Abstract
AIM
To evaluate the differences in the functional connectivity (FC) of the primary visual cortex (V1) between the youth comitant exotropia (CE) patients and health subjects using resting functional magnetic resonance imaging (fMRI) data.
METHODS
Totally, 32 CEs (25 males and 7 females) and 32 healthy control subjects (HCs) (25 males and 7 females) were enrolled in the study and underwent the MRI scanning. Two-sample t-test was used to examine differences in FC maps between the CE patients and HCs.
RESULTS
The CE patients showed significantly less FC between the left brodmann area (BA17) and left lingual gyrus/cerebellum posterior lobe, right middle occipital gyrus, left precentral gyrus/postcentral gyrus and right inferior parietal lobule/postcentral gyrus. Meanwhile, CE patients showed significantly less FC between right BA17 and right middle occipital gyrus (BA19, 37).
CONCLUSION
Our findings show that CE involves abnormal FC in primary visual cortex in many regions, which may underlie the pathologic mechanism of impaired fusion and stereoscopic vision in CEs.
Keywords: comitant exotropia, functional connectivity, primary visual cortex, spontaneous activity
INTRODUCTION
The strabismus is a common eye disease characterized by the dysfunction of eye movement[1] and binocular vision[2]. The incidence rate of strabismus is 5.65% in Eastern China in preschool children[3]. There are a variety of factors contributing to the strabismus, including congenital and acquired factors, such as genetic mutations[4], refractive errors[5], amblyopia[6] and so on. Strabismus can be classified into exotropia and esotropia based on the oblique direction. According to the change of the squint angle direction, we can classify strabismus as comitant strabismus and incomitant strabismus. Clinically, the manifestation of exotropia patients is a noticeable outward deviation of the eyes, which will not only lead to appearance problem but also cause impairment of stereo vision[7]. At present, surgery is the main treatment for exotropia[8]. Meanwhile, the surgical correction for exotropia is beneficial for the recovery of binocular visual function[9].
The esotropia patients are often associated with the impairment of stereo vision[10]. Because of the disruption in binocular visual signal correspondence in strabismus patients, the binocular activation of visual cortical neurons in the primary visual cortex [Visual area 1 (V1)] may change[2]. Receiving inputs from both eyes, the primary visual cortex is the first step of stereo vision processing[11]. A previous study demonstrated that all visual areas play important roles in disparity-defined depth[12]. Other research exhibited that neurons in the V5 have similar response to that of V1. And the V4 neurons reflect an experience of stereoscopic depth[13]. However, understanding of the abnormalities of V1 in the comitant exotropia (CE) remains unknown.
Resting-state functional magnetic resonance imaging (rs-fMRI) is an effective method to evaluate the changes of the brain neural anatomy and function in strabismus. Yan et al[14] demonstrated that the CE showed the imparied dorsal visual pathway. Strabismic patients show suppression in the primary visual cortex[15]. Other study demonstrated that the monocular deprivation by strabismus leads to the dysfunction of the V1[16]. Additionally, the V1 neurons in the strabismic group showed reduction of interocular spatial phase disparities (disparity sensitivity) and a higher prevalence of binocular inhibitory interactions[17]. Many previous studies have demonstrated that the primary angle-closure glaucoma patients and amblyopia patients showed abnormal FC in primary visual cortex[18]–[19]. Although the above mentioned studies have demonstrated that strabismus could lead to the dysfunction of the V1, the abnormal intrinsic functional connectivity (FC) between the V1 and the other cortex has not been revealed yet.
Here, our study is the first to assess the alternations in FC of V1 in CE, which might help to reveal the pathogenesis of abnormal visual fusion function in youth patients with strabismus.
SUBJECTS AND METHODS
Subjects
In total, individuals with CEs (25 males and 7 females) were enrolled in this study from Department of the Ophthalmology, the First Affiliated Hospital of Nanchang University in Jiangxi Province of China. The criteria for the study include: 1) exotropia with stereopsis defects (no visual fusion); 2) visual acuity (VA) >1.0; 3) the deflection angle of strabismus group were equal. The exclusion criteria of the study were: 1) acquired strabismus, esotropia strabismus, incomitant strabismus; 2) ocular diseases or surgery history; 3) without psychiatric disorders, cardiovascular disease and cerebral disease.
We also recruited 32 healthy control subjects (HCs) including 25 males and 7 females with matched age and education in this study. HCs were recruited based on the following criteria: 1) normal brain parenchyma on cranial MRI; 2) VA>1.0 and free of any ocular diseases; 3) absence of psychiatric diseases including depressive disorder and delusional disorder; 4) accessible to the MRI scanning.
The study were approved by the committee of the medical ethics of the Department of Ophthalmology, the First Affiliated Hospital of Nanchang University. The paticipants provided an informed consent.
Magnetic Resonance Imaging Parameters
MRI images were scanned on a 3-Tesla MR scanner (Trio, Siemens, Germany). rs-fMRI data acquisition was obtained in 8min. High-resolution T1-weighted images were acquired with a three-dimensional spoiled gradient-recalled sequence in an axial orientation and 240 functional images with each patient covering the whole brain were obtained[18],[20].
Functional Magnetic Resonance Imaging Data Processing
The data were preprocessed with Data Processing Assistant for rs-fMRI (DPARSF 2.3, http://rfmri.org/DPARSF) run on MATLAB2014b (Mathworks, Natick, MA, USA) which was based on statistical parametric mapping (SPM; http://www.fil.ion.ucl.ac.uk/spm) and the rs-fMRI Data Analysis Toolkit (REST; http://www.restfmri.net)[21]. The preprocessing steps are illustrated as follows: 1) the first ten time points were trimmed off due to the signal reaching equilibrium[22]; 2) the remaining 230 volumes of functional BOLD images were corrected for slice timing effects, motion corrected and realigned. fMRI scans with more than 2 mm maximum displacement in any direction or more than 2° angular motion were discarded[23]; 3) spatial normalized with re-sampling to 3-mm isotropic voxels. After smoothing with a 6-mm full width half maximum (FWHM) Gaussian kernel, linear trend was removed, the band-pass temporal filtering (0.01-0.08 Hz), and the covariates were then removed, including head motion parameters, whole brain, white matter (WM), and cerebrospinal fluid (CSF) signal.
Definition of the Region of Interest in BA17
We chose each side of the primary visual cortex, also known as brodmann area 17 (BA17), as region of interests (ROIs) using the software WFU Pick Atlas (http://www.ansir.wfubmc.edu/)[24], which has been used in previous studies to define ROIs[19]. The steps of defining the seed ROI of the BA17 were as follows: 1) both sides of BA17 were selected from the TD (Talairach Daemon) BA atlas; 2) the location of the left BA17 was intersected to generate the left ROI of the primary visual cortex; 3) the right ROI was obtained similarily (Table 1).
Table 1. MNI coordinates for selected seed regions.
| ROI | Seed regions | X | Y | Z |
| 1 | L V1 (BA17) | -8 | -76 | 10 |
| 2 | R V1 (BA17) | +8 | -76 | 10 |
ROI: Region of interest; MNI: Montreal Neurological Institute.
Functional Connectivity Analysis
FC analyses were performed for the left and right BA17 separately. The rs-fMRI data was analyzed by the statistical module of the dpabi (http://rfmri.org/dpabi). One-way analysis of covariance (ANCOVA) and generalized linear model (GLM) was applied to produce the FC maps, with age and gender used as covariates. Two-sample t-test was used to examine differences in FC between the CE patients and HCs [at voxel level P<0.01 and cluster level P<0.05, Gaussian random field (GRF)-corrected].
Correlation Analysis Between Brain Function and Clinical Behavior
All clinical data including the duration of CEs and VA of both eyes were collected. The correlation between the mean FC value in various brain regions and the behavioral performance in CEs groups was analyzed with correlation analysis. P<0.05 was set as statistical threshold.
Clinical Data Analysis
Two-sample Student's t-test was used for comparison between behavior data (IBM SPSS software version 20.0). P<0.05 was set as statistical threshold.
RESULTS
Demographics and Clinical Behaviors
Thirty-two CE patients (25 males, 7 females; mean age: 24.56±1.96y) and 32 health subjects volunteer individuals (25 males, 7 females; mean age: 25.16±2.68y) were recruited in the study. We did not observe significant difference in weight (P=0.483), age (P=0.316), VA-right (P=0.253) and VA-left (P=0.164) between HCs and CEs patients. Details are presented in Table 2.
Table 2. Demographics and clinical measures by group.
| Condition | CEs | HCs | t | aP |
| M/F | 25/7 | 25/7 | N/A | >0.99 |
| Age (y) | 24.56±1.96 | 25.16±2.68 | -1.011 | 0.316 |
| Weight (kg) | 58.34±3.66 | 59.00±3.78 | -0.705 | 0.483 |
| Handedness | 32R | 32R | N/A | >0.99 |
| Exotropia | 32 | N/A | N/A | N/A |
| Duration of strabismus (y) | 22.25±2.21 | N/A | N/A | N/A |
| VA-right | 1.20±0.20 | 1.14±0.19 | 1.154 | 0.253 |
| VA-left | 1.18±0.18 | 1.12±0.16 | 1.408 | 0.164 |
CEs: Comitant exotropia strabismus; HCs: Healthy controls; N/A: Not applicable; VA: Visual acuity; aIndependent t-tests comparing two groups.
Functional Connectivity Differences
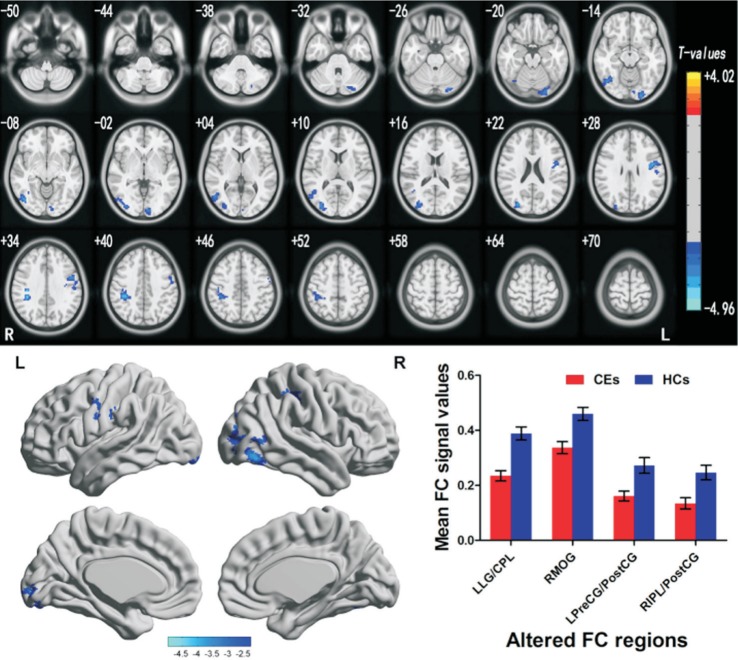
A two-sample t-test was used to determine the different FC maps between two groups. Compared with HCs (n=32), the CE patients (n=32) showed significantly lower FC with the left BA17 in left lingual gyrus/cerebellum posterior lobe (BA17, 18), right middle occipital gyrus (BA19, 37), left precentral gyrus/postcentral gyrus (BA6, 9) and right inferior parietal lobule/postcentral gyrus (BA40) (Figure 1, Table 3). On the contrary, CE patients showed significantly lower FC with the right BA17 in the right middle occipital gyrus (BA19, 37) (Figure 1, Table 3). In the meanwhile, Figures 1 and 2 showed the mean values of altered FC in the CEs and HCs. We did not observe obvious correlation between the mean FC values in different brain areas and the behavioral performance in the CEs patients (P>0.05).
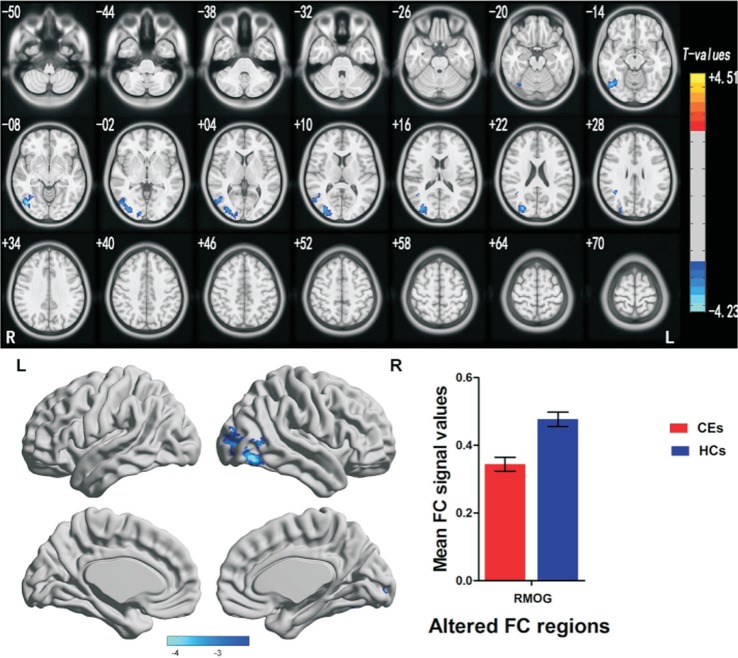
Figure 1. Brain regions demonstrating statistically significant differences between two groups in terms of FC in the left BA17.
Significant FC differences were observed in the left lingual gyrus/cerebellum posterior lobe, right middle occipital gyrus (RMOG), left precentral gyrus/postcentral gyrus, right inferior parietal lobule/postcentral gyrus, and the blue areas denote lower FC values. CEs: Comitant exotropia strabismus; HCs: Healthy controls; BA: Brodmann area; FC: Functional connectivity.
Table 3. Brain regions with significant differences in FC between CEs and HCs.
| Brain regions | BA | Cluster size | Brain region of peak MNI coordinates |
t | ||
| X | Y | Z | ||||
| ROI in left BA 17 | ||||||
| Left lingual gyrus/cerebellum posterior lobe | 17, 18 | 184 | -24 | -96 | -15 | -3.428 |
| Right middle occipital gyrus | 19, 37 | 341 | 45 | -69 | -12 | -3.886 |
| Left precentral gyrus/postcentral gyrus | 6, 9 | 166 | -45 | 0 | 30 | -3.949 |
| Right inferior parietal lobule/postcentral gyrus | 40 | 168 | 39 | -36 | 36 | -4.959 |
| ROI in right BA 17 | ||||||
| Right middle occipital gyrus | 19, 37 | 487 | 45 | -72 | -9 | -4.158 |
The significance level was set at voxel level P<0.01 and cluster level P<0.05, cluster >40 voxels, Gaussian random field theory corrected. FC: Functional connectivity; CEs: Comitant exotropia strabismus; HCs: Healthy controls; BA: Brodmann area; MNI: Montreal Neurological Institute; ROI: Region of interest.
Figure 2. Brain regions demonstrating statistically significant differences between two groups in terms of FC in the right BA17.
Significant FC differences were observed in the right middle occipital gyrus (RMOG); the blue areas denote lower FC values. CEs: Comitant exotropia strabismus; HCs: Healthy controls; BA: Brodmann area; FC: Functional connectivity.
Receiver Operating Characteristic Curve
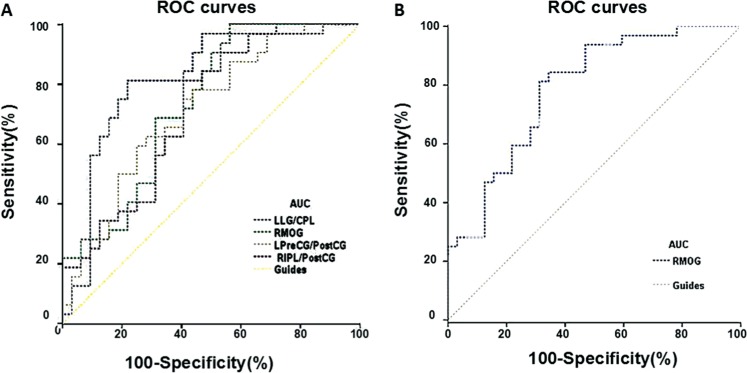
We identified FC values in different brain regions with significant differences between the CEs and HCs groups. In our study, the area under the curve (AUC) was as follows: left lingual gyrus/cerebellum posterior lob (0.821), right middle occipital gyrus (0.734), left precentral gyrus/postcentral gyrus (0.711), right inferior parietal lobule/postcentral gyrus (0.709) (CEs<HCs) (ROI in left BA17; HMs<HCs) (Figure 3A), and right middle occipital gyrus (0.786) (CEs<HCs) (ROI in right BA17; HMs<HCs) (Figure 3B), respectively.
Figure 3. ROC curve analysis of the mean FC values for altered brain regions.
ROC: Receiver operating characteristic; FC: Functional connectivity; CI: Confidence interval; HCs: Healthy controls; CEs: Comitant exotropia strabismus; LG: Lingual gyrus; CPL: Cerebellum posterior lobe; MOG: Middle occipital gyrus; PreCG: Precentral gyrus; PostCG: Postcentral gyrus; IPL: Inferior parietal lobule; R: Right; L: Left; B: Bilateral.
DISCUSSION
In this study, we discovered that young CEs patients had significantly decreased FC in the left BA17, left lingual gyrus/cerebellum posterior lobe, right middle occipital gyrus, left precentral gyrus/postcentral gyrus and right inferior parietal lobule/postcentral gyrus. Additionally, the youth CE patients showed significantly decreased FC in the right BA17 and right middle occipital gyrus.
The lingual gyrus, as a part of the V1, resides in the occipital lobe. The lingual gyrus is functional in the apprehension of vision[25] and reading[26]. It has been shown that the lingual gyrus is less activated in the infantile esotropia, indicating the fusion defects[27]. Another research demonstrated that the strabismic amblyopia patients had reduced activities in the V1[28]. Consistent with that, we also showed that the CEs had decreased FC in the left V1 and left lingual gyrus, which might reflect the defects in the processing of vision in CEs.
The middle occipital gyrus (BA19) is a visual association area involved in the stereovision function[29]. The middle occipital gyrus (MOG) also plays an important role in the category-selective attention-modulated unconscious face/tool processing[30] and spatial processing[31]. It has been shown that the intermittent exotropia showed lower activities in the MOG[32]. Consistently, we demonstrated that the CEs had significantly decreased FC in the left/right BA17 and right MOG (BA19), reflecting the defects in the right MOG in the CEs.
The precentral gyrus is located in the frontal eye fields (FEF), which is responsible for the oculomotor[33]. A previous study demonstrated that the precentral gyrus is involved in the encoding of oculomotor[34]. Moreover, another research showed that the stimulating precentral gyrus controls eye movement[35]. A recent study demonstrated that the stimulation of early visual cortex is associated with feed forward in the FEF[36]. We found that functional connectivity between left primary visual cortex and left precentral gyrus was significantly decreased in CEs, indicating the impaired interaction of the V1 and precentral gyrus in CEs. Thus, we speculated that the decrease of the FC might indicate oculormotor disorder in CEs.
The dorsal stream begins with V1, goes through V2, then to the visual area MT (middle temporal/V5) and to the inferior parietal lobe[37]. Additionally, the inferior parietal lobule (IPL) is involved in the visual categorization[38] and visual word recognition[39]. Meanwhile, the IPL plays an important role in the stereopathway[40]. Ding et al[19] showed decreased FC in the BA17 and IPL of the amblyopia patients. In this study, we demonstrated that FC in the left BA17 and right IPL was significantly decreased, which might reflect the defects in stereo vision in CEs.
Our study showed that CEs had significantly decreased FC in the primary visual cortex and other brain regions, which might provide valuable information to explain the defects in stereo vision in the youth CE patients.
There are some limitations in the study. First, the CEs patients with long-term duration might be associated with depression, mental disorders and other mental symptoms. A neuropsychological assessment in CEs patients would be necessary to dissect out the relationship of CE and neural disorders. Second, the number of youth CE patients in the study was relatively small. Increasing the number of study subject will improve the accuracy of the results. Third, the different strabismus angle of the CEs might have some impacts on the accuracy of the result.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81660158; No.81160118; No.81400372).
Conflicts of Interest: Zhu PW, None; Huang X, None; Ye L, None; Jiang N, None; Zhong YL, None; Yuan Q, None; Zhou FQ, None; Shao Y, None.
REFERENCES
- 1.Hertle RW, National Eye Institute Sponsored Classification of Eye Movement Abnormalities and Strabismus Working Group A next step in naming and classification of eye movement disorders and strabismus. J AAPOS. 2002;6(4):201–202. doi: 10.1067/mpa.2002.126491. [DOI] [PubMed] [Google Scholar]
- 2.Bui Quoc E, Milleret C. Origins of strabismus and loss of binocular vision. Front Integr Neurosci. 2014;8:71. doi: 10.3389/fnint.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Fu Z, Yu J, Ding H, Bai J, Chen J, Gong Y, Zhu H, Yu R, Liu H. Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36-72 months. Br J Ophthalmol. 2016;100(4):515–519. doi: 10.1136/bjophthalmol-2015-306999. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal AB, Feng CY, Altick AL, Quilici DR, Wen D, Johnson LA, von Bartheld CS. Altered protein composition and gene expression in strabismic human extraocular muscles and tendons. Invest Ophthalmol Vis Sci. 2016;57:5576–5585. doi: 10.1167/iovs.16-20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang SM, Chan RY, Bin Lin S, Rong SS, Lau HH, Lau WW, Yip WW, Chen LJ, Ko ST, Yam JC. Refractive errors and concomitant strabismus: a systematic review and Meta-analysis. Sci Rep. 2016;6:35177. doi: 10.1038/srep35177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemi H, Nabovati P, Dadbin N, Heidari Z, Yekta A, Jafarzadehpur E, Ostadimoghaddam H, Khabazkhoob M. The prevalence of ptosis and its association with amblyopia and strabismus in 7-year-old schoolchildren in Iran. Strabismus. 2015;23(3):126–131. doi: 10.3109/09273972.2015.1068346. [DOI] [PubMed] [Google Scholar]
- 7.Read JC. Stereo vision and strabismus. Eye (Lond) 2015;29(2):214–224. doi: 10.1038/eye.2014.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X, Ji Z, Yu H, Xu M, Xu J. Exotropia is the main pattern of childhood strabismus surgery in the south of China: a six-year clinical review. J Ophthalmol. 2016;2016:1489537. doi: 10.1155/2016/1489537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usui C, Kubota N, Maruo T. Binocular function of intermittent exotropia before and after surgery. Jpn J Ophthalmol. 2001;45(1):117. doi: 10.1016/s0021-5155(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Sato M, Ohmura T, Miyazaki Y, Wang AH, Awaya S. Binocular depth-from-motion in infantile and late-onset esotropia patients with poor stereopsis. Invest Ophthalmol Vis Sci. 1999;40(12):3031–3036. [PubMed] [Google Scholar]
- 11.Scholl B, Burge J, Priebe NJ. Binocular integration and disparity selectivity in mouse primary visual cortex. J Neurophysiol. 2013;109(12):3013–3024. doi: 10.1152/jn.01021.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neri P, Bridge H, Heeger DJ. Stereoscopic processing of absolute and relative disparity in human visual cortex. J Neurophysiol. 2004;92(3):1880–1891. doi: 10.1152/jn.01042.2003. [DOI] [PubMed] [Google Scholar]
- 13.Neri P. A stereoscopic look at visual cortex. J Neurophysiol. 2005;93(4):1823–1826. doi: 10.1152/jn.01068.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yan X, Lin X, Wang Q, Zhang Y, Chen Y, Song S, Jiang T. Dorsal visual pathway changes in patients with comitant extropia. PLoS One. 2010;5(6):e10931. doi: 10.1371/journal.pone.0010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen VJ, Tarczy-Hornoch K. Functional magnetic resonance imaging of binocular interactions in visual cortex in strabismus. J Pediatr Ophthalmol Strabismus. 2011;48(6):366–374. doi: 10.3928/01913913-20101118-01. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner SD, Vorobyov V, Sengpiel F. Limited protection of the primary visual cortex from the effects of monocular deprivation by strabismus. Cereb Cortex. 2005;15(11):1822–1833. doi: 10.1093/cercor/bhi059. [DOI] [PubMed] [Google Scholar]
- 17.Kumagami T, Zhang B, Smith EL, 3rd, Chino YM. Effect of onset age of strabismus on the binocular responses of neurons in the monkey visual cortex. Invest Ophthalmol Vis Sci. 2000;41(3):948–954. [PubMed] [Google Scholar]
- 18.Li S, Li P, Gong H, Jiang F, Liu D, Cai F, Pei C, Zhou F, Zeng X. Intrinsic functional connectivity alterations of the primary visual cortex in primary angle-closure glaucoma patients before and after surgery: a resting-state fMRI study. PLoS One. 2017;12(1):e0170598. doi: 10.1371/journal.pone.0170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding K, Liu Y, Yan X, Lin X, Jiang T. Altered functional connectivity of the primary visual cortex in subjects with amblyopia. Neural Plast. 2013;2013:612086. doi: 10.1155/2013/612086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Ye CL, Zhong YL, Ye L, Yang QC, Li HJ, Jiang N, Peng DC, Shao Y. Altered regional homogeneity in patients with late monocular blindness: a resting-state functional MRI study. Neuroreport. 2017;28(16):1085–1091. doi: 10.1097/WNR.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for "Pipeline" data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, Wang YX. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–2175. doi: 10.2147/NDT.S69681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 25.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50(5):607–614. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 2000;267(1455):1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Zhang J, Lang L, Gong Q, Liu L. Assessment of cortical dysfunction in infantile esotropia using fMRI. Eur J Ophthalmol. 2014;24(3):409–416. doi: 10.5301/ejo.5000368. [DOI] [PubMed] [Google Scholar]
- 28.Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. J Physiol (Lond) 2001;533(Pt 1):281–297. doi: 10.1111/j.1469-7793.2001.0281b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortin A, Ptito A, Faubert J, Ptito M. Cortical areas mediating stereopsis in the human brain: a PET study. Neuroreport. 2002;13(6):895–898. doi: 10.1097/00001756-200205070-00032. [DOI] [PubMed] [Google Scholar]
- 30.Tu S, Qiu J, Martens U, Zhang Q. Category-selective attention modulates unconscious processes in the middle occipital gyrus. Conscious Cogn. 2013;22(2):479–485. doi: 10.1016/j.concog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68(1):138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Bai J, Zhang J, Gong Q, Liu L. Assessment of cortical dysfunction in patients with intermittent exotropia: an fMRI study. PLoS One. 2016;8;11(8):e0160806. doi: 10.1371/journal.pone.0160806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnon D, O'Driscoll GA, Petrides M, Pike GB. The effect of spatial and temporal information on saccades and neural activity in oculomotor structures. Brain. 2002;125(Pt 1):123–139. doi: 10.1093/brain/awf005. [DOI] [PubMed] [Google Scholar]
- 34.Iacoboni M, Woods RP, Lenzi GL, Mazziotta JC. Merging of oculomotor and somatomotor space coding in the human right precentral gyrus. Brain. 1997;120(Pt 9):1635–1645. doi: 10.1093/brain/120.9.1635. [DOI] [PubMed] [Google Scholar]
- 35.Blanke O, Spinelli L, Thut G, Michel CM, Perrig S, Landis T, Seeck M. Location of the human frontal eye field as defined by electrical cortical stimulation: anatomical, functional and electrophysiological characteristics. Neuroreport. 2000;11(9):1907–1913. doi: 10.1097/00001756-200006260-00021. [DOI] [PubMed] [Google Scholar]
- 36.Cocchi L, Sale MV, L Gollo L, Bell PT, Nguyen VT, Zalesky A, Breakspear M, Mattingley JB. A hierarchy of timescales explains distinct effects of local inhibition of primary visual cortex and frontal eye fields. Elife. 2016;5.pii:e15252. doi: 10.7554/eLife.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgerald JK, Swaminathan SK, Freedman DJ. Visual categorization and the parietal cortex. Front Integr Neurosci. 2012;6:18. doi: 10.3389/fnint.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sliwinska MW, James A, Devlin JT. Inferior parietal lobule contributions to visual word recognition. J Cogn Neurosci. 2015;27(3):593–604. doi: 10.1162/jocn_a_00721. [DOI] [PubMed] [Google Scholar]
- 40.Backus BT, Fleet DJ, Parker AJ, Heeger DJ. Human cortical activity correlates with stereoscopic depth perception. J Neurophysiol. 2001;86(4):2054–2068. doi: 10.1152/jn.2001.86.4.2054. [DOI] [PubMed] [Google Scholar]