to value-added products (Käldström et al. 2014; Li and McDonald 2014). The fractionated lignin product recovered from the organic phase contained 89.6% Klason lignin with 0.76% acid soluble lignin (Table S2). Cross-contamination of sugar (< 1%) and ash (0.16%) was negligible under this experimental condition. The recovered lignin had a weight average molecular weight (Mw) of 2727 g/mol and a number average molecular weight (Mn) of 1640 g/mol, and hence a polydispersity (Mw/Mn) of 1.663. This result suggested the lignin product molecules had a non-uniform distribution of molecular weight.
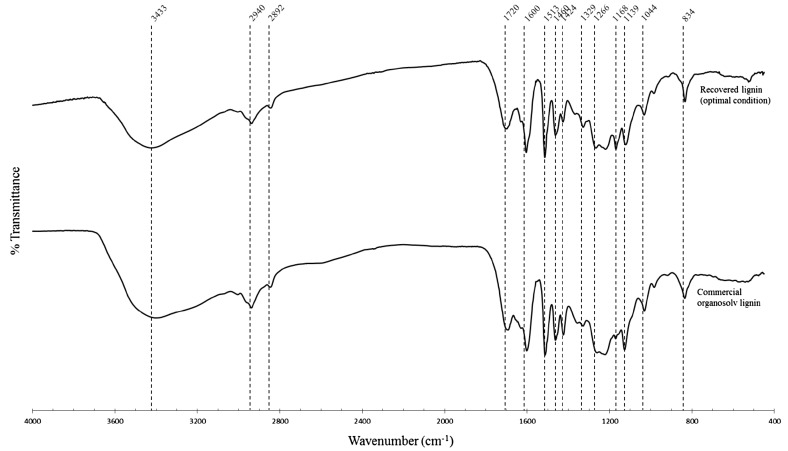
The fractionated lignin product showed a similar FT-IR spectra to commercial organosolv-extracted lignin (Fig. 4). The FT-IR spectra showed the vibration of O–H stretching at 3433 cm−1 and the signal at 2940 and 2892 cm−1, which are attributed to CH vibrational stretching in CH2 and CH3 groups. The characteristic signal of carbonyl group stretching is observed at 1720 cm−1. Strong signals at 1600, 1513, and 1424 cm−1 are assigned to aromatic skeleton vibrations in lignin. The signal at 1460 cm−1 corresponds to CH formation and bonding of methoxyl in lignin. The signal at 1168 cm−1 suggests a conjugated carbonyl group. In addition, the signals at 1266 and 1044 cm−1 are associated with guaiacyl (G) units, while syringyl (S) unit signals were observed at 1329 and 1139 cm−1. The signal at 834 cm−1 is attributed to C–H out of plane deformation. The results thus indicated no major chemical modification of the lignin structure occurred during the CF process.
Fig. 4.
Fourier-transform infrared spectroscopy (FT-IR) spectra of lignin recovered from the fractionation process under the optimal conditions compared with commercial organosolv-extracted lignin
Comparison of fractionated products from CF with formic acid promoter with previous reports
Based on the method developed in this study, a highly cellulose-enriched solid fraction with 81.8% purity with marked improvement in enzymatic digestibility was obtained with high recovery (91–99%) which was relatively high compared to previous works using the conventional CF-based processes (Imman et al. 2015). The glucose recovery achieved using the modified CF-based process with formic acid in this study (84.5%) was higher than the previously reported value using CF-based process of prairie cordgrass with the same solvent system from which 74.9% glucose recovery was obtained (Cybulska et al. 2012). Moreover, cellulose and sugar recoveries in the modified CF process in this study are higher than that obtained by other methods such as hydrothermal, steam explosion, ionic liquid and alkaline pretreatment applied to different types of materials, e.g., bamboo, rapeseed straw, giant reed and mixed softwood (Xiao et al. 2014; Lopez-Linares et al. 2015; Trinh et al. 2015; Jiang et al. 2016). The markedly high cellulose content of the solid fraction makes it promising for further processing to make microcrystalline cellulose and nanocellulose (Gilfillan et al. 2014; Hgaderi et al. 2014; de Oliveira et al. 2016), in addition to conversion to sugars for further fermentation to biofuels and commodity chemicals (Chen et al. 2015; Li et al. 2017a, b, Li et al. 2017b).
The CF process allows efficient solubilization of hemicellulose and removal of lignin into the aqueous and organic phases, respectively. The efficiencies of removing these non-cellulosic components in this study were higher than those previously reported using hydrothermal and organosolv processes (Koo et al. 2011; Hi et al. 2012; Amiri and Karimi 2015). Nearly 90% of the hemicellulose was solubilized under the optimized reaction conditions in this study, which is comparable to that obtained using conventional CF-based processes on different lignocellulosic materials, i.e., eucalyptus wood chip and rice straw (Klamrassamee et al. 2013; Imman et al. 2015). The level of furans generated as dehydration products in this study were slightly higher than the tolerable level for yeast fermentation (0.5 g/L) (Klinke et al. 2004). The hemicellulose fraction can be further detoxified (Villarreal et al. 2006), or purified by chromatography (Bozell et al. 2011) and used as a substrate for fermentation to valorized chemicals, e.g., xylitol (Jia et al. 2016), or it can be directly dehydrated to furfural (Qing et al. 2017).
The degree on lignin removal obtained in this work was in the same range as that reported previously using a H2SO4 catalyzed CF-based fractionation of prairie cordgrass (Brudecki et al. 2012) and higher than that reported for fractionation of corn stover and wheat straw using organosolv processes, which were in the range of 65–75% (Wildschut et al. 2013; Yu et al. 2013; Chen et al. 2015). Furthermore, the level of lignin removal in this study is comparable to that obtained for fractionation of wood lignocellulosic materials using a combination of organosolv and autohydrolysis treatments (Garcia et al. 2011; Romani et al. 2011). The lignin obtained in this study was not chemically modified and had a remarkably high purity, with very low cross-contamination by polysaccharide-derived products and ash. Ash was reported as a contaminant in Kraft lignin (Hussin et al. 2015), and sulfur adducts to lignin could be resulted from the conventional H2SO4-catalyzed CF process (Gellerstedt 2015; Faris et al. 2017). The high quality lignin obtained by CF with formic acid promoter is thus suitable for conversion to higher valued products such as lignin carbon fiber (Meek et al. 2016) and phenolic products (Long et al. 2014; Kim et al. 2017).
The role of formic acid in the CF process
According to our study, the use of aqueous formic acid in the solvent mixture allowed high efficiency and selectivity of the reaction which was reflected in the high yield and purity of the fractionated products compared to selected strong acid (H2SO4) and weak acid (acetic acid). Formic acid can be considered to function as an acid promoter and a solvent in the CF solvent mixture. It is assumed that the formic acid-catalyzed organosolv process involves the hydrolysis of ether and ester bonds of the α-carbons units between hemicellulose and lignin linkages (Zhao et al. 2009). The use of aqueous formic acid in organosolv has been shown to increase the rate of cleavage of α- and β-ether linkages in lignin (McDonough 1993) and resulted in the dissolution of lignin fragments with lower molecular weights (Zhang et al. 2016a, b). This can result in increase xylan dissolution under hydrothermal conditions (Ma et al. 2015).
Formic acid is a weak organic acid widely used in many industries. It has a lower b.p. than H2SO4 (100.8 and 337 °C, respectively). Moreover, formic acid is miscible with water and alcohols and can be recovered by evaporation under a depressurized condition (Li et al. 2015). It is also minimally corrosive to industrial equipment and its use in industrial processes incurs a low cost for waste treatment. Although formic acid is considered inhibitory to growth of fermenting microorganisms, this can be solved by an additional washing step after the fractionation process. The level of formic acid remaining in the solid biomass in our study (0.007 g/L, data not shown) is below the growth inhibitory level for Saccharomyces cerevisiae (Klinke et al. 2004; Cantarella et al. 2004).
Application of organic acids, e.g., formic acid and acetic acid as the sole solvent or as mixtures in organosolv fractionation of lignocellulosic biomass in the presence or absence of strong acid promoters has been reported (Snelders et al. 2014; Ferrer et al. 2013; Vanderghem et al. 2012). Shui et al. reported the use of an aqueous acetic/formic acid mixture in the presence of strong acids, e.g., HCl or H2SO4 for fractionation of crude cellulose from corn stalk with efficient removal of hemicellulose and separation of lignin (Shui et al. 2016). Formic acid in the presence of HCl as a promoter has also been used for hydrolysis of hemicellulose, and swelling cellulose fibers in preparation of cellulose nanocrystals from chemical bleached pulp (Li et al. 2015). The formic acid used in the process could be recovered by vacuum distillation with the recovery rate of > 90%.The Formiline process using formic acid as delignification agent has been reported (Zhao and Liu 2012). The spent liquor could be efficiently recycling in subsequent batches with a slightly decreasing trend in performance of the reaction. The delignified solids from sugarcane bagasse and wheat straw were shown to improve enzymatic digestibility and could be used as substrates for fermentation (Chen et al. 2015, Zhao and Liu 2012).
In our study, the use of formic acid with the solvent mixture in the CF process resulted in a miscible solvent with low boiling point (≤ 100 °C) with homogeneous solution properties which can be easily recovered using evaporation under depressurized condition compared to the conventional MIBK/ROH/H2O system. Composition of the respective solvents was shown to influence the reaction efficiency and selectivity. Increasing water content was found to increase removal efficiency of hemicelluloses. This can be relevant to the high solubility of hemicellulose and its derived products in water under pressurized and high temperature conditions where water is also auto-ionized to protonated form (Hongdan et al. 2013). The ability of ethyl acetate [referred from Hildebrand parameter, 18.6 (J/cm3)−1/2] is suitable for solubilized of lignin fraction, as its solubility parameter approach a value of around 22.5 (J/cm3)−1/2 (Zhao et al. 2017). The use of ethyl acetate as an alternative solvent was also reported to reduce the unwanted side condensation reaction of lignin due to incomplete drying of the organic fraction which can lead to the formation of pseudo-lignin higher-molecular-weight lignin (Brudecki et al. 2013). However, side hydrolysis reaction of ethyl acetate to acetic acid in the presence of acid promoter needed to be considered.
Conclusion
A modified CF-based one-step organosolv fractionation optimized for formic acid and a ternary solvent mixture is reported in this study. This process offers improved reaction efficiency and selectivity, allowing higher performance of the fractionation process for separation of lignocellulosic biopolymers in integrated biorefinery. Potential recyclability of the solvent system could further improve economics of the developed fractionation process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project was financially supported by PTT Global Chemicals and the Thailand Research Fund (RTA5980006). Manuscript proofreading by Dr. Pornkamol Unrean and Dr. Philip J. Shaw is appreciated.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1244-9) contains supplementary material, which is available to authorized users.
Contributor Information
Verawat Champreda, Phone: +66 2564 6700, Email: verawat@biotec.or.th.
Navadol Laosiripojana, Phone: +66-2872-9014, Email: navadol@jgsee.kmutt.ac.th.
References
- Amiri H, Karimi K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst Eng. 2015;38:1959–1972. doi: 10.1007/s00449-015-1437-0. [DOI] [PubMed] [Google Scholar]
- Black SK, Hames BR, Myers MD (1998) Inventors. Midwest Research Institute, Assignee. Method of separating lignocellulosic material into lignin, cellulose and dissolved sugars, United State patent US 5730837, Mar 24
- Boeriu CG, Fitigau FI, Gosselink RJA, Frissen AE, Stoutjesdijk J, Peter F. Fractionation of five technical lignins by selective extraction in green solvents and characterization of isolated fractions. Ind Crops Prod. 2014;62:481–490. doi: 10.1016/j.indcrop.2014.09.019. [DOI] [Google Scholar]
- Bozell JJ, Black SK, Myers M, Cahill D, Miller WP, Park S. Solvent fractionation of renewable woody feedstocks: organosolv generation of biorefinery process steams for the production of biobased chemicals. Biomass Bioenerg. 2011;35:4197–4208. doi: 10.1016/j.biombioe.2011.07.006. [DOI] [Google Scholar]
- Brudecki G, Cybulska I, Rosentrater K, Julson J. Optimization of clean fractionation processing as a pre-treatment technology for prairie cordgrass. Bioresour Technol. 2012;107:494–504. doi: 10.1016/j.biortech.2011.12.122. [DOI] [PubMed] [Google Scholar]
- Brudecki G, Cybulska I, Rosentrater K. Integration of extrusion and clean fractionation processes as a pre-treatment technology for prairie cordgrass. Bioresour Technol. 2013;135:672–682. doi: 10.1016/j.biortech.2012.10.132. [DOI] [PubMed] [Google Scholar]
- Cantarella M, Cantarella L, Gallifuoco A, Spera A, Alfani F. Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol Prog. 2004;20:200–206. doi: 10.1021/bp0257978. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao J, Hu T, Zhao X, Liu D. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: substrate digestibility, fermentability and structural features. Appl Energ. 2015;150:224–232. doi: 10.1016/j.apenergy.2015.04.030. [DOI] [Google Scholar]
- Cybulska I, Brudecki G, Rosentrater K, Lei H, Julson J. Catalyzed modified clean fractionation of prairie cordgrass integrated with hydrothermal post-treatment. Biomass Bioenerg. 2012;46:389–401. doi: 10.1016/j.biombioe.2012.08.002. [DOI] [Google Scholar]
- Cybulska I, Brudecki GP, Hankerson BR, Julson JL, Lei H. Catalyzed modified clean fractionation of switchgrass. Bioresour Technol. 2013;127:92–99. doi: 10.1016/j.biortech.2012.09.131. [DOI] [PubMed] [Google Scholar]
- de Oliveira FB, Bras J, Pimenta MTB, Curvelo AAS, Belgacem MN. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind Crops Prod. 2016;93:48–57. doi: 10.1016/j.indcrop.2016.04.064. [DOI] [Google Scholar]
- Du H, Liu C, Mu X, Gong W, Lv D, Hong Y, Si C, Li B. Preparation and characterization of thermally stable cellulose nano crystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose. 2016;23:2389–2407. doi: 10.1007/s10570-016-0963-5. [DOI] [Google Scholar]
- Faris AH, Rahim AA, Ibrahim MNM, Hussin MH, Alkurdi AM, Salehabadi A. Investigation of oil palm based Kraft and auto-catalyzed organosolv lignin susceptibility as a green wood adhesives. Inter J Adhesion Adhesives. 2017;74:115–122. doi: 10.1016/j.ijadhadh.2017.01.006. [DOI] [Google Scholar]
- Fengel D, Wegener G. Wood: Chemistry. Reactions, De Gruyster, Berlin: Ultrastructure; 1984. [Google Scholar]
- Ferrer A, Vega A, Rodríguez A, Jiménez L. Acetosolv pulping for the fractionation of empty fruit bunches from palm oil industry. Bioresour Technol. 2013;132:115–120. doi: 10.1016/j.biortech.2012.12.189. [DOI] [PubMed] [Google Scholar]
- Garcia A, Alriols MG, Llano-Ponte R, Labidi J. Ultrasound-assisted fractionation of the lignocellulosic material. Bioresour Technol. 2011;102:6326–6330. doi: 10.1016/j.biortech.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Gellerstedt G. Soft wood kraft lignin: raw material for the future. Ind Crops Prod. 2015;77:845–854. doi: 10.1016/j.indcrop.2015.09.040. [DOI] [Google Scholar]
- Gilfillan WN, Moghaddam L, Doherty WOS. Preparation and characterization of composites from starch with sugarcane bagasse nanofibres. Cellulose. 2014;21:2695–2712. doi: 10.1007/s10570-014-0277-4. [DOI] [Google Scholar]
- Hgaderi M, Mousavi M, Yousefi H, Labbafi M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr Polym. 2014;104:59–65. doi: 10.1016/j.carbpol.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Hi KL, Yeap SP, Mashitah MD. Pretreatment of pressed pericarp fibers (PPF) using alcohol as solvent to increase the accessibility of cellulose for cellulose production. Appl Biol Chem. 2012;55:507–514. [Google Scholar]
- Hongdan Z, Shaohua X, Shubin W. Enhancement of enzymatic saccharification of sugarcane bagasse by liquid hot water pretreatment. Bioresour Technol. 2013;143:391–396. doi: 10.1016/j.biortech.2013.05.103. [DOI] [PubMed] [Google Scholar]
- Hussin MH, Rahim AA, Ibrahim MNM, Perrin D, Brosse N. Enhanced properties of oil palm fronds (OPF) lignin fractions produced via tangential ultrafiltration technique. Ind Crops Prod. 2015;66:1–10. doi: 10.1016/j.indcrop.2014.12.027. [DOI] [Google Scholar]
- Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N. Fractionation of rice straw by a single-step solvothermal process: effects of solvents, acid promoters, and microwave treatment. Renewable Energy. 2015;83:663–673. doi: 10.1016/j.renene.2015.04.062. [DOI] [Google Scholar]
- Jia H, Shao T, Zhong C, Li H, Jiang M, Zhou H, Wei P. Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr Polym. 2016;151:676–683. doi: 10.1016/j.carbpol.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Jiang D, Ge X, Zhang Q, Li Y. Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour Technol. 2016;216:60–68. doi: 10.1016/j.biortech.2016.05.052. [DOI] [PubMed] [Google Scholar]
- Käldström M, Meine N, Farès C, Rinaldi R, Schüth F. Fractionation of ‘water-soluble lignocellulose’ into C5/C6 sugars and sulfur-free lignins. Green Chem. 2014;16:2454–2462. doi: 10.1039/C4GC00168K. [DOI] [Google Scholar]
- Katahira R, Mittal A, McKinney K, Ciesielski PN, Donohoe BS, Black SK, Johnson DK, Biddy MJ, Beckham GT. Evaluation of clean fractionation pretreatment for the production of renewable fuels and chemicals from corn stover. ACS Sustain Chem Eng. 2014;2:1364–1376. doi: 10.1021/sc5001258. [DOI] [Google Scholar]
- Kim M, Son D, Choi JW, Jae J, Suh DJ, Ha JM, Lee KY. Production of phenolic hydrocarbons using catalytic depolymerisation of empty fruit bunch (EFB)-derived organosolv lignin on Hβ-supported Ru. Chem Eng J. 2017;309:187–196. doi: 10.1016/j.cej.2016.10.011. [DOI] [Google Scholar]
- Klamrassamee T, Champreda V, Reunglek V, Laosiripojana N. Comparison of homogeneous and heterogeneous acid promoters in single-step aqueous-organosolv fractionation of eucalyptus wood chips. Bioresour Technol. 2013;147:276–284. doi: 10.1016/j.biortech.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- Koo BW, Park N, Jeong HS, Choi JW, Yeo H, Choi IG. Characterization of by-products from organosolv pretreatment of yellow poplar wood (Liriodendron tulipifera) in the presence of acid and alkali catalysts. J Ind Eng Chem. 2011;17:18–24. doi: 10.1016/j.jiec.2010.10.003. [DOI] [Google Scholar]
- Lam HQ, Bigot YL, Delmas M, Avignon G. Formic acid pulping of rice straw. Ind Crop Prod. 2001;14:65–71. doi: 10.1016/S0926-6690(00)00089-3. [DOI] [Google Scholar]
- Li H, McDonald AG. Fractionation and characterization of industrial lignins. Ind Crops Prod. 2014;62:67–76. doi: 10.1016/j.indcrop.2014.08.013. [DOI] [Google Scholar]
- Li B, Xu W, Kronlund D, Määttänen A, Liu J, Smatt JH, Peltonen J, Willför S, Mu X, Xu C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr Polym. 2015;133:605–612. doi: 10.1016/j.carbpol.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Li MF, Yang S, Sun RC. Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour Technol. 2016;200:971–980. doi: 10.1016/j.biortech.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Li H, Xiong L, Xiong L, Chen X, Wang C, Qi G, Huang C, Luo M, Chen X. Enhanced enzymatic hydrolysis and acetone-butanol-ethanol fermentation of sugarcane bagasse by combined diluted acid with oxidate ammonolysis pretreatment. Bioresour Technol. 2017;228:257–263. doi: 10.1016/j.biortech.2016.12.119. [DOI] [PubMed] [Google Scholar]
- Li MF, Yu P, Li SX, Wu XF, Xiao X, Bian J. Sequential two-step fractionation of lignocellulose with formic acid organosolv followed by alkaline hydrogen peroxide under mild conditions to prepare easily saccharified cellulose and value-added lignin. Energy Convers Manage. 2017;148:1426–1437. doi: 10.1016/j.enconman.2017.07.008. [DOI] [Google Scholar]
- Lin H, Yeh CE, Hong GB, Lee MJ. Enhancement of liquid phase splitting of water + ethanol + ethyl acetate mixtures in the presence of a hydrophilic agent or an electrolyte substance. Fluid Phase Equilib. 2005;237:21–30. doi: 10.1016/j.fluid.2005.08.009. [DOI] [Google Scholar]
- Liu C, Li B, Du H, Lv D, Zhang Y, Yu G, Mu X, Peng H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr Polym. 2016;151:716–724. doi: 10.1016/j.carbpol.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Long J, Zhang Q, Wang T, Zhang X, Xu Y, Ma L. An efficient and economical process for lignin depolymerisation in biomass-derived solvent tetrahydrofuran. Bioresour Technol. 2014;154:10–17. doi: 10.1016/j.biortech.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Lopez-Linares JC, Ballesteros I, Touran J, Cara C, Castro E, Ballesteros M, Romero I. Optimization of uncatalyzed steam explosion pretreatment of rapeseed straw for biofuel production. Bioresour Technol. 2015;190:97–105. doi: 10.1016/j.biortech.2015.04.066. [DOI] [PubMed] [Google Scholar]
- Ma J, Ji Z, Chen JC, Zhou X, Kim YS, Xu F. The mechanism of xylans removal during hydrothermal pretreatment of poplar fibers investigated by immunogold labeling. Planta. 2015;242:327–337. doi: 10.1007/s00425-015-2313-5. [DOI] [PubMed] [Google Scholar]
- McDonough TJ. The chemistry of organosolv delignification. Tappi J. 1993;76:186–193. [Google Scholar]
- Meek N, Penumadu D, Hosseinaei O, Harper D, Young S, Rials T. Synthesis and characterization of lignin carbon fiber and composites. Compos Sci Technol. 2016;137:60–68. doi: 10.1016/j.compscitech.2016.10.016. [DOI] [Google Scholar]
- Qin L, Liu ZH, Li BZ, Dale BE, Yuan YJ. Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresour Technol. 2012;112:319–326. doi: 10.1016/j.biortech.2012.02.134. [DOI] [PubMed] [Google Scholar]
- Qing Q, Guo Q, Zhou L, Wan Y, Xu Y, Ji H, Gao X, Zhang Y. Catalytic conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Bioresour Technol. 2017;226:247–254. doi: 10.1016/j.biortech.2016.11.118. [DOI] [PubMed] [Google Scholar]
- Reisinger M, Tirpanalan O, Huber F, Kneifel W, Novalin S. Investigations on a wheat bran biorefinery involving organosolv fractionation and enzymatic treatment. Bioresour Technol. 2014;170:53–61. doi: 10.1016/j.biortech.2014.07.068. [DOI] [PubMed] [Google Scholar]
- Romani A, Garrote G, Lopez F, Parajo JC. Eucalyptus globulus wood fractionation by autohydrolysis and organosolv delignification. Bioresour Technol. 2011;102:5896–5904. doi: 10.1016/j.biortech.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Sathitsuksanoh N, Zhu Z, Zhang Y-HP. Cellulose solvent- and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour Technol. 2012;117:228–233. doi: 10.1016/j.biortech.2012.04.088. [DOI] [PubMed] [Google Scholar]
- Segal L, Creely L, Martin AE. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractormeter. Text Res J. 1959;29:786–794. doi: 10.1177/004051755902901003. [DOI] [Google Scholar]
- Sindhu R, Binod P, Satyanagalakshmi K, Janu KU, Sajna KV, Kurien N, Sukumaran RK, Pandey A. Formic acid as a potential pretreatment agent for the conversion of sugarcane bagasse to bioethanol. Appl Biochem Biotechnol. 2010;162:2313–2323. doi: 10.1007/s12010-010-9004-2. [DOI] [PubMed] [Google Scholar]
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory NREL/TP-510-42623
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory NREL/TP-510-42618
- Snelders J, Dornez E, Mlayah BB, Huijgen WJJ, de Wild PJ, Gosselink RJA, Gerritsma J, Courtin CM. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour Technol. 2014;156:275–282. doi: 10.1016/j.biortech.2014.01.069. [DOI] [PubMed] [Google Scholar]
- Sun FF, Wang L, Hong J, Ren J, Du F, Hu J, Zhang Z, Zhou B. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour Technol. 2015;187:354–361. doi: 10.1016/j.biortech.2015.03.051. [DOI] [PubMed] [Google Scholar]
- Trinh LTP, Lee YJ, Lee JW, Lee HJ. Characterization of ionic liquid pretreatment and the bioconversion of pretreated mixed softwood biomass. Biomass Bioenerg. 2015;81:1–8. doi: 10.1016/j.biombioe.2015.05.005. [DOI] [Google Scholar]
- Vanderghem C, Brostaux Y, Jacquet N, Blecker C, Paquot M. Optimization of formic/acetic acid delignification of Miscanthus x giganteus for enzymatic hydrolysis using response surface methodology. Ind Crops Prod. 2012;35:280–286. doi: 10.1016/j.indcrop.2011.07.014. [DOI] [Google Scholar]
- Villarreal MLM, Prata AMR, Felipe MGA, Almeida E Silva JB. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzyme Microbial Technol. 2006;40:17–24. doi: 10.1016/j.enzmictec.2005.10.032. [DOI] [Google Scholar]
- Wildschut J, Smit AT, Reith JH, Huijgen WJJ. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour Technol. 2013;135:58–66. doi: 10.1016/j.biortech.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Xiao X, Bian J, Li MF, Xu H, Xiao B, Sun RC. Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour Technol. 2014;159:41–47. doi: 10.1016/j.biortech.2014.02.096. [DOI] [PubMed] [Google Scholar]
- Yu G, Li B, Liu C, Zhang Y, Wang H, Mu X. Fractionation of the main components of corn stover by formic acid and enzymatic saccharification of solid residue. Ind Crops Prod. 2013;50:750–757. doi: 10.1016/j.indcrop.2013.08.053. [DOI] [Google Scholar]
- Zhang J, Deng H, Lin L, Sun Y, Pan C, Liu S. Isolation and characterization of wheat straw lignin with a formic acid process. Bioresour Technol. 2010;101:2311–2316. doi: 10.1016/j.biortech.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Zhang M, Qi W, Liu R, Su R, Wu S, He Z. Fractionating lignocellulose by formic acid: characterization of major components. Biomass Bioenerg. 2010;34:525–532. doi: 10.1016/j.biombioe.2009.12.018. [DOI] [Google Scholar]
- Zhang K, Pei Z, Wang D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemical: a review. Bioresour Technol. 2016;199:106–114. doi: 10.1016/j.biortech.2015.08.102. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Harrison MD, Rackemann DW, Doherty WOS, O’Hara M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016;18:360–381. doi: 10.1039/C5GC02034D. [DOI] [Google Scholar]
- Zhao X, Liu D. Fractionating pretreatment of sugarcane bagasse by aqueous formic acid with direct recycle of spent liquor to increase cellulose digestibility-the Formiline process. Bioresour Technol. 2012;117:25–32. doi: 10.1016/j.biortech.2012.04.062. [DOI] [PubMed] [Google Scholar]
- Zhao X, Cheng K, Liu D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbial Biotechnol. 2009;82:815–827. doi: 10.1007/s00253-009-1883-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li S, Wu R, Liu D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels Bioprod Bioref. 2017;11:567–590. doi: 10.1002/bbb.1768. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.