Abstract
AIM
To evaluate the efficacy of prophylactic administration of topical non-steroidal anti-inflammatory drugs (NSAIDs) on macular edema following cataract surgery in diabetic patients, and to compare between types of NSAIDs (ketorolac tromethamine 0.4% and nepafenac 0.1%).
METHODS
Group 1 (control) received artificial tears substitute as a placebo group, group 2 (nepafenac) received topical nepafenac 0.1%, and group 3 (ketorolac) received topical ketorolac tromethamine 0.4%. Patients were examined postoperatively after completing one week, one month, two months and three months' intervals for evaluating cystoid macular edema (CME) development. The main study outcomes were achieving the best corrected visual acuity (BCVA) and change in the central macular thickness (CMT) measured with optical coherence topography (OCT).
RESULTS
Eighty eyes of 76 patients were included in this study. BCVA showed a statistically significant difference at the third month postoperative follow up between the control group and the NSAIDs groups (P=0.04). There was an increase in the CMT in all cases starting from postoperative first week until third month. CMT showed a statistically significant difference between control group and NSAIDs groups from postoperative first month until third month (P=0.008, 0.027, 0.004). There was no statistically significant difference between nepafenac and ketorolac groups in BCVA and OCT CMT.
CONCLUSION
Prophylactic preoperative and postoperative NSAIDs may have a role in reducing the frequency and severity of CME in diabetic eyes following cataract surgery.
Keywords: diabetes mellitus, cataract surgery, central macular thickness, non-steroidal anti-inflammatory drugs, ketorolac, nepafenac
INTRODUCTION
Currently, phacoemulsification and intra-capsular intraocular lens (IOL) is one of the most performed surgical procedures[1]. Cataract in diabetic patients with its high prevalence and earlier age of development is considered as a major problem against adequate fundus examination and laser photocoagulation therapy in addition to decreasing visual acuity (VA)[2].
Macular thickening is a well-known post-operative complication after cataract surgery, even with uncomplicated surgery[3]. Clinical cystoid macular edema (CME) can be identified on biomicroscopic examination and associated with decreasing VA. After phacoemulsification surgery, clinical CME has been reported to be less than 3% in healthy population[4]. Subclinical CME is diagnosed with fluorescein angiography as leakage from perifoveal dilated capillaries without VA affection. After uncomplicated phacoemulsification in healthy individuals the incidence of subclinical CME has been reported to be less than 20%[3].
The pathophysiology of diabetic retinopathy (DR) is mainly microangiopathy in which small blood vessels are affected by occlusion and leakage[5]. The blood retinal barrier in diabetic eyes is impaired to a variable degree and is responsible for developing post-operative CME depending on many factors including duration, severity of disease, presence of retinopathy and previous treatment with photocoagulation or steroid injection[6]. Post-operative CME had been reported with its strong association with non-insulin dependent diabetes mellitus (NIDDM) more than insulin dependent diabetes mellitus (IDDM)[7]. Many studies reported that retinopathy and macular changes in diabetic patients were accelerated by cataract surgery[8]. But other studies attributed this acceleration to the natural course of diabetic disease[6],[9].
Fluorescein angiography is considered a golden standard for diagnosing macular edema, however, quantification of fluorescein leakage is difficult. Optical coherence topography (OCT) nowadays, has an upper hand in the diagnosis of macular edema because of its advantages as a non-invasive device, in addition to detecting macular edema quantitatively[10] as well as qualitatively[11].
Recently, an increased evidence that prostaglandins (PGs) play a role in pathogenesis of DR and macular edema is reported[12]. In spite of using corticosteroids as a golden standard for treating ocular inflammation, it was associated with adverse effects that warrant their judicious use. Multiple side effects of topical steroids made it unsafe for extended periods. Topical corticosteroids side effects included suppression of host immune response which increases the susceptibility to microbial infections, retardation of corneal epithelium and stromal wound healing, and rise in intraocular pressure (IOP)[13]. Non-steroidal anti-inflammatory drugs (NSAIDs) are potent anti-inflammatory medications which decrease pro-inflammatory PGs by their inhibitory effect on cyclooxygenase (COX)[12]. The therapeutic efficacy of topical NSAIDs might have a role in the treatment of allergic conjunctivitis, post-operative inflammation, pain and macular edema in addition to stability of intraoperative dilated pupil[14]. Also, intravitreal diclofenac was effective in the treatment of diffuse diabetic macular edema (DME) up to 12wk compared to therapeutic effects of intravitreal triamcinolone on retinal thickness[15].
This study was conducted to evaluate the efficacy of prophylactic administration of topical NSAIDs on macular edema following cataract surgery in diabetic patients and to compare between types of NSAIDs (ketorolac tromethamine 0.4% and nepafenac 0.1%) in preventing macular edema in diabetic patients undergoing phacoemulsification cataract surgery.
SUBJECTS AND METHODS
Patient Enrollment
This comparative prospective randomized clinical trial (RCT) was conducted at Mansoura Ophthalmic Center, Mansoura University from December 2015 to January 2017. The study protocol was approved by Medical Research Ethics Committee, Faculty of Medicine, Mansoura University (No.MS/15.12.09) and informed consent was obtained from each participant in the study after assuring confidentiality. Inclusion criteria were diabetic patients with senile immature cataract scheduled to undergo cataract extraction and posterior chamber IOL implantation. Exclusion criteria were: 1) anterior segment pathology (corneal opacities, pseudo-exfoliation syndrome, dense cataract interfering with OCT imaging); 2) posterior segment pathology, e.g. DR, previous retinal photocoagulation therapy, DME, age-related macular degeneration, retinal vascular diseases or history of uveitis, intraoperative complications including: posterior capsular rupture, vitreous loss or IOL not implanted in the capsular bag; 3) postoperative complications: e.g. increased IOP, leaking incision, inflammation or corneal edema; 4) patients using concomitant medication such as topical or systemic NSAIDs, steroids which may interfere with the assessment of the study outcome measures, topical prostaglandin analogues; 5) hypersensitivity to the NSAID drug class; 6) previous intraocular surgery and ocular trauma to the same eye.
Study Protocol
Preoperatively, patients had been subjected to meticulous ophthalmic examination, including history related to the duration, control and medications of diabetes mellitus and previous ocular surgery or trauma, measurement of best corrected visual acnity (BCVA), anterior segment examination by slit lamp, fundus examination using noncontact Volk lens +78 D, IOP using applanation tonometer, A-scan and B-scan ultrasound and baseline spectral-domain OCT of the macula scan using Topcon 3D 1000OCT (Hasunuma-cho, Itabashi-Ku, Tokyo, Japan).
Postoperative follow up at first day using slit lamp was done to exclude any patient with postoperative pathology as severe corneal edema, anterior chamber flare and cells, abnormal IOL position or abnormal IOP.
Postoperative follow up at first week, first month, second month and third month was done in regard with BCVA assessment and OCT to measure the central macular thickness (CMT).
Simple random sample by method of sealed envelopes was used; every patient in the study had the same chance to be allocated to any of the 3 studied groups: group 1, patients received artificial tears substitute as placebo group in the operative eye four times a day (breakfast, lunch, dinner and before bedtime); group 2, patients received nepafenac 0.1% (one drop three times a day; breakfast, lunch and before bedtime); group 3, patients received ketorolac tromethamine 0.4% (one drop four times a day; breakfast, lunch, dinner and before bedtime).
They began medications for two days preoperatively and continued for two months postoperatively. In addition, patients received standard regimen of topical steroid-antibiotic drops 4 times daily for a week and then tapered over the next 3wk.
Surgical Technique
All phaco-operations were performed by the same experienced surgeon. The standardized surgical technique was clear corneal incision, phacoemulsification using divide and conquer technique, and posterior chamber foldable IOL implantation into the capsular bag.
Statistical Analysis
Data was fed to the computer and analyzed using IBM SPSS (statistical package for social science) software package version 20.0. Qualitative data were described using number and percent. Quantitative data were described using median (minimum and maximum) for non-parametric data and mean, standard deviation for parametric data after testing normality using Kolmogrov-Smirnov test. Significance of the obtained results was judged at the 5% level.
The used tests were Chi-square test & Monte Carlo for categorical variables to compare between different groups, Student's t-test for parametric quantitative variables to compare between two studied groups, one way ANOVA test for parametric quantitative variables to compare between more than two studied groups and Mann-Whitney test for non-parametric quantitative variables to compare between two studied groups.
RESULTS
Demographic Data and Clinical Characteristics
Data was collected and recorded from December 2015 to January 2017 at Mansoura Ophthalmic Center. A total of 80 eyes of 76 patients were enrolled in this study and underwent surgery (40 eyes in control group, 20 in nepafenac group, and 20 in ketorolac group). Comparison between patients of the three groups regarding the age and sex revealed no statistically significant differences.
All patients enrolled in this study had good control of diabetes mellitus, half of them on insulin (40 eyes) and other half (40 eyes) on oral medications. The difference in the preoperative BCVA was statistically insignificant between the studied groups with P=0.28 (Table 1).
Table 1. Demographic data and clinical characteristics among 3 studied groups.
| Parameters | Control group (n=40) | Nepafenac group (n=20) | Ketorolac group (n=20) | Test of significance |
| Age (y) | 58.4±9.25 | 57.9±10.1 | 63.35±7.7 | F=2.28; P=0.11 |
| Sex | χ2=2.27; P=0.32 | |||
| M | 19 (47.5) | 6 (30.0) | 6 (30.0) | |
| F | 21 (52.5) | 14 (70.0) | 14 (70.0) | |
| Eye | χ2=0.0; P=1.0 | |||
| Left | 16 (40.0) | 8 (40.0) | 8 (40.0) | |
| Right | 24 (60.0) | 12 (60.0) | 12 (60.0) | |
| General health | ||||
| Hypertension | 19 (47.5) | 8 (40.0) | 9 (45.0) | χ2=2.97; P=0.23 |
| DM drugs | χ2=5.32; P=0.07 | |||
| Insulin injection | 21 (52.5) | 13 (65.0) | 6 (30.0) | |
| Oral antidiabetic drugs | 19 (47.5) | 7 (35.0) | 14 (70.0) | |
| HbA1c | 7.06±0.38 | 7.06±0.33 | 7.18±0.4 | F=0.206; P=0.82 |
| Preoperative BCVA (logMAR) | 1.21±0.3 | 1.07±0.28 | 1.16±0.29 | F=1.28; P=0.28 |
DM: Diabetes mellitus; HbA1c: Hemoglobin A1c.
n (%)
Comparison Between Control Group and NSAIDs Group Best corrected visual acuity
The difference in the BCVA was statistically insignificant between the two groups at the preoperative assessment, post-operative first week and first month. However, the BCVA improved at first week in control group to 0.25 (0.2-0.8) from 1.1 (0.6-1.5) preoperatively and in NSAIDs group to 0.3 (0.2-0.8) from 1.1 (0.6-1.5) preoperatively. There was statistically significant differences in comparison between patients of the two groups regarding the BCVA three months postoperatively with P=0.04 (Table 2).
Table 2. Comparison of BCVA among control and NSAIDs groups.
| Visual acuity | Control group (n=40) | NSAIDs group (n=40) | Test of significance |
| Preoperative | 1.1 (0.6-1.5) | 1.1 (0.6-1.5) | Z=1.32; P=0.18 |
| 1wk postop. | 0.25 (0.2-0.8) | 0.3 (0.2-0.8) | Z=0.75; P=0.46 |
| 1mo postop. | 0.2 (0.0-0.6) | 0.3 (0.0-0.6) | Z=1.37; P=0.17 |
| 2mo postop. | 0.2 (0.0-0.6) | 0.2 (0.0-0.6) | Z=1.08; P=0.28 |
| 3mo postop. | 0.0 (0.0-0.6) | 0.0 (0.0-0.3) | Z=2.01; P=0.04a |
aP value statistically significant <0.05.
median (min-max), logMAR
Central macular thickness
OCT CMT was listed in Table 3. The difference in the preoperative OCT CMT was statistically insignificant between both groups with thickness ≤250 µm.
Table 3. Comparison of central macular thickness between control and NSAIDs groups.
| Central macular thickness | Control group(n=40) | NSAIDs group(n=40) | Test of significance |
| Preoperative | 225.89±15.2 | 229.48±19.6 | t=0.89; P=0.38 |
| 1wk postop. | 245.22±26.26 | 231.48±28.7 | t=1.98; P=0.052 |
| 1mo postop. | 255.7±33.2 | 234.5±26.16 | t=2.7; P=0.008b |
| 2mo postop. | 260.41±55.8 | 235.43±24.21 | t=2.26; P=0.027a |
| 3mo postop. | 267.5±61.2 | 231.7±21.5 | t=3.03; P=0.004b |
aP value statistically significant <0.05; bHigh statistically significant at P value <0.01.
mean±SD, µm
At the first postoperative month, CMT started to show high statistically significant difference between control group (255.7±33.2 µm) and NSAIDs group (234.5±26.16 µm). CMT difference remained highly statistically significant at the third month postoperatively between control and NSAIDs groups with P=0.004 (Table 3).
Changes in central macular thickness from preoperative to 3rd month
There was a high statistically significant difference between the two studied groups regarding median CMT changes with high median change in control group 22 µm (-26.0, 188.0) in comparison to NSAIDs group 1.00 µm (-54.0, 39.0) (Table 4).
Table 4. Comparison of central macular thickness changes from preoperative to 3rd month postoperative between control and NSAIDs groups.
| Central macular thickness | Control group (n=40) | NSAIDs group (n=40) | Test of significance |
| Preoperative | 225.89±15.2 | 229.48±19.6 | t=0.89; P=0.38 |
| 3mo postoperative | 267.5±61.2 | 231.7±21.5 | t=3.03; P=0.004b |
| Changes foveal thickness, median (min, max) | 22.0 (-26.0, 188.0) | 1.0 (-54.0, 39.0) | Z=3.3; P=0.001b |
bHigh statistically significant at P value <0.01.
mean±SD, µm
Comparison Between Nepafenac and Ketorolac Groups
Best corrected visual acuity
The difference in the BCVA was statistically insignificant between the two NSAIDs groups at preoperative assessment and post-operative follow up visits (Table 5).
Table 5. Comparison of BCVA among nepafenac and ketorolac groups.
| BCVA | Nepafenac group (n=20) | Ketorolac group(n=20) | Test of significance |
| Preoperative | 1.0 (0.6-1.5) | 1.1 (0.6-1.5) | Z=1.04; P=0.32 |
| 1wk postop. | 0.3 (0.2-0.8) | 0.3 (0.2-0.6) | Z=1.05; P=0.29 |
| 1mo postop. | 0.2 (0.0-0.6) | 0.3 (0.0-0.6) | Z=1.5; P=0.13 |
| 2mo postop. | 0.0 (0.0-0.3) | 0.25 (0.0-0.6) | Z=1.92; P=0.055 |
| 3mo postop. | 0.0 (0.0-0.2) | 0.0 (0.0-0.3) | Z=1.36; P=0.17 |
median (min-max), logMAR
Central macular thickness
The preoperative difference in the OCT CMT was statistically insignificant between two groups with thickness ≤250 µm (Table 6).
Table 6. Comparison of central macular thickness between NSAIDs groups.
| Central macular thickness | Nepafenac group(n=20) | Ketorolac group(n=20) | Test of significance |
| Preoperative | 231.6±18.2 | 227.5±21.1 | t=0.65; P=0.52 |
| 1wk postop. | 236.53±29.9 | 225.36±26.9 | t=1.08; P=0.29 |
| 1mo postop. | 234.7±28.7 | 234.33±24.4 | t=0.04; P=0.9 |
| 2mo postop. | 238.0±26.5 | 232.08±21.4 | t=0.65; P=0.52 |
| 3mo postop. | 230.8±22.5 | 232.8±21.04 | t=0.25; P=0.8 |
mean±SD, µm
At third month postoperative, the difference in the CMT was statistically insignificant between NSAIDs groups with P=0.8.
Changes in central macular thickness from preoperative to 3rd month postoperatively
There was a statically insignificant difference between Nepafenac and ketorolac groups regarding median CMT changes with P=0.71 (Table 7).
Table 7. Comparison of central macular thickness changes from preoperative to the third postoperative month between nepafenac and ketorolac groups.
| Central macular thickness | Nepafenac group (n=20) | Ketorolac group (n=20) | Test of significance |
| Preoperative thickness | 231.6±18.2 | 227.5±21.1 | t=0.65; P=0.52 |
| 3mo postoperative | 230.8±22.5 | 232.8±21.04 | t=0.25; P=0.8 |
| Changes in central macular thickness, median (min, max) | 1.0 (-54.0, 33.0) | 3.5 (-36.0, 39.0) | Z=0.37; P=0.71 |
mean±SD, µm
Complications
No cases had shown complications related to the medication (NSAIDs) used in this study as: prolonged bleeding time, keratitis, epithelial breakdown, delayed wound healing or hypersensitivity to NSAIDs.
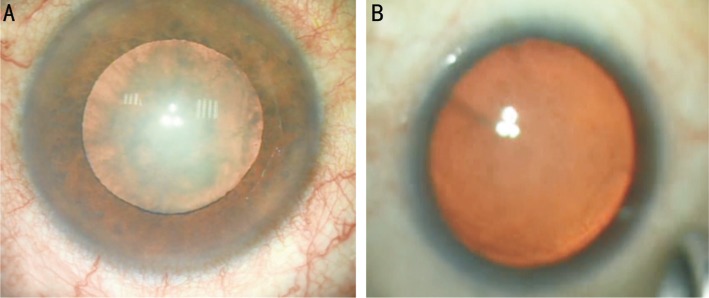
Twelve eyes of 40 (30%) in control group had an intraoperative poorly dilated pupil with no case was detected in NSAIDs groups (Figure 1).
Figure 1. Comparing poorly dilated pupil in control group (A) and full dilated pupil with NSAIDs (B).
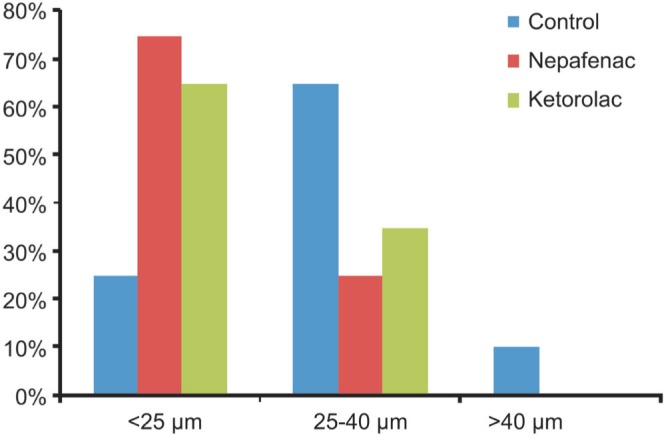
Four eyes of 40 (10.0%) in control group had an increased macular edema >40 µm and no cases were detected in NSAIDs groups. Figure 2 showed the percentage of eyes with postoperative changes in CMT≤25 µm, between 25 and 40 µm, and ≥40 µm in comparison to preoperative thickness in all groups. The highest percentage of increased CMT>25µm was observed in control group.
Figure 2. Percentage of eyes with change in central macular thickness at 3mo postoperatively compared to preoperative thickness.
DISCUSSION
This prospective, RCT demonstrated the value of addition of two days pre-surgically and two months postoperatively topical NSAIDs as a prophylactic regimen to the topical corticosteroids after uncomplicated phacoemulsification in diabetic patients. The effect appears obviously on OCT CMT and BCVA. In addition to that, it also compared the efficacy of nepafenac and ketorolac eye drops on macular thickness.
The reason behind the selection of diabetic patients undergoing cataract surgery to be included in this study; is the fact that, changes in the blood retinal barriers in diabetic patients carry a high risk of development of postoperative macular edema in those patients even if they had no preoperative DR or DME[16]. Patients with DR were excluded from the current study to avoid acceleration of DR which was documented in many studies[6],[9]. In addition, to make a clear cut between DME and macular edema related to the cataract surgery.
Following the same concept, we decided to use the CMT to assess the development of postoperative macular edema. Pseudophakic CME is usually presented with a central macular edema and cystoid fluid accumulating in the central macula. In contrast, DME typically had a higher retinal volume and diffuse or focal retinal thickening[17].
Perhaps the most important finding in this study was that the addition of preoperative NSAIDs to the standard therapeutic corticosteroid regimen used after uncomplicated cataract surgery decreased the occurrence of postoperative CME. Incidence of CME or OCT CMT was evident with significant differences between control group and NSAIDs groups. No cases of clinical CME (increased CMT>40 µm from preoperative baseline) were detected in groups received NSAIDs, in contrast, clinical CME was detected in 10% of patients in the control group.
In a similar study by Wittpenn et al[18] evaluating the efficacy of prophylactic ketorolac in addition to steroid versus steroid only on macular thickness after uncomplicated phacoemulsification surgery; the authors reported an increase of the macular thickness in the control group with 1.8% of patients had clinical CME (increase CMT>40 µm from preoperative baseline). The current study showed that 10% of patients in control group had clinical CME with significant increase in macular thickness in comparison to NSAIDs groups. Wittpenn et al[18] reported 11.5% of patients in control group had an increase in the foveal thickness 25-40 µm and 8.4% in ketorolac group, while in the current study a higher incidence of increased CMT was noticed and it was 65% of patients in control group, 25% in nepafenac group and 35% in ketorolac group. Wittpenn et al[18] evaluated macular thickening in low risk patients for 4wk only and used NSAIDs 4 drops over one hour preoperatively in both control and therapeutic groups to maintain mydriasis during surgery which could explain the decrease in CME incidence in comparison to the current study.
The effect of increased macular thickness after standard phacoemulsification on BCVA was another important clinical issue. This study supported the fact that OCT measured CMT had adverse effect on BCVA. However, it should be noted that postoperative BCVA was excellent immediately after phacoemulsification surgery. Similar to the current study, Singh et al[19] reported that postoperative BCVA was affected in correlation with OCT macular thickness.
The present study found no statistically significant differences in macular thickness between Nepafenac and Ketorolac groups.
The effect of Ketorolac on the incidence of CME seen in this study was consistent with the results of the study of Donnenfeld et al[20] which evaluated Ketorolac effect that was taken by patients either 3d, one day or one hour preoperatively and continued postoperatively with corticosteroids for all groups. The authors reported that CME developed in 12% of patients in the group which did not receive ketorolac and 4% in the group that received ketorolac one hour preoperatively. In contrast none of the patients received ketorolac for one day or 3d preoperatively developed CME. The study did not exclude patients with intraoperative complications but excluded any preoperative risk factors for CME.
Also, the effect of nepafenac on the incidence of CME seen in the present study was demonstrated in Singh et al's[19], study that evaluated the effect of nepafenac which was given one day preoperatively to day 90 postoperatively. In their study, nepafenac had a statistically significant effect in the prevention of macular thickening compared with vehicle group. Macular thickening ≥30 µm was 16.7% of patients in control group versus 2.4% in nepafenac group. The study was conducted on 263 non-proliferative diabetic retinopathy (NPDR) patients and was continued for 90d follow up.
Tzelikis et al[21] reported that the use of prophylactic ketorolac or nepafenac were not effective in the prevention of macular thickening after uneventful cataract surgery compared with placebo. In their study, macular thickness >25 µm was reported in 7% of placebo group, 6% of nepafenac group and 3% in ketorolac group. This study excluded any preoperative risk factors for CME and intraoperative complications.
Hayashi et al[16] compared changes in macular edema in eyes with DR and in eyes without DR after phacoemulsification surgery. They found that the degree of DME changes was more prominent in eyes with DR than in eyes with no DR. Macular edema increased up to 3mo after cataract surgery, but thereafter decreased gradually. In the present study all cases had no DR preoperatively and macular thickening was reported postoperatively and the difference was statistically significant between the control group and NSAIDs groups.
A database study of about 81 thousand eyes from patients with diabetes documented an increasing risk of new macular edema after surgery even in the absence of DR[22]. Also, multicenter database study across 19 centers from an electronic medical record system including 4850 eyes undergoing cataract surgery in patients with diabetes with no history of DME prior to study start demonstrated that the rate of developing treatment-requiring DME increased sharply in the year after cataract surgery. Risk of treatment-requiring DME was associated with pre-operative grade of retinopathy: being 1.0% in diabetic patients without DR pre-operatively, 5.4% in mild NPDR, 10.0% in moderate NPDR, 13.1% in severe NPDR and 14.9% in proliferative diabetic retinopathy[23]. In contrast to this database, the current study documented an increasing risk of treatment requiring CME to 10%. The current study included a small number of diabetic patients (compared to the above mentioned study) without DR undergoing phacoemulsification with postoperative follow up only for 3mo.
Pollack et al[24] documented that 32% of eyes without pre-existing DR developed CME after extracapsular cataract extraction. In the present study 10% (4 cases out of 40 in control group) developed CME and 0 in NSAIDs group. Clinical macular edema in the present study was less than that shown in Pollack et al[24], due to good control of HbA1c and using phacoemulsification technique.
The present study revealed that 30% of cases in the control group had shown poor pupillary dilatation during surgery, on the other hand, no such cases were reported in the NSAIDs group. This phenomenon could be explained by the anti-inflammatory effect of NSAIDs which guard against the effect of surgical trauma including corneal incision, phacoemulsification, instrument manipulations and irrigation which stimulate ocular inflammation and release of PGs, leukotrienes, lipoxins, hepoxylins, and platelet-activating factor which induce conjunctival hyperemia, miosis, pain, change in IOP, posterior synechiae, and CME. Many studies had demonstrated the effectiveness of different types of topical NSAIDs in preventing miosis during cataract surgery as (flurbiprofen, nepafenac 0.1%)[25] or ketorolac[26].
The limitations of the present study include: absence of fluorescein angiography for the study patients which can detect small abnormalities in blood retinal barriers. The OCT used in this study was a noninvasive maneuver and easy to be applied to large numbers of patients but it detects only changes in thickness ≥10 µm without any information about blood retinal barriers problems. However, optical coherence tomography angiography could give helpful data about micro-vascular changes, and might be used in the future studies. There was no collected data about the duration of surgery or phacoemulsification parameters which might play a role in the macular thickening.
In conclusion, this study revealed the prophylactic effect of topical NSAIDs in reducing the frequency and severity of CME in diabetic patients undergoing cataract surgery; and showed insignificant difference between ketrolac and nepafenac eye drops in the prevention of macular edema.
Acknowledgments
Conflicts of Interest: Alnagdy AA, None; Abouelkheir HY, None; El-Khouly SHE, None; Tarshouby SM, None.
REFERENCES
- 1.Finger RP, Kupitz DG, Fenwick E, Balasubramaniam B, Ramani RV, Holz FG, Gilbert CE. The impact of successful cataract surgery on quality of life, household income and social status in South India. PLoS One. 2012;7(8):e44268. doi: 10.1371/journal.pone.0044268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erie JC. Rising cataract surgery rates: demand and supply. Ophthalmology. 2014;121(1):2–4. doi: 10.1016/j.ophtha.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Mentes J, Erakgun T, Afrashi F, Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. 2003;217(6):408–412. doi: 10.1159/000073070. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Cai L, Sun Z, Ye H, Fan Q, Zhang K, Lu W, Lu Y. Risk factors for and diagnosis of pseudophakic cystoid macular edema after cataract surgery in diabetic patients. J Cataract Refract Surg. 2017;43(2):207–214. doi: 10.1016/j.jcrs.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Abazari A, Ghazi NG, Karcioglu ZA. Diabetic Eye Disease. Diabetes and Kidney Disease. New York: Springer; 2014. pp. 153–161. [Google Scholar]
- 6.Krepler K, Biowski R, Schrey S, Jandrasits K, Wedrich A. Cataract surgery in patients with diabetic retinopathy: visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):735–738. doi: 10.1007/s00417-002-0530-7. [DOI] [PubMed] [Google Scholar]
- 7.Flesner P, Sander B, Henning V, Parving HH, Dornonville de la Cour M, Lund-Andersen H. Cataract surgery on diabetic patients. A prospective evaluation of risk factors and complications. Acta Ophthalmol Scand. 2002;80(1):19–24. doi: 10.1034/j.1600-0420.2002.800105.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Xu Q, Du Y, Wu X. Does phacoemulsification speed the progression of diabetic retinopathy? A meta-analysis. Int J Clin Exp Med. 2016;9:8874–8882. [Google Scholar]
- 9.Squirrell D, Bhola R, Bush J, Winder S, Talbot J. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol. 2002;86(5):565–571. doi: 10.1136/bjo.86.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119(8):1135–1142. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 11.Soliman W, Sander B, Jørgensen TM. Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand. 2007;85(6):613–617. doi: 10.1111/j.1600-0420.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2):108–133. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 13.McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011;31(1):4–12. doi: 10.1097/IAE.0b013e3181fd9740. [DOI] [PubMed] [Google Scholar]
- 15.Elbendary AM, Shahin MM. Intravitreal diclofenac versus intravitreal triamcinolone acetonide in the treatment of diabetic macular edema. Retina. 2011;31(10):2058–2064. doi: 10.1097/IAE.0b013e31822a042a. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular oedema after phacoemulsification surgery. Eye. 2009;23(2):389–396. doi: 10.1038/sj.eye.6703022. [DOI] [PubMed] [Google Scholar]
- 17.Munk MR, Jampol LM, Simader C, Huf W, Mittermüller TJ, Jaffe GJ, Schmidt-Erfurth U. Differentiation of diabetic macular edema from pseudophakic cystoid macular edema by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(11):6724–6733. doi: 10.1167/iovs.15-17042. [DOI] [PubMed] [Google Scholar]
- 18.Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, Acular LS for Cystoid Macular Edema (ACME) Study Group A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146(4):554–560. doi: 10.1016/j.ajo.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Singh R, Alpern L, Jaffe GJ, Lehmann RP, Lim J, Reiser HJ, Sall K, Walters T, Sager D. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clin Ophthalmol. 2012;6:1259–1269. doi: 10.2147/OPTH.S31902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnenfeld ED, Perry HD, Wittpenn JR, Solomon R, Nattis A, Chou T. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic-response curve. J Cataract Refract Surg. 2006;32(9):1474–1482. doi: 10.1016/j.jcrs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Tzelikis PF, Vieira M, Hida WT, Motta AF, Nakano CT, Nakano EM, Alves MR. Comparison of ketorolac 0.4% and nepafenac 0.1% for the prevention of cystoid macular oedema after phacoemulsification: prospective placebo-controlled randomised study. Br J Ophthalmol. 2015;99(5):654–658. doi: 10.1136/bjophthalmol-2014-305803. [DOI] [PubMed] [Google Scholar]
- 22.Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC. United Kingdom Pseudophakic Macular Edema Study Group. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016;123(2):316–323. doi: 10.1016/j.ophtha.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Denniston AK, Chakravarthy U, Zhu H, Lee AY, Crabb DP, Tufail A, Bailey C, Akerele T, Al-Husainy S, Brand C. The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group, Report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol. 2017;101(12):1673–1678. doi: 10.1136/bjophthalmol-2016-309838. [DOI] [PubMed] [Google Scholar]
- 24.Pollack A, Leiba H, Bukelman A, Oliver M. Cystoid macular oedema following cataract extraction in patients with diabetes. Br J Ophthalmol. 1992;76(4):221–224. doi: 10.1136/bjo.76.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Mondal KK, Roy SS, Gayen S, Ghosh A, De RR. Comparison of preoperative nepafenac (0.1%) and flurbiprofen (0.03%) eye drops in maintaining mydriasis during small incision cataract surgery in patients with senile cataract: a randomized, double-blind study. Indian J Pharmacol. 2015;47(5):491–495. doi: 10.4103/0253-7613.165201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diakonis VF, Kontadakis GA, Anagnostopoulos AG, Yesilirmak N, Waren DP, Cabot F, Yoo SH, Donaldson KE. Effects of short-term preoperative topical ketorolac on pupil diameter in eyes undergoing femtosecond laser-assisted capsulotomy. J Refract Surg. 2017;33(4):230–234. doi: 10.3928/1081597X-20170111-02. [DOI] [PubMed] [Google Scholar]