Abstract
AIM
To analyze and compare five different variables over one year follow-up (1wk, 1, 3, 6 and 12mo): anterior capsule (AC), and posterior capsule (PC) area densitometry values, AC and PC linear densitometry values, and AC opening area reduction ratio after femtosecond laser-assisted cataract surgery.
METHODS
This was a prospective comparative study. Seventy-one patients underwent femtosecond laser-assisted cataract surgery on single eye between June 2014 and December 2015. A 5.0 mm diameter laser assisted anterior capsulotomy was performed on all eyes. In every post-surgery evaluation, AC opacificaction (ACO) and PC opacification (PCO) density levels were provided by Oculus Pentacam®HR using area and linear densitometry methods. Digital images were captured with a slit-lamp Topcon photographic camera and IMAGEnet® 5 software. The AC opening area on the digital images was measured using the Sketchandcalc area calculator and converted to reduction ratio levels.
RESULTS
Using Pearson correlation coefficient (PCC), we found no correlation (r=-0.091, P=0.46) in the twelfth month assessment between the evolution of ACO area densitometry values and PCO area densitometry values considered as independent variables. We found no correlation, using PCC (r=-0.096, P=0.43) between the evolution of ACO linear densitometry values and PCO linear densitometry values, in the twelfth month visit, working both as independent variables. AC linear densitometry levels and AC area densitometry levels continued to grow strongly from sixth to twelfth months. Analysis of the values of AC opening area reduction ratio (1wk, 1, 3, 6, 12mo) revealed statistically significant differences between the values of successive examinations but the magnitude of the change decreased. In the final period of monitoring between six and twelve months the magnitude of change was low.
CONCLUSION
Our results show strong increases of Scheimpflug ACO densitometry values from the sixth to the twelfth month while capsulorhexis area reduction ratio levels displayed a considerable decrease. We found no correlation between ACO area and linear densitometry values and PCO area and linear densitometry values, in the twelfth month examination, working as independent variables.
Keywords: anterior capsule opacification, area densitometry, femtosecond laser-assisted cataract surgery, linear densitometry, Pentacam®HR Scheimpflug, posterior capsule pacification
INTRODUCTION
Posterior capsule opacification (PCO) remains the most common postoperative cause of impaired visual acuity after removing a cataract using phacoemulsification. Until recently the primary clinical importance of anterior capsule opacification (ACO) and capsulorhexis contraction was that they could be an impediment during examination of the peripheral fundus. It has been recognized that the retraction of the anterior capsule might increase the shrink-wrap effect of the capsular bag, which in turn might reduce the PCO development. It was debatable whether it was indeed desirable to reduce the risk for PCO at the expense of a higher ACO rate and anterior capsular opening shrinkage. Only in case of infrequent severe anterior capsule opacification syndrome (ACOS) was ACO associated with pseudo-phacodonesis and occasionally lens dislocation.
A new category of intraocular lenses (IOLs) was introduced in 2003 when the Food and Drug Administration approved multifocal lenses for use in the United States. Nowadays, the increased use of multifocal and toric IOLs requires good centration of these IOLs to achieve good visual outcomes. Decreasing ACO is especially important to avoid a secondary IOL optic tilt and decentration. These tilts and decentrations are generally asymptomatic with monofocal lenses but more frequently symptomatic when multifocal and toric lenses have been implanted.
Many research studies have been conducted into the treatment and prevention of PCO and ACO and several risk factors have been identified for predisposing the development of PCO and ACO[1]–[3]. These studies are based on measurements of ACO with subjective scales. Due to the clinical significance of the two types of capsule opacification an objective quantification of ACO and PCO is highly important in assessing the effectiveness of trials[3]–[9].
Based on the results of a previous study we proposed area and linear densitometry measurements (continuous objective quantitative levels) provided by Pentacam®HR-Scheimpflug as a more appropriate method for use in trials than slit-lamp-based photographic ACO grading systems (subjective ordinal levels)[10]. This Scheimpflug imaging is fast, simple to perform, and has an easier learning curve compared with slit-lamp-based photographic ACO grading systems. It provides ACO and PCO densitometry measurements as a rapid, objective, easy, and repeatable means of assessment.
To our knowledge, this is the only study which makes a prospective comparison of anterior capsule Scheimpflug densitometry levels and posterior capsule densitometry levels measured in a 1y follow-up after femtosecond laser-assisted cataract surgery. The results of the following prospective comparative study will be of great help in understanding the behavior of these variables during the first year after surgery.
The hypothesis of this study is that the anterior capsular opening area reduction ratio measured by area meters and the values of opacity of anterior capsule and posterior capsule after cataract surgery measured by Schempflug densitometry can show progression after 6mo.
The aim of the study was to analyze and compare five different variables over one year follow-up (1wk, 1, 3, 6, 12mo): anterior capsule area densitometry, posterior capsule area densitometry, anterior capsule linear densitometry, posterior capsule linear densitometry[10] provided by Oculus Pentacam®HR densitometry software and anterior capsular opening area reduction ratio[11]–[12] after femtosecond laser-assisted cataract surgery.
SUBJECTS AND METHODS
This prospective comparative study looked at 71 eyes of 71 cases, who underwent femtosecond laser-assisted cataract surgery at Donostia University Hospital, Spain, between June 2014 and December 2015.
The study conformed to the tenets of the Declaration of Helsinki and was approved by the Health Area Ethics Committee of Donostia University Hospital. Informed consent was obtained from all patients prior to participation.
We performed 71 femtosecond laser-assisted cataract operations on 71 patients (32 men and 39 women) using the VICTUS™ femtosecond laser platform. A femtosecond laser guided 5.0 mm capsulotomy and lens fragmentation was performed on all eyes. After a conventional phacoemulsification an anterior capsule polishing was performed. Next, a 6.0 mm optic IOL was inserted into a capsular bag inflated with 1% hyaluronate sodium. Finally, the surgeon confirmed that the IOLs were into the bag position at the end of operation[3]. In order to avoid any bias ascribed to IOLs due to different scatter light intensities from the anterior IOL surface, the same type of acrylic hydrophobic non-tinted square edged IOL, the enVista™ (Model MX60), was implanted on all eyes[4],[13]. Postoperatively, all patients received similar routine medication comprising topical of diclofenac sodium, tobramycin and dexamethasone 4 times daily for a week.
Before cataract surgery all patients responded to an exhaustive questionnaire regarding their clinical history and a thorough ophthalmologic examination. We also included applanation tonometry, slit-lamp evaluation and ophthalmoscopy through dilated pupils. Every patient involved in the study gave prior consent in writing and was fully aware of the nature of the trials. Every patient met the following inclusion criteria; none displayed any of the exclusion criteria.
Inclusion Criteria
Aged between 65 and 85y with a pupillary diameter of more than 6.0 mm after complete pharmacological mydriasis; eyes that have undergone femtosecond laser-assisted cataract surgery without any intraoperative complication; no tears in the circular anterior capsulotomy and enVista™ IOL in the correct intracapsular position at the end of operation.
Exclusion Criteria
Insufficient pharmacological pupil dilation (less than 6.0 mm measured by Haab's pupillometer) and corneal opacities-both could alter the post-surgery evaluation; history of previous ocular disease; risk of intraoperative floppy iris syndrome; uveitis, glaucoma, pseudoexfoliation syndrome or pigment retinitis; corneal endothelial dystrophies and degeneration; intraocular tumors; history of ocular surgical procedures; surgical intraoperative and early postoperative complications related to surgery in this study; unfavorable capsular bag conditions for the implementation of an IOL, history of rheumatic disease and systemic connective disease; we also excluded chronically-ill patients taking immunomodulators, immunosuppressive drugs, corticosteroids or anti-inflammatory drugs.
Post-surgery evaluation of patients was performed in the first week, the first and the third, sixth and twelfth months. During these examinations anterior capsular opening area reduction ratios were quantified and ACO and PCO densitometry levels (area and linear) were measured.
Postoperative Anterior Capsular Opening Size Reduction Ratio Levels
This measured on trans-illumination digital images acquired from dilated pupils. The continuous curvilinear capsulorhexis (CCC) area on the digital images was measured in mm2 using the Sketchandcalc area calculator (https://www.sketchandcalc.com/)[10].
In all operations a 5.0 mm capsulotomy was performed using the VICTUS™ femtosecond laser platform corresponding to an initial area of 19.635 mm2. Digital images were obtained on dilated pupil eyes in every post-surgery evaluation and acquired with a slit lamp Topcon photographic device and saved by IMAGEnet® 5 software. On the digital images the CCC area was measured in mm2 using the Sketchndcalc area calculator (https://www.sketchandcalc.com/). A 5.0 mm capsulotomy has 19.635 mm2. This initial anterior capsular opening area was considered to be 100% of the area for all patients. We obtained anterior capsular opening area and calculate reduction ratio levels in every post-surgery evaluation[11]–[12].
ACO and PCO Densitometry Levels (Pentacam®HR Densitometry Software)
Densitometry levels of the anterior capsule and posterior capsule were taken using the new Pentacam®HR densitometry software. Twenty-five Scheimpflug images were acquired during one scan. A second frontal pupillary camera aligned the images and compensated for ocular movement. The measurement process lasted less than two seconds and minute eye-movements were captured and corrected simultaneously.
Imaging was performed in a darkened clinical assessment room with a luminance level = or <1 cd/m2 in all cases, measured by a Gossen Starlite 2 m. The Pentacam®HR calculates a quality specification score (QS). Only images captured with a QS of over 95 were accepted for the study. Pentacam®HR provides densitometry software including lens densitometry using the new Pentacam nucleus staging (PNS) software and three densitometry methods (area, linear and peak) available for measuring lens capsules[14]–[15].
In our study, we used two densitometry methods provided by the new Oculus Pentacam®HR densitometry software (area densitometry and linear densitometry). In all post-surgery examinations, we measured the ACO and PCO densitometry levels taken from Scheimpflug images at four meridians: 0°, 45°, 90°, 135°[16]–[20]. The true ACO level was considered to be the average of the ACO densitometry levels on both sides of the anterior capsule at each of the four meridians[16]–[20]. The true PCO level was considered to be the average of the densitometry levels at each of the four meridians.
The densitometry measurements are expressed in gray scale units (GSU). The GSU scale is calibrated by proprietary software, which establishes a minimum light scatter of 0 (maximum transparency) and maximum light scatter of 100 (minimum transparency). In our study, anterior capsule area densitometry is measured on a fixed rectangular area of 0.2 mm×0.5 mm. This specified area is located manually on both sides of the anterior capsule in each of the four selected meridians from the Scheimpflug images; the GSU levels appear on the screen instantly. Scheimpflug imaging is used to calculate the anterior capsule linear densitometry as follows: a line of 0.5 mm is drawn through both sides of the anterior capsule on each of the selected meridians and GSU levels; the GSU levels appear on the screen instantly (Figure 1).
Figure 1. In our study posterior capsule area densitometry is measured on a fixed rectangular area of 0.2 mm×2 mm.
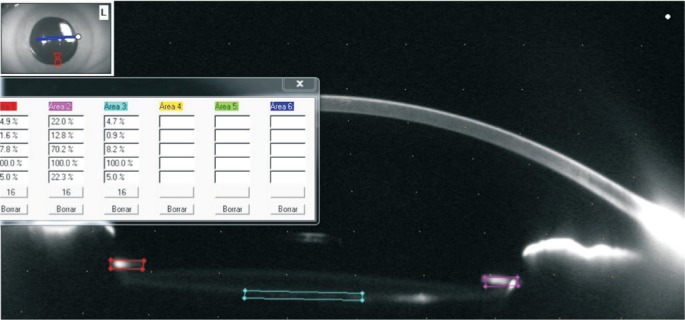
This specified area is located manually on the central posterior capsule in each of the four selected meridians from the Scheimpflug images; the GSU levels appear on the screen instantly. Scheimpflug imaging is used to calculate the posterior capsule linear densitometry as follows: a 2 mm line is drawn on the posterior capsule in each of the four selected meridians and GSU levels appear on the screen instantly. All measurements were performed by two optometrists who had no prior knowledge of the aims of this study.
Statistical Analysis
The data was compared using the 22.0 version of the SPSS software. We used Pearson's coefficient to analyze the correlation between the different variables under study.
RESULTS
One year after surgery two patients failed to complete follow-up due to personal reasons. Data from the sixty-nine (97.18%) remaining patients who consequently completed all postoperative examinations were considered for analysis.
The average age and standard deviation of the patients was 72.93 and 5.06, in an age range of 65 to 83y. There were 31 men and 38 women.
After analysis of all ACO and PCO post-surgery evaluations (1wk, 1, 3, 6, 12mo) we found the results appearing below (Table 1). Anterior capsule linear densitometry levels and anterior capsule area densitometry levels continue to grow strongly from the sixth to the twelfth month. Analysis of the values of anterior capsular opening area reduction ratio (1wk, 1, 3, 6, 12mo) reveals statistically significant differences between the values of successive examinations, but the magnitude of the change decreases. In the last period between the sixth month examination and the first year examination the magnitude of the change is very low (Table 2).
Table 1. Anterior capsule and posterior capsule area and linear densitometry values and CCC area reduction ratio values.
| Date of examinations | 1st week | 1st month | 3rd month | 6th month | 12th month |
| aAC area densitometry | 4.21±0.59 | 9.21±5.95 | 14.33±9.87 | 16.74±10.52 | 20.80±13.53 |
| aAC linear densitometry | 4.97±0.87 | 11.82±7.92 | 18.56±13.21 | 22.05±14.23 | 26.42±16.24 |
| bCCC area reduction ratio | 2.43±3.7 | 7.78±8.71 | 10.39±9.39 | 11.80±10.31 | 12.12±10.52 |
| aPC area densitometry | 4.00±0.4 | 4.05±0.56 | 4.20±0.68 | 4.56±1.07 | 5.95±1.18 |
| aPC linear densitometry | 4.90±0.6 | 4.95±0.76 | 5.12±0.90 | 5.70±1.55 | 6.39±1.80 |
AC: Anterior capsule; PC: Posterior capsule; CCC: Continuous curvilinear capsulorhexis. aThe densitometry values are expressed in gray scale units (GSU). Zero expresses maximum transparency, and 100 expresses minimum transparency; b19.635 mm2 is considered to be 100% of the area and reduction ratio is calculated in every post-surgery evaluation.
mean±SD
Table 2. Magnitude of change over all examinations of anterior capsular opening area reduction ratio.
| Date of examinations | Magnitude of change | P |
| 1st week-1st month | 5.3 | <0.0005 |
| 1st month-3rd month | 2.6 | <0.0005 |
| 3rd month-6th month | 1.4 | <0.005 |
| 6th month-1st year | 0.3 | 0.007 |
However, in the case of ACO area and linear densitometry levels the magnitudes of change did not follow a downward pattern in subsequent examinations. Using a general linear model of repeated measurements, we found that the changes in the ACO area and linear densitometry levels are statistically significant (P>0.0005) over all the examinations. Using Pearson's coefficient, we found a positive correlation between area and linear densitometry values of ACO (PCC=0.95 and P<0.0005) in the change of magnitudes from the first month examination to the first year examination. We found a positive correlation between area and linear densitometry values of PCO (PCC=0.9 and P<0.0005) in the change of magnitudes from the first month examination and the first year examination.
There is a correlation between anterior capsule area densitometry values and anterior capsular opening area reduction ratio (PCC=0.4 and P<0.001) in the change of magnitudes between the first week examination and the first year examination.
We found no correlation between anterior capsule opening size reduction ratio levels and anterior capsule density levels provided by the two different Oculus Pentacam®HR densitometry methods (area, linear), working both as independent variables, in the changes of values from the sixth to the twelfth month.
There is no correlation between posterior capsule area and linear densitometry values and anterior capsular opening area reduction ratio in the change in magnitudes between the first week examination and the first year examination.
There is no correlation between posterior capsule area and linear densitometry values and anterior capsule area and linear densitometry values in the change of magnitudes between the first week examination and the first year examination.
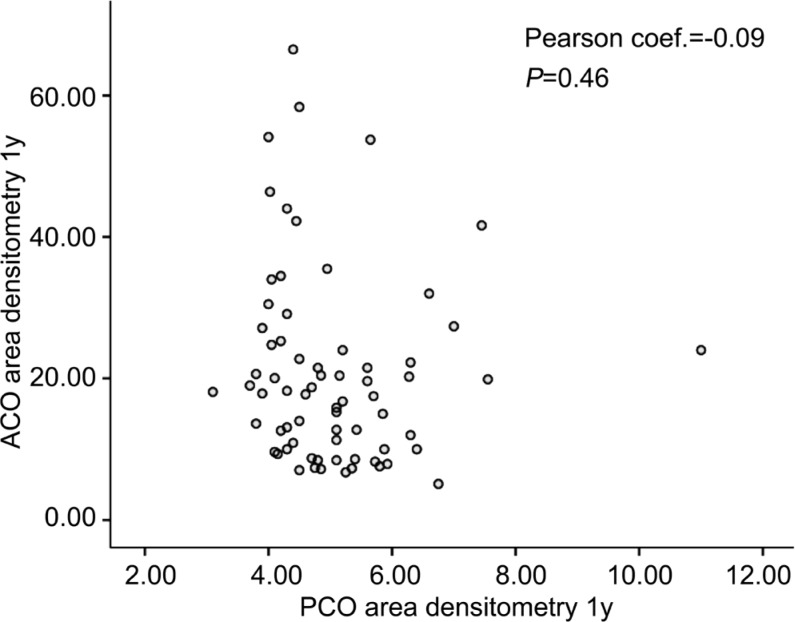
We found no correlation between the changes in ACO area densitometry values and PCO area densitometry values in the twelfth month examination using PCC (r=-0.091, P=0.46; Figure 2).
Figure 2. No correlation between the changes in ACO and PCO area densitometry values in the twelfth month visit.
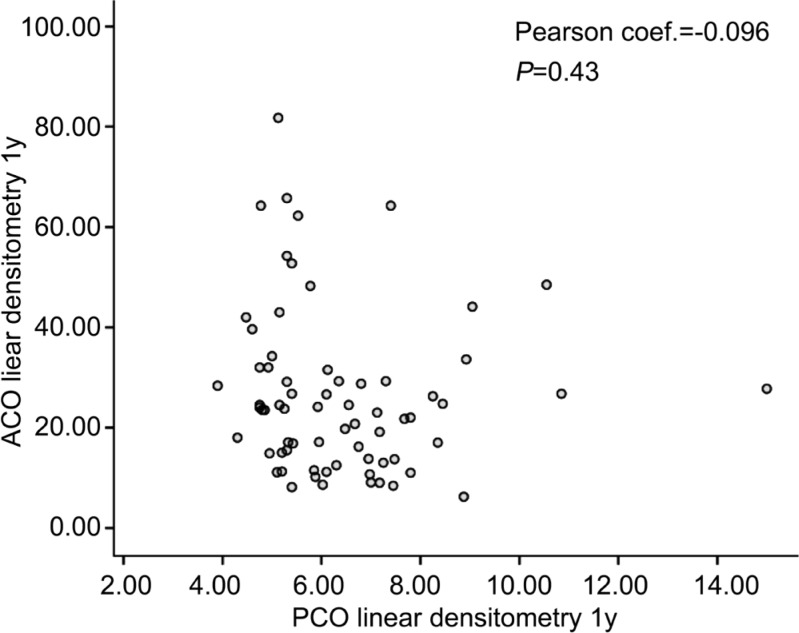
We found no correlation between the changes in ACO linear densitometry values and PCO linear densitometry values, in the twelfth month visit using PCC (r=-0.096, P=0.43; Figure 3).
Figure 3. No correlation between the changes in ACO and PCO linear densitometry values in the twelfth month visit.
DISCUSSION
Our data shows an increase in ACO Scheimpflug densitometry levels from the sixth to the twelfth month. We also found an increase in anterior capsule opening size reduction ratio levels; however the differences were increasingly smaller. Using a general linear model of repeated measurements, we found in both cases that the changes are statistically significant (P<0.005) in both cases over the entire examination period.
After a 6mo follow-up, Kimura et al[11] described in 1998 that the anterior capsule opening shrank rapidly during the first month after acrylic IOL implantation followed by a slower progressive reduction in the subsequent 6mo. This publication demonstrated that ACO sets in by the first postoperative month and continues for 6mo and then ceases.
In our opinion subjective clinical quantification methods and slit-lamp based photographic grading systems have not been able to detect the increase of capsule opacification from six to twelfth months; in our study, however, the new Oculus Pentacam®HR Scheimpflug densitometry (area and linear) software has detected this increase. Slit-lamp based photographic grading systems do not discriminate between the degree of opacity in the anterior capsule and the degree in the posterior capsule located behind the anterior capsule area. Slit-lamp based photographic grading systems are subjective ACO quantification methods that provide qualitative ordinal values and consequently are not accurate measurement systems for establishing a relationship between ACO and PCO[5],[10].
The Pentacam®HR software calculates three types of lens capsule densitometry (area, linear and peak) and provides density levels as an easy, rapid and repeatable assessment. In 2016, Alberdi et al[10] found that the density levels provided by area and linear densitometry methods have a strong positive correlation with the values obtained by the slit-lamp based photographic subjective scale after analysis using Spearman's Rho testing[10]. Using the same analysis they found no correlation between peak densitometry and the slit-lamp based photographic subjective scale[10]. According to this study Peak densitometry is not an appropriate method for the clinical evaluation of ACO[10].
In our opinion, area and linear densitometry levels provided by Scheimpflug camera are quantitative continuous objective values and have great potential in documenting increases in ACO in longitudinal studies as well as for epidemiologic studies and clinical trials[10].
Scheimpflug image acquisition must be performed according to standard procedures to avoid the appearance of artefacts and enable subsequent analysis. ACO densitometry measurements may be impossible or distorted by external factors such as insufficient mydriasis or corneal opacities. The device provides a quality specification score which takes into account the alignment, area covered, and ocular motion. This quality specification score helps users assess the validity of each image acquisition.
Marcantonio and Vrensen[21] studied cell biology and demonstrated that the anterior epithelial cells (A-cells) that line the anterior capsule are probably important in the pathogenesis of ACO and fibrous PCO, since the primary type of response of these cells is to undergo fibrous metaplasia[21]. Nowadays there is uniform consensus that A-cell proliferation and trans-differentiation play a fundamental role in the pathogenesis of ACO and fibrous PCO, besides the importance of equatorial cells (E cells) lining the equatorial zone in the pathogenesis of proliferative PCO resulting from Elschnig Pearls proliferation[22]–[24].
After an exhaustive review of the literature we could not find any reports of an association between anterior capsule opening opacification and fibrous PCO development. Hayashi et al[25] demonstrated in 2004 that there was no correlation between the size of the anterior capsule opening area one day after surgery and the degree of PCO one year after surgery (r=0.041, P=0.79). They demonstrated that the percentage reduction in the anterior capsule opening area from one day to one year after surgery did not correlate with the degree of PCO one year after surgery (r=-0.08, P=0.60)[23]. In their opinion, the contraction of the anterior capsule opening and PCO after cataract surgery can not be explained by a common mechanism[24]. In this previous study, the density of the posterior capsule was calculated using the Peak height of the axial densitometry on the four meridians[24]. In our opinion Peak densitometry is not an appropriate method for the clinical evaluation of ACO nor PCO[10].
Since fibrous PCO development and ACO seem to share similar mechanisms, there could be a significant correlation between the postoperative ACO densitometry levels and the fibrous PCO severity[10],[25]. In our opinion, the accuracy of methods used to date to quantify the degree of ACO and PCO is questionable. A more precise measurement of ACO different variables and PCO different types is essential to understand the relationship of both processes[10],[25]. In our study, we propose area and linear Scheimpflug densitometry methods for measuring ACO and PCO to establish a correlation between both processes. However, despite using new methods of measurement, we found no correlation between posterior capsule density levels provided by the two different Oculus Pentacam®HR densitometry methods (area and linear), anterior capsular opening area reduction ratio in the change in magnitudes between the first month and the first year examinations. There is no correlation between posterior capsule area and linear densitometry values, anterior capsule area and linear densitometry values in the change in magnitudes between the first month and the first year examinations. The data shows a strong increase in anterior capsule Scheimpflug densitometry levels from the sixth to the twelfth month while the anterior capsular opening size reduction ratio levels CCC decrease markedly. There is no correlation between posterior capsule area and linear densitometry values and anterior capsule area and linear densitometry values in the change in magnitudes between the first month and the first year examinations.
Acknowledgments
Conflicts of Interest: Alberdi T, None; Mendicute J, None; Bascarán L, None; Barandika O, None; Ruiz-Ederra J, None.
REFERENCES
- 1.Werner L, Pandey SK, Escobar-Gomez M, Visessook N, Peng Q, Apple DJ. Anterior capsule opacification: A histological study comparing different IOL styles. Ophthalmology. 2000;107:463–467. doi: 10.1016/s0161-6420(99)00088-3. [DOI] [PubMed] [Google Scholar]
- 2.Werner L, Pandey SK, Apple DJ, Escobar-Gomez M, McLendon L, Macky TA. Anterior capsule opacification: correlation of pathologic findings with clinical sequelae. Ophthalmology. 2001;108(9):1675–1681. doi: 10.1016/s0161-6420(01)00674-1. [DOI] [PubMed] [Google Scholar]
- 3.Vasavada AR, Raj SM, Shah GD, Nanavaty MA. Posterior capsule opacification after lens implantation. Expert Rev Ophthalmol. 2013;8(2):141–149. [Google Scholar]
- 4.Minami K, Honbo M, Mori Y, Kataoka Y, Miyata K. Area densitometry using rotating Scheimpflug photography for posterior capsule opacification and surface light scattering analyses. J Cataract Refract Surg. 2015;41:2444–2449. doi: 10.1016/j.jcrs.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Sacu S, Findl O, Menapace R, Georgopoulos M, Buehl W, Rainer G. Assessment of anterior capsule opacification: photographic technique and quantification. J Cataract Refract Surg. 2002;28(2):271–275. doi: 10.1016/s0886-3350(01)01273-1. [DOI] [PubMed] [Google Scholar]
- 6.Prinz A, Vecsei-Marlovits PV, Sonderhof D, Irsigler P, Findl O, Weingessel B. Comparison of posterior capsule opacification between a 1-piece and a 3-piece microincision intraocular lens. Br J Ophthalmol. 2013;97(1):18–22. doi: 10.1136/bjophthalmol-2012-301899. [DOI] [PubMed] [Google Scholar]
- 7.Aslam TM, Dhillon B, Werghi N, Taguri A, Wadood A. Systems of analysis of posterior capsule opacification. Br J Ophthalmol. 2002;86(10):1181–1186. doi: 10.1136/bjo.86.10.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal D, Jain R, Brar GS, Grewal SP. Pentacam tomograms: a novel method for quantification of posterior capsule opacification. Invest Ophthalmol Vis Sci. 2008;49(5):2004–2008. doi: 10.1167/iovs.07-1056. [DOI] [PubMed] [Google Scholar]
- 9.Praveen MR, Vasavada AR, Shah GD, Shah AR, Khamar BM, Dave KH. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: a case-control study. Eye (Lond) 2014;28(6):720–727. doi: 10.1038/eye.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberdi T, Mendicute J, Bascarán L, Goñi N, Barandika O, Ruiz-Ederra J. Anterior capsule opacification after femtosecond laser-assisted cataract surgery: Clinical classification versus Scheimpflug device densitometry values. J Cataract Refract Surg. 2016;42(6):826–832. doi: 10.1016/j.jcrs.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Kimura W, Yamanishi S, Kimura T, Sawada T, Ohte A. Measuring the anterior capsule opening after cataract surgery to assess capsule shrinkage. J Cataract Refract Surg. 1998;24(9):1235–1238. doi: 10.1016/s0886-3350(98)80018-7. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Oshika T, Numaga J, Hayashi Y, Oshiro M, Yuguchi T, Kaiya T. Anterior capsular contraction after cataract surgery in eyes of diabetic patients. Br J Ophthalmol. 2001;85:21–23. doi: 10.1136/bjo.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Kato S, Miyata K, Honbo M, Nejima R, Kitano S, Amano S, Oshika T. Limitation of Scheimpflug videophotography system in quantifying posterior capsule opacification after intraocular lens implantation. Am J Ophthalmol. 2004;137(4):732–735. doi: 10.1016/j.ajo.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J Cataract Refract Surg. 2006;32(3):456–459. doi: 10.1016/j.jcrs.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 15.Grewal DS, Brar GS, Grewal SP. Correlation of nuclear cataract lens density using Scheimpflug images with Lens Opacities Classification System III and visual function. Ophthalmology. 2009;116(8):1436–1443. doi: 10.1016/j.ophtha.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Lasa MS, Datiles MB, 3rd, Magno BV, Mahurkar A. Scheimpflug photography and postcataract surgery posterior capsule opacification. Ophthalmic Surg. 1995;26(2):110–113. [PubMed] [Google Scholar]
- 17.Hayashi K, Hayashi H, Nakao F, Hayashi F. In vivo quantitative measurement of posterior capsule opacification after extracapsular cataract surgery. Am J Ophthalmol. 1998;125(6):837–843. doi: 10.1016/s0002-9394(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi H, Hayashi K, Nakao F, Hayashi F. Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol. 1998;116(12):1579–1582. doi: 10.1001/archopht.116.12.1579. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Hayashi H, Nakao F, Hayashi F. Reproducibility of posterior capsule opacification measurement using Scheimpflug videophotography. J Cataract Refract Surg. 1998;24:1632–1635. doi: 10.1016/s0886-3350(98)80355-6. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Hayashi H. Posterior capsule opacification after implantation of a hydrogel intraocular lens. Br J Ophthalmol. 2004;88(2):182–185. doi: 10.1136/bjo.2003.023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye (Lond) 1999;13(Pt 3b):484–488. doi: 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- 22.Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of αV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med. 2014;18(4):656–670. doi: 10.1111/jcmm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldred JA, Hodgkinson LM, Dawes LJ, Reddan JR, Edwards DR, Wormstone IM. MMP2 activity is critical for TGFβ2 -induced matrix contraction-implications for fibrosis. Invest Ophthalmol Vis Sci. 2012;53(7):4085–4098. doi: 10.1167/iovs.12-9457. [DOI] [PubMed] [Google Scholar]
- 24.Chandler HL, Haeussler DJ, Jr, Gemensky-Metzler AJ, Wilkie DA, Lutz EA. Induction of posterior capsule opacification by hyaluronic acid in an ex vivo model. Invest Ophthalmol Vis Sci. 2012;53(4):1835–1845. doi: 10.1167/iovs.11-8735. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi Y, Kato S, Fukushima H, Numaga J, Kaiya T, Tamaki Y, Oshika T. Relationship between anterior capsule contraction and posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2004;30(7):1517–1520. doi: 10.1016/j.jcrs.2003.11.045. [DOI] [PubMed] [Google Scholar]