Abstract
Background
Pregnant women treated with methadone as opioid maintenance therapy are more likely than women treated with buprenorphine to deliver preterm. Preterm birth is associated with less risk of neonatal abstinence syndrome (NAS). We sought to assess the role of preterm birth as a mediator of the relationship between in utero exposure to methadone and NAS compared with buprenorphine.
Methods
We studied 716 women receiving methadone or buprenorphine and delivering live-born infants at Magee-Womens Hospital, Pittsburgh, Pennsylvania (2013-2015). We implemented inverse probability weighted marginal structural models to isolate the role of preterm birth (<37 weeks’ gestation). Weights accounted for confounding by maternal age, race, insurance, parity, delivery year, marital, employment, hepatitis C, and smoking status.
Results
Approximately 57% of the cohort were treated with methadone. Preterm birth was more common in methadone exposed pregnancies (25% versus 14%). The incidence of NAS treatment was higher in methadone-compared with buprenorphine-exposed infants (65% versus 49%), and term compared with preterm births (64% versus 36%). For every 100 infants live-born to mothers treated for opioid dependence, there were 13 excess cases of NAS among infants exposed to methadone compared with buprenorphine (adjusted risk difference [RD] 13.3, 95% confidence interval [CI] 5.7, 20.9). Among term births, this increased to 17 excess cases of NAS in methadone- compared with buprenorphine-exposed (RD 16.7, 95% CI 9.3, 24.0).
Conclusion
The further increased risk of NAS associated with methadone use versus buprenorphine in term deliveries emphasises the utility of buprenorphine in clinical settings aimed at decreasing NAS.
Keywords: buprenorphine, methadone, neonatal abstinence syndrome, preterm birth, mediation analysis
Introduction
Neonatal abstinence syndrome (NAS), or postnatal opioid withdrawal, affected nearly 6 of every 1000 live-born US infants in 20121 — a five-fold increase since 2000.2 NAS is associated with long-term physical and behavioral complications3, and cost the US health system an estimated $1.5 billion in 2012 alone.1 The marked increase in NAS parallels increases in opioid use dependence in pregnancy,4 and the two recommended opioid maintenance therapies, buprenorphine and methadone.5 Determining which treatment regimen will maternal and infant outcomes, including reducing risk of NAS, is a public health priority.
Buprenorphine is associated with improved perinatal outcomes compared with methadone, most notably reporting less NAS and shorter duration of neonatal treatment.6-9 Though the majority of extant studies are in agreement, methadone remains the mainstay of care in the US,10 and barriers to access buprenorphine treatment persist.11 Prescription of buprenorphine as opioid maintenance therapy in an outpatient setting requires that the physician obtain a waiver from the Controlled Substances Act.12 In 2012, only 2.2% of all US physicians applied for and received the waiver and were therefore able to treat patients with buprenorphine.13
Inherent biases in prescribing preferences, access to treatment options, and necessity of tailoring treatment to the individual beyond risk of NAS, have continued to fuel the debate on optimal treatment in pregnancy. The role of gestational age in these associations, however, has not been investigated - a problem frequently encountered in perinatal epidemiologic studies.14 Gestational age is often adjusted in regression models, when treated as a confounder, or excluded from the model, thereby assessing the total association. Such approaches ignore the complexities of this relationship. When evaluating the relationship between opioid maintenance therapy and NAS, the interplay among treatment, NAS and gestational age is important. Methadone treatment has been associated with an increased risk of preterm birth compared with buprenorphine treatment or no treatment,7,15-18 and preterm infants exhibit a lower incidence and reduced severity of NAS compared with term infants.19-21 If preterm birth is a mediator of this relation, the estimated increased risk of NAS associated with methadone compared with buprenorphine may be an underestimate among term infants.
We aimed to estimate the association between opioid maintenance therapy and NAS, independent of the effect of opioid maintenance therapy on preterm birth. We hypothesised that the increased risk of NAS associated with methadone exposure compared with buprenorphine would be stronger among term than preterm births. If true, these results will support the expansion of buprenorphine use and access in pregnancy, making more treatment options available to individualize treatment.
Methods
Data source
Magee-Womens Hospital is one of the largest maternity hospitals in Pennsylvania with approximately 10 000 deliveries annually. Pregnant women initiating new opioid maintenance therapy at Magee-Womens Hospital can self-select treatment with methadone or buprenorphine in accordance with the American Congress of Obstetricians and Gynecologists recommendations provided they meet prescribing requirements for both.5 Women who conceive while receiving opioid maintenance therapy are normally maintained on their medication regimen.
Study cohort
The study cohort consisted of all live-born, singleton deliveries to women exposed to methadone or buprenorphine as opioid maintenance therapy on the day of delivery at Magee-Womens Hospital from 2013-2015. Using International Classification of Diseases, Ninth and Tenth Revision codes for drug- dependent (ICD-9 64831) or drug- complicated delivery (ICD-10 O99324) we identified 872 drug-dependent pregnancies (Figure 1). Of these, 745 had documentation of opioid maintenance therapy with either buprenorphine or methadone on the day of delivery. We restricted the cohort further to live-born, singleton pregnancies and therefore excluded 6 fetal deaths and 9 pairs of twins. Twins were excluded due to known differences in gestational ages and the dearth of information on NAS in twin pregnancies. We retained 716 pregnancies (691 women) in the final analytic sample. The Institutional Review Board at the University of Pittsburgh approved this study.
Figure 1.
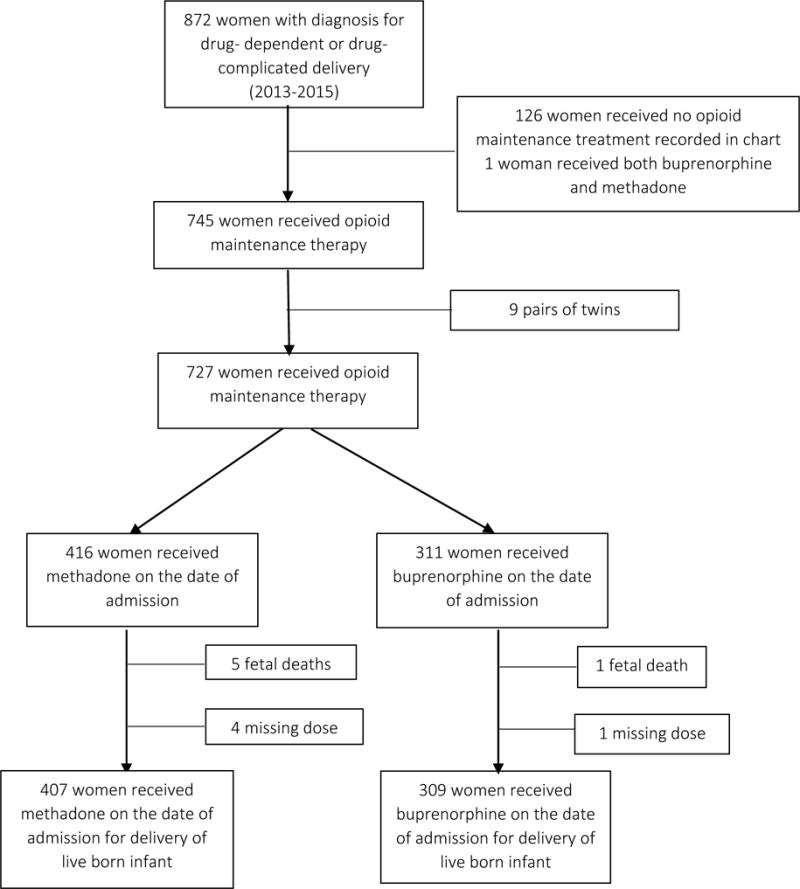
Flow diagram describing sample population (n=716, 2013-2015*Note: 25 women with 2 pregnancies).
Opioid maintenance medications
We determined maternal exposure to opioid maintenance therapy with pharmacy billing claims, then extracted dosing information directly from the medical chart. Women treated with Subutex® (buprenorphine, n=299; Reckitt Benckiser Pharmaceuticals Inc., VA) or Suboxone® (buprenorphine + naloxone, n=10; Reckitt Benckiser Pharmaceuticals Inc., VA) were considered buprenorphine-treated. In utero exposure to buprenorphine was the referent in all analyses. We selected treatment on the day of delivery as the exposure of interest and used this as a surrogate of pregnancy exposure as opioid exposure closest to the time of delivery is thought to have the highest impact on NAS risk22 and we lacked data on entire treatment trajectories.
Neonatal abstinence syndrome
We identified cases of NAS using pharmacy-billing codes indicating infant pharmacologic treatment with morphine. Therefore, our analysis only accounts for NAS cases that were severe enough to be treated, using treatment as a surrogate of NAS. At Magee-Womens Hospital all infants with known exposure to opioids, both illicit and maintenance, remain in the hospital for five to seven days post-delivery for continuous monitoring for NAS. Infants are scored using the Finnegan Neonatal Abstinence Scoring Tool23 every 3 to 4 hours; those with an average score of eight or greater for three consecutive assessments receive treatment with morphine. In our cohort, receipt of morphine was highly correlated with ICD code indicating “Drug Withdrawal Syndrome in Newborn” (kappa>0.99).
Preterm birth
Preterm birth was the mediator in each analysis. For consistency with the literature, we defined preterm birth as live-born delivery prior to 37 weeks’ gestation documented in the pharmacy billing records.24 We were unable to discern between spontaneous and induced labor and therefore considered both in our definition of preterm birth. Gestational age was determined using the best obstetric estimate from ultrasound or last menstrual period when ultrasound was unavailable. All pregnancies had documented gestational age from 20 to 42 weeks at delivery.
Preterm birth meets the criteria as a potential mediator of the association between opioid maintenance therapy and NAS as (i) methadone has been shown to be associated with preterm birth both in comparison to buprenorphine7,17,18 and to no opioid maintenance therapy;15,16 and (ii) preterm infants develop less, or less severe, NAS compared with term infants after exposure to methadone.19-21,25
Covariates
We obtained data on medication use, maternal characteristics and pregnancy outcomes from electronic pharmacy records at Magee-Womens Hospital. Information missing from electronic pharmacy records was informed directly from patient charts and birth certificates. Data were therefore a combination of clinical billing codes, documentation by a health professional, and self-report.
Maternal characteristics in the cohort included maternal age, race (Black, White, or other), education (less than high school, high school graduate or equivalent, some college, or college graduate), marital status (yes or no), employment status (employed or unemployed), type of insurance (private or public), and prepregnancy body mass index (BMI, kg/m2). BMI was calculated as prepregnancy weight in kilograms divided by height in meters squared and was categorised as underweight (BMI <18.5), normal weight (BMI 18.5-24), overweight (BMI 25-29), or obese (BMI ≥30).26 Data pertinent to the pregnancy included parity, hepatitis c status (positive or negative), smoking status (smoked at any time in pregnancy), birthweight, congenital anomalies, and year of delivery.
Statistical analysis
The analytic strategy was to assess the total adjusted association between opioid maintenance therapy and NAS treatment, then to define the controlled direct effect of opioid therapy on NAS treatment by removing the effect of preterm birth.27 The difference between these associations represented the effect of preterm birth. Causal diagrams were used to identify potential confounders of the overall relationship between opioid maintenance therapy and NAS, and of the preterm birth-NAS association.28,29 Variables identified as potential confounders of the opioid maintenance therapy-NAS total association included maternal age, race, marital status, employment status, type of insurance, parity, hepatitis c status, smoking status, and year of delivery. Final models of the preterm birth- NAS association were adjusted for parity, maternal race, age, smoking status, and marital status.
We first assessed interactions between treatment and preterm birth. Log-binomial models regressing NAS against treatment (methadone versus buprenorphine) were performed with and without the treatment and an interaction term for treatment-by-preterm birth. Because the risk ratio changed by less than 10% with the inclusion of the interaction term, interaction between exposure and mediator was considered insignificant and was not included in the final models.
The primary analysis evaluating mediation by preterm birth adjusted for confounders marginally using inverse probability weighting.27 This approach can estimate the direct effects in the presence or absence of exposure-induced mediator-outcome confounding. To execute this analytic approach, we used two log-binomial regression models weighted by stabilised inverse probability weights. The weights were generated from modelling methadone exposure then preterm birth as a mediator. Weights were calculated as:
where X denotes treatment (X=1 if methadone or X=0 if buprenorphine), M represents preterm birth (M=1 if term birth or M=0 if preterm birth), and C represents potential confounders included in the model (described above). In the models, Y indicated NAS (Y=1 if infant treated for NAS or Y=0 no treatment for NAS). The numerators represent the predicted probabilities from logistic regression models of treatment and preterm birth and the denominators replicate this model but adjusted for the confounding variables. All stabilised weights had a mean of one with no extreme values.
Weights were then incorporated into two log-Binomial regression models: (i) modelling the total effect of methadone treatment on NAS compared with buprenorphine; (ii) the controlled direct effect of methadone on NAS among term births. Results were reported on both the risk difference (RD) and risk ratio (RR) scale. Standard errors were obtained using robust variance estimators, which accounts for pseudo-clustering induced by the inverse probability weights, and the correlation within women with multiple pregnancies.30 Finally, the proportion increase in the association in term births was calculated as the absolute value of: [(Total effect−Controlled Direct Effect)/Total effect] × 100.31
To confirm the results, we implemented a mediation analysis conditionally adjusting for the same variables using the generalised product method.32 While this approach can accommodate exposure-mediator interactions, it cannot account for mediator-outcome confounders affected by the exposure. However, as demonstrated in our causal diagrams, we suspected that no such mediator-outcome confounders were present, and the analyses suggested no exposure-mediator interactions with the available, measured confounders. If these assumptions are true, the generalised product method should yield results identical to the inverse probability weighted approach.
Sensitivity analysis
We previously demonstrated minimal impact from unmeasured confounding by severity of addiction on the total association between opioid maintenance therapy and NAS33; however, we undertook a sensitivity analysis to evaluate the role of unmeasured confounding by prepregnancy BMI on the Controlled Direct Effect. We implemented an approach developed by VanderWeele et al34 that addresses unmeasured confounding, and used this to simultaneously address the impact of missing data for maternal prepregnancy BMI. We classified BMI as obese (BMI≥30 kg/m2) vs not obese (BMI<30 kg/m2). Classification of missing data and thereby prevalence of obesity by opioid maintenance therapy and preterm birth were varied for evaluation. Analyses were first conducted assuming all missing maternal weights were obese (Supporting Information: Sensitivity Analysis 1 and 3), then conducted once again assuming all women with missing weights were not obese (Supporting Information: Sensitivity Analyses 2 and 4). Next, because the degree of missing BMI may vary by preterm birth status, we considered different values for the prevalence of obesity based on preterm birth status (Supporting Information: Sensitivity Analyses 3a-4b).
Results
In the cohort, 57% (n=407) women were treated with methadone and the remaining 43% (n=309) with buprenorphine on the day of delivery. Nearly 20% of the final sample was born preterm, and 58% developed NAS. Women with a preterm birth were more likely than women with a term delivery to have less than a high school education, smoke during pregnancy, have a higher parity (Table 1). Race, maternal age, measured prepregnancy BMI, marital, employment, and hepatitis C status were not different between groups. The preterm infants were lighter at birth and more often had a birth defect than infants delivered at term. The incidence of NAS treatment was higher in methadone compared with buprenorphine-exposed infants (65% vs. 49%), and term infants compared with preterm infants [64% (363/570) versus 36% (52/146); Table 2]. Rates of preterm birth were also higher in methadone versus buprenorphine treated women [25% (103/407) versus 14% (43/309)].
Table 1.
Demographic characteristics of women diagnosed with drug-dependent deliveries of singletons based on preterm birth status at Magee-Women Hospital in Pittsburgh, Pennsylvania (2013-2015, n=716)
| Characteristic | Preterm birth n (%) |
Term birth n (%) |
|---|---|---|
| Total number of births | 146 | 570 |
| Opioid maintenance therapy | ||
| Buprenorphine | 43 (29.5) | 266 (46.7) |
| Methadone | 103 (70.5) | 304 (53.3) |
| Race | ||
| White | 136 (93.2) | 539 (94.6) |
| Black | 7 (4.8) | 20 (3.5) |
| Other/Unknown | 3 (2.0) | 11 (1.9) |
| Mother’s age [Mean (SD)] | 29.2 (4.7) | 28.7 (4.8) |
| Mother’s education | ||
| Less than high school | 35 (24.0) | 93 (16.3) |
| High school graduate or GED completed | 52 (35.6) | 252 (44.2) |
| Some college credit | 34 (23.3) | 112 (19.7) |
| College graduate | 20 (13.7) | 100 (17.5) |
| Unknown | 5 (3.4) | 13 (2.3) |
| Prepregnancy BMI [Mean (SD)]a | 24.6 (6.5) | 24.4 (5.5) |
| BMI categorya,b | ||
| Underweight | 5 (7.8) | 23 (7.9) |
| Normal weight | 38 (59.4) | 172 (58.9) |
| Overweight | 21 (32.8) | 97 (33.2) |
| Obese | 8 (5.5) | 45 (7.9) |
| Married | 19 (13.0) | 74 (13.0) |
| Employed | 52 (35.6) | 222 (39.0) |
| Smoked during pregnancy | 129 (88.4) | 456 (80.0) |
| Parity | ||
| Nulliparous | 43 (29.5) | 181 (31.8) |
| 1-2 previous pregnancies | 67 (45.9) | 292 (51.2) |
| Greater than 2 pregnancies | 36 (24.6) | 97 (17.0) |
| Hepatitis C positive | 15 (10.3) | 77 (13.5) |
| Birthweight [Mean (SD)] | 2091 (588) | 3043 (459) |
| Gestational age at delivery [Mean (SD)] | 33.6 (3.0) | 39.0 (1.2) |
| Diagnosed with congenital anomaly | 21 (14.4) | 56 (9.8) |
SD=standard deviation; GED=general educational development; BMI=body mass index
Prepregnancy BMI based on n=356.
Prepregnancy BMI defined as underweight (<18.5 kg/m2), normal weight (18.5-24 kg/m2), overweight (25-29 kg/m2), or obese (≥30 kg/m2).
Table 2.
Risk of neonatal abstinence syndrome (NAS) by opioid maintenance treatment and preterm birth status
| Treatment | Term births
|
Preterm births
|
Total births | ||
|---|---|---|---|---|---|
| With NAS | Without NAS | With NAS | Without NAS | ||
| Methadone, n (%) | 223 (55) | 81 (20) | 40 (10) | 63 (15) | 407 (100) |
| Buprenorphine, n (%) | 140 (45) | 126 (41) | 12 (4) | 31 (10) | 309 (100) |
| Total | 363 (51) | 207 (29) | 52 (7) | 94 (13) | 716 (100) |
Associations between type of opioid maintenance treatment and NAS are displayed in Table 3. On the absolute risk scale, for every 100 live-born infants exposed to opioid maintenance therapy in utero, there were 13 more cases of NAS among infants exposed to methadone compared with buprenorphine (RD 13.3, 95% CI 5.7, 20.9). When the mediating role of preterm birth was accounted for, the RD increased to 16.7 (95% CI 9.3, 24.0). These findings suggested an estimated 25% increase in the association among term births.
Table 3.
Opioid maintenance therapy (OMT) and neonatal abstinence syndrome (NAS) association and OMT-NAS association not attributable to preterm birth in women exposed to opioid maintenance therapy at Magee-Womens Hospital, 2013 to 2015 using inverse probability weighted marginal structural models (n=716)
| Events (n) |
Population at risk (n) |
Risk (%) | Risk difference (%) (95% confidence interval) |
Risk ratio (95% confidence interval) |
Proportion explained on risk difference scale | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total association | Association not attributed to preterm birth | Total association | Association not attributed to preterm birth | Proportion of total avoided by preterm birth | ||||
| Methadone | 263 | 407 | 64.6 | 13.3 (5.7, 20.9) |
16.7 (9.3, 24.0) |
1.26 (1.10, 1.45) |
1.34 (1.17, 1.53) |
24.9% |
| Buprenorphine | 152 | 309 | 49.2 | 0.0 (Reference) |
0.0 (Reference) |
1.00 (Reference) |
1.00 (Reference) |
|
Linear risk models adjusted for parity, maternal race, age, employment status, smoking status, marital status, hepatitis c status, private versus public insurance, and year of delivery
Poisson regression models adjusted for parity, maternal race, age, employment status, smoking status, marital status, hepatitis c status, private versus public insurance, and year of delivery
Assessing the associations on a relative scale resulted in a total increased relative risk of NAS of 1.26 (95% CI 1.10, 1.45) for women treated with methadone compared with buprenorphine. When the mediating role of preterm birth was accounted for, the relative risk of NAS increased to 1.34 (95% CI 1.17, 1.53). The results on the relative scale support the findings of an increased risk of NAS with methadone compared with buprenorphine that was stronger among term births. Results were not meaningfully different when the generalised product method was used to assess mediation (proportion explained on RD scale=24.8% vs. 24.9%; supplemental Table 2).
Sensitivity analysis for unmeasured confounding
Bias estimates ranged from 0.99 to 1.02 depending on classification of missing BMI suggesting that the true association for the Controlled Direct Effect lies between 1.31 to 1.35. This demonstrates that, under our bias-analysis specifications, the potential confounding due to unmeasured BMI is minimal (Supporting Information). Results were not meaning different when the prevalence of obesity was varied based on preterm birth status.
Comment
Principal findings
Results from this study support earlier findings that risk of NAS is decreased in buprenorphine- compared with methadone-exposed infants. We advanced this research by further decomposing the association between methadone treatment and NAS compared with buprenorphine and finding that the association was stronger among term births compared with preterm births. As prolongation of pregnancy to term delivery is preferable when possible, this conclusion supports expanded use of, and access to, buprenorphine in women eligible for this therapy. Sensitivity analyses results suggest these findings are subject to minimal bias from unmeasured confounding and missing prepregnancy BMI.
Interpretation
This study expands upon previous work arguing the need to properly address gestational age in studying the association between opioid maintenance therapy and NAS,35 by being the first to describe the role of preterm birth and to quantify to what extent it may influence the association. Regression adjustment for gestational age, an approach often implemented in the literature,8,364 is inappropriate. Due to temporality, gestational age at delivery is a potential result of opioid maintenance therapy- and cannot be a predictor of treatment type.
Strengths of the study
Despite these limitations, our approach is characterised by several strengths. First, we found the same results using inverse probability weighted regression and the generalised product method, which suggests that our findings are robust to model misspecification. Second, we performed an empirically informed sensitivity analysis that simultaneously evaluated the extent to which unmeasured confounding by prepregnancy BMI impacted our results and the role of differential missingness of this variable. Results demonstrated little to no effect from such biases. We relied on pharmacy records only for identification of women receiving opioid maintenance therapy; each treatment type and dose was confirmed by extraction directly from the chart for all 716 women. Finally, to date, this is the largest study comparing these opioid maintenance therapies in actively treated pregnant women at one institution in the US.
Limitations of the data
As with all studies using observational data, interpreting the associations causally require assumptions of positivity, no interference, exchangeability, and counterfactual consistency.37 In this work, positivity and no interference pose little to no threats to the validity of the inferences. Positivity requires the presence of both exposed and unexposed term and preterm infants in all confounder strata. This assumption is verifiable, and held in the setting, as evidenced by the distribution of our stabilised inverse probability weights. No interference requires that the outcome of any given infant is not affected by the opioid maintenance therapy or preterm birth status of any other infant, and is a reasonable assumption to make. Exchangeability requires no uncontrolled information, selection, or confounding bias. As with other studies, we were unable to control for the prescribing preference for methadone versus buprenorphine. However, we have previously reported that unmeasured confounding by severity of addiction had little impact on the association between methadone and NAS compared with buprenorphine.33
We also lacked data on treatment trajectories, urine toxicology data, and gestational age at initiation and therefore assumed that treatment remained constant throughout pregnancy. Though this could introduce immortal time bias if women receiving opioid maintenance therapy were not converted to treatment until after 37 weeks,38 a detailed chart review we undertook in a subset of this cohort (n=200) found that no women were initiated on treatment after 36 weeks.33 The absence of trajectories also prohibited the presentation of true ‘directed’ acyclic graphs. We were unable to establish temporality of certain associations. For example, it is reasonable to assume that lack of insurance could influence treatment type if a woman cannot afford certain treatments. Conversely, it is also reasonable to assume that a woman receiving methadone may be less likely to be employed and therefore have no insurance. Finally, a lack of treatment history also prevented the evaluation of cumulative exposure. If NAS is influenced by a cumulative effect or sensitive exposure window we were unable to asses this. We chose to utilize the day of delivery as our exposure of interest as complete pregnancy treatment data were unavailable and because this is thought to be the most strongly associated with NAS.22
A noteworthy limitation of this work is our assumption that the relationships between opioid maintenance therapy-preterm birth and preterm birth-NAS are causal. Though a large body of work supports the notion that methadone affects preterm birth,7,15-18 research devoted to better understanding the mechanisms by which gestational age influences NAS is needed. Information on this relationship is limited as the pathophysiologic response associated with NAS is not fully understood. Therefore, it remains unknown whether the association between gestational age and NAS is attributable to a bias existing as Finnegan Scores were developed for term infants alone and symptoms in preterm may vary, or if a true difference in response to opioid exposure exists. This complication jeopardizes the validity of the counterfactual consistency assumption, an assumption that is commonly violated with measures of gestational age and preterm birth.39 There are many potential biologic mechanisms explaining why preterm infants may experience less NAS, including opioid receptor network immaturity, differential development of neurotransmitters, increased placental transfer of the opioid as pregnancy progresses, less fatty tissues available for methadone distribution in preterm infants, and/or less cumulative exposure to opioids.40 Lastly, we did not have data specifying if preterm births were spontaneous or induced, nor conditions associated with each (e.g. preeclampsia).
Conclusions
Buprenorphine is not the ideal treatment for all women seeking care; numerous social, behavioral, and biological factors must be considered when initiating opioid maintenance therapy. Nevertheless, our study emphasizes that it is crucial to accurately assess the risk of NAS associated with each treatment, while appropriately accounting for gestational age, in order to inform clinical practice and guide treatment decisions for pregnant women initiating care. Though previous research has established less risk of NAS associated with buprenorphine, we found that the increased risk of NAS after methadone exposure in utero compared with buprenorphine was stronger among a population of term than preterm births. These results support expanded use of buprenorphine as opioid maintenance therapy with the aim of decreasing NAS, adding additional incentive to providers and insurance companies to expand access through prescribing availability and medication coverage. Future work is needed to assess the impact of gestational age on additional maternal and infant outcomes known to be associated with opioid maintenance therapy as NAS is not the only outcome influencing treatment decisions.
Supplementary Material
Acknowledgments
This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Obstetric-Fetal Pharmacology Research Center (HD047905]. Lara Lemon is also a Ruth Kirschstein T-32 grant recipient. Robert W. Platt holds the Albert Boehringer I Chair at McGill University, and a Chercheur-national (National Scholar) award from the Fonds de Recherche du Québec – Santé.
The authors thank Drs. Allison Serra and Neggin Mokhtari for their contributions to the data collection for this study.
Footnotes
DR. LARA S LEMON (Orcid ID : 0000-0001-6806-7787)
References
- 1.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. Journal of Perinatology. 2015;35(8):650–5. doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. Journal of the American Medical Association. 2012;307(18):1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 3.Maguire DJ, Taylor S, Armstrong K, Shaffer-Hudkins E, Germain AM, Brooks SS, et al. Long-term outcomes of infants with neonatal abstinence syndrome. Neonatal Network. 2016;35(5):277–86. doi: 10.1891/0730-0832.35.5.277. [DOI] [PubMed] [Google Scholar]
- 4.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–65. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 5.ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstetrics and Gynecology. 2012;119(5):1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 6.Brogly SB, Saia KA, Walley AY, Du HM, Sebastiani P. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. American Journal of Epidemiology. 2014;180(7):673–86. doi: 10.1093/aje/kwu190. [DOI] [PubMed] [Google Scholar]
- 7.Zedler BK, Mann AL, Kim MM, Amick HR, Joyce AR, Murrelle EL, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111(12):2115–2128. doi: 10.1111/add.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England Journal of Medicine. 2010;363(24):2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, et al. Treating women who are pregnant and parenting for opioid use disorder and the concurrent care of their infants and children: Literature review to support national guidance. Journal of Addiction Medicine. 2017;11(3):178–190. doi: 10.1097/ADM.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young JL, Martin PR. Treatment of opioid dependence in the setting of pregnancy. The Psychiatric Clinics of North America. 2012;35(2):441–60. doi: 10.1016/j.psc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Kermack A, Flannery M, Tofighi B, McNeely J, Lee JD. Buprenorphine prescribing practice trends and attitudes among New York providers. Journal of Substance Abuse Treatment. 2017;74:1–6. doi: 10.1016/j.jsat.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie D, et al. Growth in buprenorphine waivers for physicians increased potential access to opioid agonist treatment, 2002-11. Health Affairs. 2015;34(6):1028–34. doi: 10.1377/hlthaff.2014.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of Family Medicine. 2015;13(1):23–6. doi: 10.1370/afm.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheon JA, Savitz DA. Invited Commentary: Influenza, influenza immunization, and pregnancy-It’s about time. American Journal of Epidemiology. 2016;184(3):187–91. doi: 10.1093/aje/kww042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary BJ, Donnelly JM, Strawbridge JD, Gallagher PJ, Fahey T, White MJ, et al. Methadone and perinatal outcomes: a retrospective cohort study. American Journal of Obstetrics and Gynecology. 2011;204(2):139.e1–9. doi: 10.1016/j.ajog.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Norgaard M, Nielsson MS, Heide-Jorgensen U. Birth and neonatal outcomes following opioid use in pregnancy: A Danish population-based study. Substance Abuse : Research and Treatment. 2015;9(Suppl 2):5–11. doi: 10.4137/SART.S23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurst KE, Zedler BK, Joyce AR, Sasinowski M, Murrelle EL. A Swedish population-based study of adverse birth outcomes among pregnant women treated with buprenorphine or methadone: Preliminary findings. Substance Abuse : Research and Treatment. 2016;10:89–97. doi: 10.4137/SART.S38887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer MC, Johnston AM, Crocker AM, Heil SH. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. Journal of Addiction Medicine. 2015;9(2):81–6. doi: 10.1097/ADM.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruwanpathirana R, Abdel-Latif ME, Burns L, Chen J, Craig F, Lui K, et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatrica. 2015;104(5):e188–94. doi: 10.1111/apa.12910. [DOI] [PubMed] [Google Scholar]
- 20.Dysart K, Hsieh HC, Kaltenbach K, Greenspan JS. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. Journal of Perinatal Medicine. 2007;35(4):344–6. doi: 10.1515/JPM.2007.063. [DOI] [PubMed] [Google Scholar]
- 21.Doberczak TM, Kandall SR, Wilets I. Neonatal opiate abstinence syndrome in term and preterm infants. The Journal of Pediatrics. 1991;118(6):933–7. doi: 10.1016/s0022-3476(05)82214-0. [DOI] [PubMed] [Google Scholar]
- 22.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. British Medical Journal. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive Diseases. 1975;2(1-2):141–58. [PubMed] [Google Scholar]
- 24.Institute of Medicine Committee on Understanding Premature B, Assuring Healthy O. The National Academies Collection: Reports funded by National Institutes of Health. In: Behrman RE, Butler AS, editors. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academies Press (US) National Academy of Sciences; 2007. [PubMed] [Google Scholar]
- 25.Liu AJ, Jones MP, Murray H, Cook CM, Nanan R. Perinatal risk factors for the neonatal abstinence syndrome in infants born to women on methadone maintenance therapy. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2010;50(3):253–8. doi: 10.1111/j.1479-828X.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 26. (World Health Organization Technical Report Series).Obesity: preventing and managing the global epidemic Report of a WHO consultation. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 27.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20(1):18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 28.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. Bioorganic & Medicinal Chemistry Medical Research Methodology. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandoli G, Palmsten K, Flores KF, Chambers CD. Constructing causal diagrams for common perinatal outcomes: Benefits, limitations and motivating examples with maternal antidepressant use in pregnancy. Paediatric and Perinatal Epidemiology. 2016 Sep;30(5):521–8. doi: 10.1111/ppe.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–38. [Google Scholar]
- 31.VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology. 2013;24(1):175–6. doi: 10.1097/EDE.0b013e3182781410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological Methods. 2013;18(2):137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemon LS, Caritis SN, Venkataramanan R, Platt RW, Bodnar LM. Methadone versus buprenorphine for opioid use dependence and risk of neonatal abstinence syndrome. Epidemiology. doi: 10.1097/EDE.0000000000000780. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21(4):540–551. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brogly SB, Hahn KA, Diaz SH, Werler M. Confounding of the comparative safety of prenatal opioid agonist therapy. Journal of Addiction Research & Therapy. 2015;6(4) doi: 10.4172/2155-6105.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiegand SL, Stringer EM, Stuebe AM, Jones H, Seashore C, Thorp J. Buprenorphine and naloxone compared with methadone treatment in pregnancy. Obstetrics and Gynecology. 2015;125(2):363–8. doi: 10.1097/AOG.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 37.Naimi AI, Cole SR, Kennedy EH. An introduction to G methods. International Journal of Epidemiology. 2017;46(2):756–762. doi: 10.1093/ije/dyw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matok I, Azoulay L, Yin H, Suissa S. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Research Part A, Clinical and Molecular Teratology. 2014;100(9):658–62. doi: 10.1002/bdra.23271. [DOI] [PubMed] [Google Scholar]
- 39.Basso O, Naimi AI. Commentary: from estimation to translation: interpreting mediation analysis results in perinatal epidemiology. Epidemiology. 2015;26(1):27–9. doi: 10.1097/EDE.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 40.Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547–61. doi: 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


