Abstract
The degree of behavioral control that an organism has over a stressor is a potent modulator of the stressor’s impact; controllable stressors produce none of the neurochemical and behavioral sequelae that occur if the stressor is uncontrollable. Research demonstrating the importance of control and the neural mechanisms responsible has been conducted almost entirely with male rats. It is unknown if behavioral control is stress blunting in females, and whether or not a similar resilience circuitry is engaged. Female rats were exposed to controllable, yoked uncontrollable, or no tailshock. In separate experiments, behavioral (juvenile social exploration, fear, and shuttle box escape) and neurochemical (activation of dorsal raphe serotonin and dorsal raphe-projecting prelimbic neurons) outcomes, which are sensitive to the dimension of control in males, were assessed. Despite successful acquisition of the controlling response, behavioral control did not mitigate dorsal raphe serotonergic activation and behavioral outcomes induced by tailshock, as it does in males. Moreover, behavioral control failed to selectively engage prelimbic cells that project to the dorsal raphe as in males. Pharmacological activation of the prelimbic cortex restored the stress-buffering effects of control. Collectively, the data demonstrate stressor controllability phenomena are absent in females and that the protective prelimbic circuitry is present but not engaged. Reduced benefit from coping responses may represent a novel approach for understanding differential sex prevalence in stress-related psychiatric disorders.
Keywords: rat, medial prefrontal cortex, anxiety, learned helplessness, serotonin
Graphical abstract
Coping behaviors potently modulate the impact of adverse life events, with controllable stressors blunting or eliminating many of the neurochemical and behavioral sequelae that occur if the stressor is uncontrollable. Here we report in female rats that the 1) stress-buffering effects of control/coping are absent and that 2) coping-related circuitry previously described in males (medial prefrontal cortex (mPFC) top-down inhibition over stress-responsive structures) is not engaged.
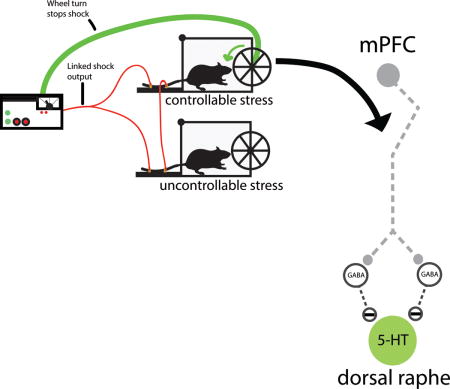
Introduction
Stress-related disorders such as depression, generalized anxiety, and post-traumatic stress disorder (PTSD) have a higher incidence in women than men (Blazer et al., 1994; Kessler et al., 1994; Kessler, 1997; Haskell et al., 2010). Although the direct mechanisms that drive these sex differences are unclear, they may emerge, in part, from different appraisal or coping processes in response to adverse life events (Ptacek et al., 1994; Matud, 2004; Kelly et al., 2008). A key feature of coping is the perceived or actual behavioral control over some aspect of the adverse event, which can be isolated and studied in animals so that the underlying neural mechanisms can be explored. This can be accomplished by employing a triad design in which one subject can terminate each of a series of tailshocks by performing a wheel-turn escape response (escapable shock, ES). Another subject is yoked to the ES subject and so each tailshock terminates whenever the ES subject turns the wheel. Here, turning the wheel has no consequence (inescapable shock, IS), and a third subject does not receive shock (home cage control, HC). Thus, the physical aspects of the adverse event (intensity, duration, etc.) are identical for ES and IS subjects, but the ability to exert behavioral control over its termination differs. There are numerous behavioral outcomes that typically follow IS (exaggerated freezing, shuttle box escape deficits, reduced juvenile social exploration, impaired fear extinction, etc.) that do not develop after ES (termed “stressor controllability effects”) (Maier & Watkins, 2005; Baratta et al., 2007). That is, the presence of behavioral control blunts the impact of the stressor.
Research directed at understanding the neural mechanisms underlying stressor controllability effects has focused on two lines of research: the mechanisms by which uncontrollable stressors produce their behavioral outcomes, and how control over physically identical stressors prevents them and produces resiliency. The former has indicated that the activation of the serotonergic (5-HT) dorsal raphe nucleus (DRN) and its projections to effector regions (e.g., amygdala) are critical for producing the behavioral consequences of IS (Maier & Watkins, 2005; Maier, 2015). Work on the second issue has established that the protective effects of control are mediated through the medial prefrontal cortex (mPFC), particularly the prelimbic (PL) subregion that provides top-down inhibitory control over stress-responsive structures such as the DRN (Maier, 2015). That is, when control is present, activation of the DRN is potently reduced by projections from the PL, thereby preventing the behavioral outcomes of stress.
Despite advances in identifying the critical mechanisms involved in stressor controllability effects, virtually all the work has been conducted in male subjects. We know of only one study that has varied stressor controllability in females, and interestingly, control was without an effect. Shors et al. (2007) reported that the presence of behavioral control did not reduce the impact of the stressor on neurogenesis. However, neither behavior nor DRN activation were examined. Prior work has identified sex differences in the neural responses to acute stressors (Kudielka & Kirschbaum, 2005; Iwasaki-Sekino et al., 2009; Lin et al., 2009), including those that involve mPFC and 5-HT systems (Shansky et al., 2004; Mitsushima et al., 2006), but invariably these stressors have been uncontrollable and a group for which the stressor is controllable was not included. The typical comparison is between subjects exposed to an uncontrollable stressor and a no stress home cage group. Therefore, it is unknown if controllability confers protection in females. The present set of experiments sought to determine if the protective effects afforded by behavioral control are present in females and if the mPFC circuitry that mediates the behavioral sequelae of controllability in males is also engaged.
Materials and methods
Subjects
A total of 172 adult female Sprague–Dawley rats (225–250 g; Envigo, Indianapolis, IN, USA) were pair housed on a 12-h light–dark cycle (lights on at 0600 h). Food (standard laboratory chow) and water were available ad libitum. Rats were allowed to acclimate to colony conditions for at least one week prior to experimentation. Stress treatment and behavioral testing were conducted between 0900 and 1400 h. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavior
Wheel-turn escape/yoked IS procedure
For manipulation of controllability, subjects were run in a triad design. One subject of each triad received ES, a second received yoked IS, and a third received no tailshock (HC). Each rat was placed in a Plexiglas box (14 × 11 × 17 cm) with a wheel mounted in the front. The tail was secured to a Plexiglas rod extending from the back of the box, and affixed with two copper electrodes and electrode paste. Each tailshock session consisted of 100 trials of tailshock (33 × 1.0, 33 × 1.3, 34 × 1.6 mA) on a variable interval 60-s schedule. Initially, the shock was terminated by a quarter turn of the wheel. When trials are completed in less than 5 s, the response requirement was increased by one-quarter turn of the wheel, up to a maximum of four full turns of the wheel. The requirement was reduced if the trial was not completed in less than 5 s. If the requirement was not reached in less than 30 s, the shock was terminated and the requirement was reduced to one-quarter turn of the wheel. For yoked IS rats, the onset and offset of each tailshock is identical to that of ES.
Shock-elicited freezing and shuttle box escape
Shock-elicited freezing and shuttlebox escape performance were assessed in shuttle boxes (50.8 × 25.4 × 30.48 cm; Coulbourn Instruments, Holliston, MA, USA) using procedures previously described (Amat et al., 2005; Strong et al., 2011). Twenty-four hours after stress treatment, subjects were placed into shuttle boxes and allowed to explore for 5 min. Rats then received two 0.7 mA foot shocks delivered through both sides of the grid floor. Foot shocks were terminated when the subject crossed over to the opposite side of the shuttle box through a small archway (fixed ratio 1, FR-1). Following the second FR-1 trial, shock-elicited freezing was observed for 20 min. Shock-elicited freezing is a measure of fear conditioned to cues present in the shuttle box (Fanselow & Lester, 1988). Each subject’s behavior was scored every 10 s as being either freezing or not freezing. Freezing was defined as the absence of all movement except that required for respiration. The observer was blind with regard to treatment condition, and inter-rater reliability has been calculated to be greater than 0.92. FR-1 foot shock rather than fixed duration foot shock was used so as to avoid introducing inescapable shocks into the escape testing procedure. ES and IS subjects do not differ with regard to FR-1 escape latencies (Amat et al., 2005; Strong et al., 2011), and so this aspect does not introduce a confound.
This observation period was followed by three further FR-1 escape trails and then 25 FR-2 escape trails. For each FR-2 escape trial, subjects were required to cross to the other side of the shuttle box and back to the initial side in order to terminate the shock. Foot shocks occurred with an average inter-trial interval of 60 s and each shock terminated after 30 s if an escape response had not occurred. FR-1 and FR-2 escape latencies were detected by infrared sensors located in the two shuttle box compartments, and data recorded by a connected PC. Escape failures were defined as escape latencies equal to or greater than 25 s.
Juvenile social exploration (JSE)
As in prior work (Christianson et al., 2009), each experimental adult rat was allocated to a separate plastic cage with a wire lid and bedding in a brightly lit testing room. Twenty-four hours before stress treatment rats were removed from the colony, transferred to the testing room, and placed into the plastic cage. After 60 min the adult rat was added to an interaction cage that contained a juvenile stimulus rat (28–35 days old, female Sprague-Dawley). Investigative behaviors, including sniffing, pinning, and allogrooming, initiated by the adult rat were timed by an observer blind to experimental condition. Following the 3 min baseline test, the adult rat was returned to its home cage. The JSE baseline test was used to habituate rats to the procedure and to screen for subjects with atypical baseline exploration values. A second identical 3-min JSE test occurred 24 h following stress treatment. Juveniles were used for multiple tests, but never more than once for the same adult rat. Total interaction time and percent change from baseline were calculated.
Surgery
All stereotactic surgeries were carried out under isoflurane (5% induction, 2% maintenance in 2.5 L/min O2; Piramal Critical Care, Bethlehem, PA, USA) anesthesia. Following surgery, subjects received subcutaneous injections of a nonsteroidal anti-inflammatory for analgesia (meloxicam, 0.5 mg/kg; Vetmedica, St. Joseph, MO, USA) and an antibiotic (Combi-Pen-48, 0.25 ml/kg; Bimeda, Oakbrook Terrace, IL, USA). Subjects remained in a recovery box with heating pad until ambulatory before returning to the colony. Subjects were given two weeks to recover from surgery before experimentation.
Intra-DRN fluorogold
Injection of the retrograde tracer fluorogold (FG; Fluorochrome, Denver, CO, USA) was performed using the same procedure previously described (Dolzani et al., 2016). Briefly, a small craniotomy (1 × 1 mm) was first made in the skull over the DRN. A stainless steel needle with beveled tip (31 gauge; Hamilton Company, Reno, NV, USA) was directed to the DRN (A/P: −8.0 and D/V: −6.7 mm from skull) and a 2% FG solution (in 0.9% sterile saline) was infused at a rate of 0.075 μl/min (0.2 μl total volume) using a UMP3 microinjection pump (World Precision Instruments, Sarasota, FL, USA). The tracer was allowed to diffuse for an additional 10 min before the needle was withdrawn.
Intra-PL cannulation
Rats were implanted with dual cannula guides for microinjections (26 gauge) with a 1 mm center-to-center distance (Plastics One, Roanoke, VA, USA). The tips of the cannulae were aimed at the PL (A/P: +2.6; M/L: ±0.5; D/V: −1.8 mm from pial surface) and secured to the skull with stainless steel screws and dental cement. Dummy cannulae were inserted into each guide cannula and held in place with a fitted dust cap (Plastics One).
Drug microinfusion
The GABAA receptor antagonist, picrotoxin (Tocris Bioscience, Bristol, UK), was dissolved in 0.9% sterile saline. Dual microinjectors (33 gauge; Plastics One) attached to PE 50 tubing were inserted through the guides. The other end of the tubing was connected to a 25 μl Hamilton syringe that was attached to a microinjection unit (Model 5000; Kopf Instruments, Tujunga, CA, USA). Animals received 0.5 μl of either picrotoxin (100 ng) or saline in each side of the PL. The volume was injected over a period of 30 s, and the injectors were left in place for 2 min to allow for diffusion.
Immunohistochemistry (IHC)
Enzyme-based IHC double labeling of DRN 5-HT and Fos (Grahn et al., 1999; Amat et al., 2005) and PL FG and Fos (Baratta et al., 2009) were performed as described previously. Two hours following the last tail shock subjects were deeply anesthetized with an overdose of anesthesia and transcardially perfused with ice-cold physiological saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB). Brains were removed and post-fixed overnight in 4% paraformaldehyde in PB, then transferred to 30% sucrose in 0.1 M PB and stored at 4 °C until sectioning. Serial tissue sections (30 μm) were obtained in a −20 °C cryostat and placed in a cryoprotectant solution until immunohistochemical processing.
Immunolabeling for Fos and 5-HT were conducted sequentially in DRN sections. Staining for Fos was conducted first using the avidin-biotin-horseradish peroxidase (ABC) method. Briefly, sections were incubated for 24 h at room temperature (RT) with anti-Fos primary antibody (1:15,000, rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following primary antibody incubation, sections were incubated for 2 h at RT with a biotinylated secondary antibody (1:200, goat anti-rabbit; Jackson ImmunoResearch, West Grove, PA, USA), then incubated in ABC for 1 h at RT, and finally sections were exposed to a solution containing 3,3′-diaminobenzidine (DAB), cobalt chloride, nickel ammonium sulfate, ammonium chloride, and glucose oxidase in PB. The peroxidase reaction was initiated by the addition of a glucose solution that reacted with the tissue for approximately 8–10 min.
DRN sections were further processed for 5-HT using rabbit polyclonal antisera directed against 5-HT (1:10,000; ImmunoStar, Hudson, WI, USA) for 48 h at 4°C. Sections were subsequently exposed to non-biotinylated secondary antibody (1:200, goat anti-rabbit; Jackson ImmunoResearch), rabbit peroxidase anti-peroxidase (PAP, 1:500; Sigma), and DAB solution without staining intensifiers.
Immunolabeling for Fos and FG in PL sections was conducted sequentially as well. Fos labeling was processed as described above followed by tissue incubation in anti-FG antibody (1:50,000, rabbit polyclonal; Fluorochrome) for 48 h at 4°C. Following primary antibody incubation, slices were then incubated in non-biotinylated secondary antibody (1:200, goat anti-rabbit; Jackson ImmunoResearch) and rabbit PAP (1:500; Sigma) before the chromagen was developed with a NovaRED substrate kit for peroxidase (Vector Laboratories, Burlingame, CA, USA).
Imaging and analysis
Tissue sections were imaged and quantified by an observer blind to group treatment using an Olympus BX-61 microscope with a digital color DP73 camera (Olympus America, Center Valley, PA, USA). For the DRN double labeling experiment, tissue sections representing rostral, middle, and caudal subregions of the DRN were analyzed (1.36, 1.00, and 0.70 mm anterior to interaural zero, respectively.) The total number of 5-HT-positive (+) cells, Fos+, and 5-HT+ cells expressing Fos were quantified (Olympus cellSens software). For the PL-to-DRN pathway activation experiment, serial PL tissue sections were collected for quantification of the total number of FG+, Fos+, along with the percentage of FG+ cells that express Fos. In addition, the locations of FG deposits in the DRN were verified with the same microscope using epifiuorescence illumination with a mercury lamp coupled to a DAPI filter.
Estrous cycle determination
A vaginal smear was taken prior to stress treatment. A blunt-tipped eyedropper filled with a small amount of 0.9% sterile saline was inserted into the vagina. Fluid was quickly expelled 2–3 times to gently wash off and collect vaginal cells (approximately 0.25−0.5 mL). A drop was placed onto a glass slide and immediately examined with a 40× objective lens. Characteristic changes in the cytological appearance of the smears were used to identify the cycle stage: diestrus (I/II), proestrus, and estrus.
Statistics
Data analysis was performed with StatView software (SAS Institute, Cary, NC, USA) and a Holm-Bonferroni correction calculator (Gaetano, 2013). The effect of treatment was analyzed with repeated-measures (trial block behavioral data), one-way (Stress), two-way (Stress and Drug), or mixed-design (Stress and DRN subregion measures) analysis of variance (ANOVA). Main effects and interactions were considered statistically significant if p < 0.05. When appropriate, post hoc analyses and planned comparisons were performed using Holm-Bonferroni correction for multiple comparisons. Values in graphs are represented as mean ± SEM.
Results
Behavioral control in females does not buffer against the behavioral outcomes of tailshock
Importantly, female ES subjects rapidly acquired the wheel-turn escape (control) response. As in prior studies with males (Amat et al., 2005), the number of wheel turns required to terminate the shock (in quarter turns of the wheel) was increased as female subjects became more proficient at escape (see Materials and methods). Thus, to measure quality of escape performance, the response requirement attained (that is, the number of quarter turns to terminate the shock) and the latency to terminate each tailshock were assessed (Fig. S1). Acquisition and performance was at least as efficient as is typical of males. The following day all rats received two FR-1 trials in a shuttle box and then freezing to the environmental context was measured for 20 min (Fig. 1A). As is typical in males, IS led to exaggerated freezing following the two foot shocks relative to HC controls. Unexpectedly, ES did not blunt this stress-induced enhancement of freezing as it does in males, rather female ES subjects also displayed equally enhanced freezing. Repeated measures ANOVA showed significant main effects of Stress (F 2,26 = 13.239, p < 0.001) and Trial Block (F 9,234 = 14.254, p < 0.001, n = 9–10/group). Post-hoc analyses indicated that ES and IS did not differ from each other, but did differ from HC (ps < 0.05).
Figure 1.
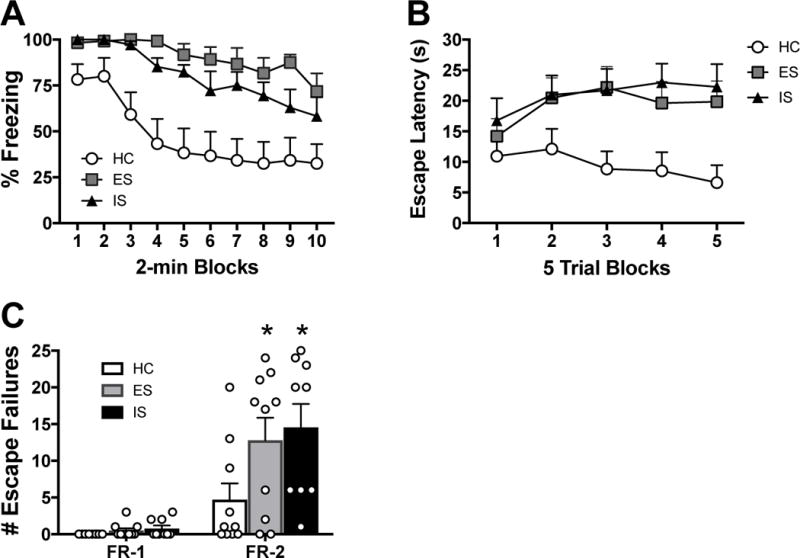
Controllable stress does not mitigate the impact of stress on shock-elicited freezing and shuttle box escape. Subjects received escapable shock (ES), inescapable shock (IS), or home cage control (HC) followed by behavioral testing 24 h later. (A) Percent freezing, in 2 min blocks, immediately following 2 foot shocks in a shuttle box. (B) Shuttle box escape latencies across blocks of five FR-2 escape trials. (C) Number of FR-1 and FR-2 escape failures. Data are mean (± SEM), *p < 0.05 compared to HC.
Shuttle box escape followed a similar pattern. Both IS and ES interfered with escape performance (increased escape latencies) on FR-2 trials compared to HC, and to the same degree (Figs. 1B, C). Repeated measures ANOVA indicated significant main effects of Stress (F 2,26 = 5.244, p = 0.012) and Trial Block (F 5,130 = 20.126, p < 0.001) and a significant Stress × Trial Block interaction (F 10,130 = 1.908, p = 0.049). Furthermore, ES and IS also impacted the total number of escape failures during FR-2, but not FR-1, trials (Stress: F 2,26 = 3.584, p = 0.042). Post-hoc analyses showed that ES and IS subjects had increased escape latencies and escape failures during FR-2 trials compared to HC (ps < 0.05), but did not differ from each other.
A second cohort received a baseline test of social exploration with a female juvenile conspecific (28–35 days old, Sprague-Dawley) 24 h prior to stress treatment, and another social exploration test 24 h after ES, IS, or HC. In agreement with previous studies in males, IS reduced exploration time (expressed as the percentage of baseline) when subjects were tested again 24 h post stress treatment (Fig. 2). Once again, ES did not mitigate the stress-induced reduction in social interaction in females. A one-way ANOVA identified a significant main effect of Stress (F 2,22 = 11.355, p < 0.001, n = 8–9/group). ES and IS showed marked reductions in exploration compared to HC (ps < 0.01), but did not differ from one another (p = 0.855). The lack of benefit from ES persisted even when tail shock intensities were reduced by ~30% (Fig. S2). It should be noted that rats in all stages of the estrous cycle (diestrus I/II, proestrus, estrus) were represented in each condition, although the design was not sufficiently powered to conclusively detect the impact of estrous phase. Individual plots of subject data by estrous phase are shown in Supplementary Figure 3.
Figure 2.
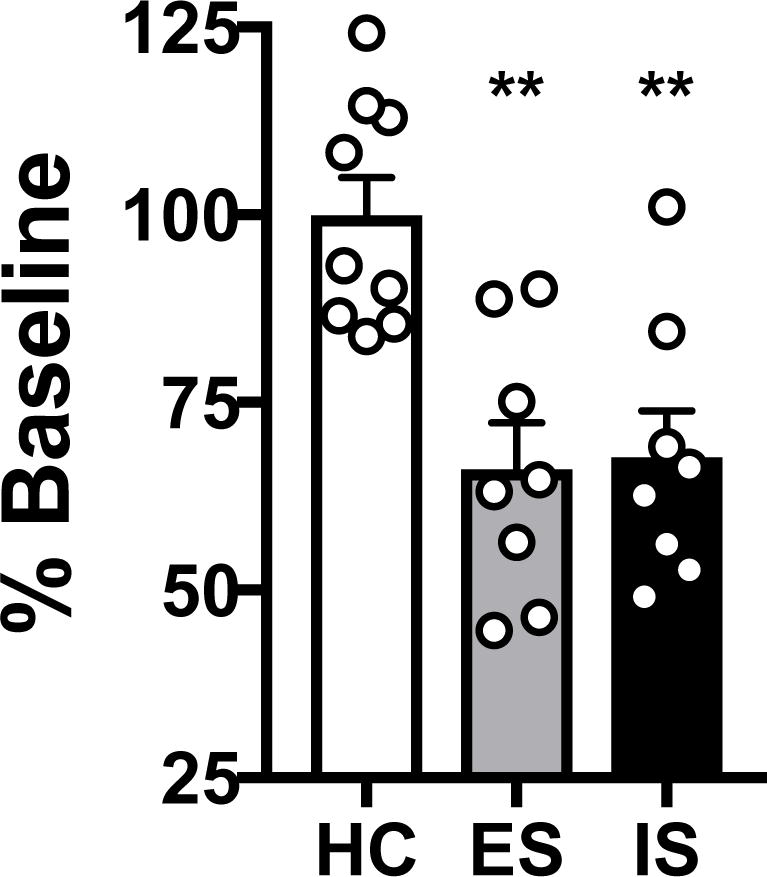
Controllable stress does not prevent stress-induced reduction of juvenile social exploration. A 3 min juvenile social exploration test was given 24 h after escapable shock (ES), inescapable shock (IS), or home cage control (HC). Bar graphs represent mean (± SEM) social exploration expressed as the percentage of baseline exploration, **p < 0.01 compared to HC.
Behavioral control in females does not mitigate stress-induced DRN 5-HT activation
We have previously reported in males that IS potently activates DRN 5-HT neurons (as assessed by Fos protein expression in 5-HT-labeled neurons), but that physically identical ES produces far less activation (Grahn et al., 1999; Amat et al., 2005). Furthermore, this controllability effect on 5-HT activation is especially prominent in the middle and caudal regions of the DRN in males. In the present experiment, female rats received ES, IS, or HC and were sacrificed 2 h after the stress session. As expected, the total number of 5-HT-labeled cells did not differ between groups in any of the DRN subregions (Table 1). A mixed-design ANOVA revealed a main effect of Stress (F 2,30 = 14.126, p < 0.001, n = 11/group) and a significant Stress × DRN subregion interaction (F 4,60 = 5.884, p < 0.001) for total Fos. Both ES and IS, relative to HC, increased the number of Fos-positive cells in each subregion of the DRN (ps < 0.001, Table 1). This was also true for the percentage of 5-HT-labeled neurons expressing Fos (Fig. 3). A mixed-design ANOVA showed a main effect of Stress (F 2,30 = 13.752, p < 0.001), and a significant interaction between Stress and DRN subregion (F 4,60 = 3.842, p = 0.008). Both ES and IS increased the number of double labeled cells compared to HC (ps < 0.001), however ES and IS did not differ from one another (p = 0.602). Thus, there was no effect of stressor controllability on DRN 5-HT activation in females.
Table 1.
5-HT and Fos Expression in DRN Subregions
Summary data showing the overall number of 5-HT- and Fos-positive cells in DRN subregions of escapable (ES), inescapable (IS), and home cage control (HC) subjects. Brain tissue was collected 2 h after stressor exposure,
| Marker | Region | Treatment | ||
|---|---|---|---|---|
|
| ||||
| HC | ES | IS | ||
| 5-HT | Rostral | 98.18(8.91) | 105.82(14.08) | 88.27(8.38) |
| Middle | 111.45(9.40) | 121.91(6.72) | 119.00(13.76) | |
| Caudal | 87.55(9.35) | 104.82(9.26) | 92.64(12.17) | |
|
| ||||
| Fos | Rostral | 5.00(1.72) | 41.36(6.85)a | 46.55(9.83)a |
| Middle | 1.82(0.74) | 22.36(4.42)a | 21.27(4.39)a | |
| Caudal | 2.00(0.80) | 9.45(1.77)a | 9.09(1.91)a | |
p < 0.01 compared to HC.
Figure 3.
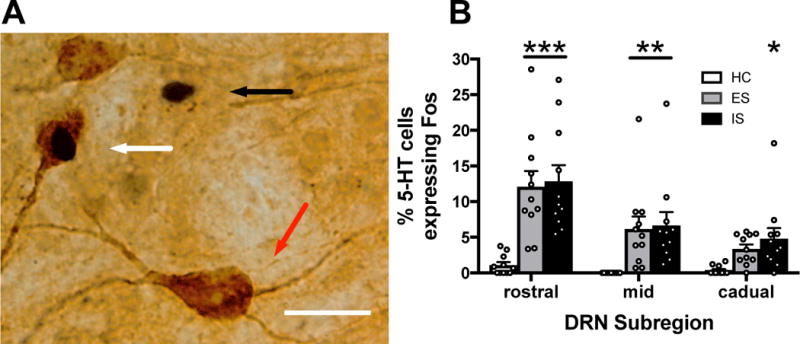
No effect of stressor controllability on DRN activation. (A) A representative brightfield photomicrograph showing a Fos-immunoreactive (ir) nucleus (black arrow), a 5-HT-ir soma (red arrow) and a double-labeled (Fos and 5-HT) neuron (white arrow) in the DRN following tail shock. (B) Percentage of 5-HT-labeled cells expressing Fos in rostral, middle, and caudal regions of the DRN for escapable shock (ES), inescapable shock (IS), or home cage control (HC) groups. Bar graphs represent mean (± SEM), *p < 0.05, **p < 0.01, ***p < 0.001 compared to HC. Scale bar represents 25 μm.
Behavioral control does not activate DRN-projecting PL neurons in females
In males, the buffering effects of behavioral control require activation of the pathway from the PL to the DRN, which provides top-down inhibition over DRN 5-HT activity (Amat et al., 2005; Amat et al., 2006; Baratta et al., 2009). One possibility for the lack of benefit from behavioral control in females is that the DRN-projecting neurons in the PL are simply not engaged by control. It is even possible that the PL does not project to the DRN as the existing anatomy studies have used males. In order to test these possibilities, the retrograde tracer, FG, was injected into the DRN followed by stress treatment (ES, yoked IS, or HC) 2 weeks later. Subjects were sacrificed and brains taken 2 h following the completion of the stress session.
Figure 4A shows a representative FG deposit in the DRN, and Figure 4B shows representative Fos and FG labeling in the PL. FG deposits tended to be restricted to the middle and caudal regions of the DRN. Only subjects with minimal or no FG deposit beyond the DRN were included in the final analysis. There was robust retrograde labeling, indicating the existence of a PL-to-DRN pathway in females. Importantly, there were no significant differences between groups in the number of FG-positive cells in the PL. HC subjects showed almost no Fos expression in the PL. In contrast, ES and IS led to a robust Fos enhancement (F 2,19 = 5.911, p = 0.010, n = 6–8/group, Figure 4C). Post hoc analyses showed that ES (p = 0.016) and IS (p = 0.029) significantly increased the number of Fos-positive cells relative to HC, although the two stress groups did not differ in amount (p = 0.829).
Figure 4.
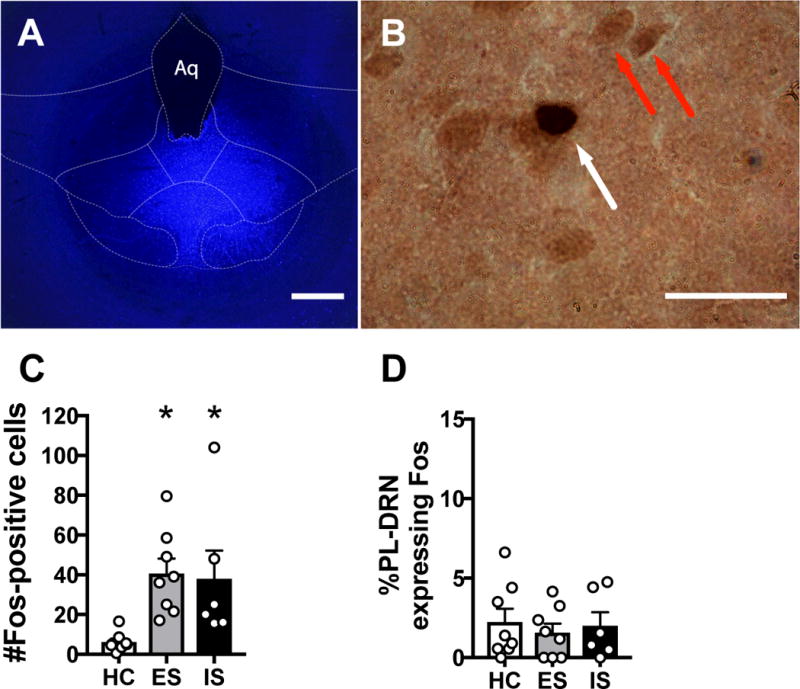
Behavioral control does not activate the PL to DRN pathway. (A) Fluorescent photomicrograph showing a representative fluorogold (FG) deposit in the DRN. (B) Representative brightfield photomicrograph showing a FG-immunoreactive (ir) soma (red arrow) and a double-labeled (FG and Fos) neuron (white arrow) in the PL following tail shock. (C) Total number of Fos-positive cells in the PL. (D) Percentage of FG-ir neurons expressing Fos in the PL following escapable shock (ES), inescapable shock (IS), or home cage control (HC). Bar graphs represent mean ± SEM, *p < 0.05 compared to HC. Scale bars represent 500 μm in A and 25 μm in B.
The percentage of DRN-projecting PL neurons co-expressing Fos was analyzed using a one-way ANCOVA; no significant main effect of Stress (F 2,16 = 0.783, p = 0.474), Number of FG-positive cells (covariate; F 1,16 = 2.425, p = 0.139), or interaction between Stress and Number of FG-positive cells were revealed (F 2,16 = 0.714, p = 0.505). In males, ES but not IS activates the PL-to-DRN pathway, but here all groups showed a similar lack of PL-to-DRN pathway activation. Thus, unlike prior studies with males, behavioral control did not selectively engage the PL-to-DRN pathway in females.
Pharmacological activation of the PL during stress exposure provides protection
Given that in females there is a PL-to-DRN pathway but it is not engaged by ES to blunt the behavioral outcomes of the tailshocks, we asked whether stress-buffering could be induced with pharmacological activation of the PL during stress as it is in males (Amat et al., 2008). Subjects received a social exploration baseline test with a female juvenile conspecific 24 h prior to stress. The next day, either the GABA receptor antagonist picrotoxin (100 ng/hemisphere) or vehicle was microinfused into the PL 30 min prior to the initiation of stress treatment, as has been done in males (Amat et al., 2008). Cannulae placement within the PL are shown in Figure 5A. As in a prior study with males (Amat et al., 2008), intra-PL picrotoxin did not interfere with wheel turn escape performance (Fig. S4). Repeated-measures ANOVA did not indicate any difference between picrotoxin and vehicle-treated ES subjects on response requirement (F 1,14 = 0.231, p = 0.638, n = 8/group) or escape latency (F 1,14 = 0.706, p = 0.415). The following day both vehicle-treated ES and IS groups showed reduced exploration (Fig. 5B), as above. Intra-PL picrotoxin had no effect on HC subjects but prevented the stress-induced reduction in social exploration for both ES and IS subjects. ANOVA revealed significant main effects of Stress (F 2,42 = 10.048, p < 0.001, n = 8/group) and Drug (F 1,42 = 15.770, p < 0.001) and a significant Stress × Drug (F 2,42 = 3.573, p = 0.037) interaction. Post hoc analysis indicated that ES-Vehicle and IS-Vehicle differed from all the other groups (ps < 0.01), which did not differ among themselves.
Figure 5.

Prelimbic (PL) activation prevents stress-induced reduction in social exploration. (A) Schematic representation of cannula placements within the PL. Numerals indicate in mm distance from bregma. For clarity, only cannulae from picrotoxin-treated groups are shown. (B) Escapable shock (ES), inescapable shock (IS), and home cage control (HC) subjects received intra-PL picrotoxin (PTX) or vehicle (VEH) 30 min prior to stress exposure. A 3 min juvenile social exploration test was given 24 h later. Bars represent mean (± SEM) social exploration expressed as the percentage of baseline exploration, **p < 0.01 compared to HC of the same drug treatment.
Discussion
The present experiments sought to determine whether the stress-blunting properties of behavioral control, as well as the neural mechanisms that mediate these effects, are present in female rats as they are in males. The behavioral results of the present experiments conducted in females were clear. Control over the stressor had no impact on shock-induced behavioral outcomes. That is, ES produced potentiated freezing and poor escape behavior equal to that produced by IS. During the 20 min freezing scoring period in the shuttle box we did not observe any sex-specific alternative fear responses, such as rapid “darting” movements, that have previously been reported in female rats (Gruene et al., 2015). One potential explanation for this discrepancy is that female darting behavior typically increases as signaled foot shock pairings progress, while only two non-signaled foot shocks were used in the current study.
The identical pattern was observed for juvenile social exploration assessed 24 h following stressor exposure. Both ES and IS led to a similar reduction in social exploration with a female juvenile. Although we did not include males here for a side-by-side comparison, we have conducted these experiments many times in males with the identical procedures, equipment, and laboratory conditions, as well as male groups that were participating in other experiments at the very same time as the female experiments. Thus, we are confident that the differences found are due to sex. It should also be noted that the behavioral end-points used here in females are the same end-points as most often used with males in both behavior and circuitry experiments.
The lack of benefit afforded by behavioral control in females is in sharp contrast to what has been observed in males, and suggests that the neural processing of control differs between the sexes. Multiple lines of evidence in males have shown that IS, compared to equal ES, induces a greater activation of DRN 5-HT and that this activation is critical for producing the behavioral sequelae that follow IS such as interference with shuttle box escape, exaggerated freezing, and decreased social exploration (Maswood et al., 1998; Grahn et al., 1999; Maier & Watkins, 2005). These outcomes are prevented in male ES subjects because the experience of control engages prefrontal top-down inhibition over DRN 5-HT activity (Hajos et al., 1998; Varga et al., 2001; Amat et al., 2005). Indeed, the PL-DRN pathway is selectively activated by the presence of control and inactivation of the PL during ES eliminates the protective effects of control (Baratta et al., 2009; Christianson et al., 2014). Here we show that females respond to behavioral control differently. Consistent with the above behavioral results, controllable stress in females failed to blunt stress-induced activation of DRN 5-HT. Across DRN subdivisions, ES and IS females showed equally elevated Fos expression in 5-HT-labeled neurons, relative to HC controls.
The absence of a modulating effect of control on DRN 5-HT may be due to a lack of top-down inhibition provided by the PL. This could occur because such an anatomical pathway does not exist in females, or because the pathway exists but is not activated by behavioral control. An intra-DRN deposit of a fluorescent retrograde tracer led to robust labeling in the PL, indicating the presence of this pathway in females, however it was not engaged by behavioral control. Unlike males, co-expression of Fos protein in DRN-projecting PL neurons was minimal in female ES subjects and did not differ from HC controls. This finding further supports the notion that neural processing of control differs in females.
The failure of control to provide protection is striking given that female ES subjects rapidly acquired the controlling (wheel-turn) response and maintained optimal responding throughout the entire tailshock session (Figs. S1, S4). The escape response is an instrumental response, and research in the field of instrumental learning, almost all of which has occurred in the appetitive rather than the aversive domain, suggests that there are two separable neural systems involved in the encoding of instrumental responses (Horvitz, 2009; Balleine & O’Doherty, 2010). One system, termed the “habit system”, supports the acquisition of inflexible, stimulus-response associations that are insensitive to contingencies (e.g., the difference between the conditional probability of reward in the presence of the response and the conditional probability of reward in the absence of the response). The other system, called the “action-outcome system”, encodes response-outcome associations and is sensitive to contingencies and leads to what can be called an “expectation”. Importantly, the habit system involves a circuit between the sensorimotor cortex and the dorsal lateral striatum, while the action-outcome system involves a corticostriatal loop that includes the PL and the dorsal medial striatum (DMS). The concepts of action-outcome contingency learning and behavioral control are formally identical (degree of behavioral control defined as the difference between the conditional probability of shock termination in the presence and absence of a response) (Maier & Seligman, 1976; Liljeholm et al., 2011), and activation of the PL and DMS during acquisition of the controlling escape response are required for the protective effects of behavioral control in males (Amat et al., 2014). Perhaps in females the encoding of the controlling response is biased towards the habit system rather than the PL-DMS action-outcome system.
Evidence regarding this possibility awaits further research, nor is it clear why females would fail to engage the PL-DMS act-outcome system to acquire the controlling response. In this regard, the work of Arnsten and colleagues suggests that high levels of catecholamine release in the mPFC, such as those that occur during acute stress, impair mPFC top-down regulation while simultaneously strengthening habit system function (Shansky et al., 2006; Arnsten, 2009; Fournier et al., 2017). Sex differences in basal levels and/or stress-evoked release of catecholamines have been reported (Mitsushima et al., 2006; Staiti et al., 2011), with levels generally increased in females compared to male rats. Enhanced sensitivity of the locus coeruleus, the sole source of prefrontal norepinephrine, to acute stress has also been reported in females, an effect found to be independent of hormonal status (Curtis et al., 2006). Future studies should address whether tail shock-induced release of catecholamines in the PL during behavioral control differs between male and female ES subjects, and whether in females it a) produces a shift in instrumental learning to a habit process that is insensitive to contingency and/or b) directly impairs PL regulation of DRN 5-HT activity. Consistent with this notion that the PL is taken “off-line” in female ES subjects, we found that pharmacological activation of the PL during stress treatment prevented the subsequent reduction in social exploration (Fig. 5), further suggesting that stress-buffering PL circuitry is present in females but is simply not engaged by behavioral control.
Establishing sex-based differences/similarities in the mechanisms that underlie the brain’s response to stress is a research imperative for addressing clinical phenomena in which women are more susceptible than men to develop stress-related disorders (Shansky, 2015). Much of the preclinical work involving female animals has led to mixed results, with many studies failing to recapitulate the directionality of sex differences observed in the clinical population. That is, females often are not more susceptible to the impact of stressors (Lin et al., 2008; Shansky, 2015). However, virtually all of this work employed stressors that are uncontrollable and thus involved comparison of groups exposed to an uncontrollable stressor and non-stressed control groups. In the present work as well, the uncontrollable stressor (IS) produced about the same magnitude of behavioral effects when compared to prior studies with males. Here we demonstrate that the stress-buffering effects of behavioral control characterized in males are absent in females, and that the neural circuit components that respond to coping with stress differ between the sexes. The present findings suggest an important role for stressor controllability in understanding sex-based differences in stress research and highlight the importance of using models of stress resilience for understanding mechanisms of susceptibility.
Supplementary Material
Acknowledgments
The authors thank Jose Amat for technical assistance and comments on the manuscript. The authors would also like to thank Dr. James Orth and the MCDB Light Microscopy Core Facility for their support. This work was supported by NIH Grants R01 MH050479 (SFM), R21 MH106817 (MVB), T32 MH016880 (SDD), and a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (MVB).
Abbreviations
- 5-HT
5-hydroxytryptamine
- ANOVA
analysis of variance
- DAB
3,3′-diaminobenzidine
- DMS
dorsal medial striatum
- DRN
dorsal raphe nucleus
- ES
escapable shock
- FG
fluorogold
- FR
fixed-ratio
- HC
home cage control
- IHC
immunohistochemistry
- IS
inescapable shock
- JSE
juvenile social exploration
- mPFC
medial prefrontal cortex
- PAP
peroxidase anti-peroxidase
- PB
sodium phosphate buffer
- PL
prelimbic cortex
- PTSD
post-traumatic stress disorder
- RT
room temperature
Footnotes
DR. MICHAEL VINCENT BARATTA (Orcid ID : 0000-0001-7273-1994)
Section: Behavioural Neuroscience
Conflict of Interest Statement
The authors report no biomedical financial interests or potential conflicts of interest.
Author Contributions
MVB and SFM, conception and design, writing and editing the manuscript; MVB, NRL, IPF, SDD, and LEC, execution of experiments and data acquisition; MVB, AMT, and SFM, analysis and interpretation of data; MVB, LRW, and SFM project supervision.
Data Accessibility Statement
All primary data are archived at the University of Colorado Boulder and available upon request.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Christianson JP, Aleksejev RM, Kim J, Richeson KR, Watkins LR, Maier SF. Control over a stressor involves the posterior dorsal striatum and the act/outcome circuit. Eur J Neurosci. 2014;40:2352–2358. doi: 10.1111/ejn.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, Watkins LR, Maier SF. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci. 2014;8:348. doi: 10.3389/fnbeh.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–450. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR, Maier SF. Activation of a habenulo-raphe circuit is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat. eNeuro. 2016;3 doi: 10.1523/ENEURO.0229-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS, editors. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. Lawrence Erlbaum Associates; Hillsdale, NJ, US: 1988. [Google Scholar]
- Fournier M, d’Arripe-Longueville F, Radel R. Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology. 2017;77:275–283. doi: 10.1016/j.psyneuen.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Gaetano J. Holm-Bonferroni sequential correction: An EXCEL calculator (1.1) 2013 Retrieved from https://www.researchgate.net/publication/236969037_Holm-Bonferroni_Sequential_Correction_An_EXCEL_Calculator.
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Haskell SG, Gordon KS, Mattocks K, Duggal M, Erdos J, Justice A, Brandt CA. Gender differences in rates of depression, PTSD, pain, obesity, and military sexual trauma among Connecticut War Veterans of Iraq and Afghanistan. J Womens Health (Larchmt) 2010;19:267–271. doi: 10.1089/jwh.2008.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Stimulus-response and response-outcome learning mechanisms in the striatum. Behav Brain Res. 2009;199:129–140. doi: 10.1016/j.bbr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Price LH, Carpenter LL. Sex differences in the use of coping strategies: predictors of anxiety and depressive symptoms. Depress Anxiety. 2008;25:839–846. doi: 10.1002/da.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Liljeholm M, Tricomi E, O’Doherty JP, Balleine BW. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J Neurosci. 2011;31:2474–2480. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Westenbroek C, Bakker P, Termeer J, Liu A, Li X, Ter Horst GJ. Effects of long-term stress and recovery on the prefrontal cortex and dentate gyrus in male and female rats. Cereb Cortex. 2008;18:2762–2774. doi: 10.1093/cercor/bhn035. [DOI] [PubMed] [Google Scholar]
- Maier SF. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: theory and evidence. J Exp Psychol General. 1976;105:3–46. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matud MP. Gender differences in stress and coping styles. Pers Individ Dif. 2004;37:1401–1415. [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Ptacek JT, Smith RE, Dodge KL. Gender differences in coping with stress - when stressor and appraisals do not differ. Pers Soc Psychol Bull. 1994;20:421–430. [Google Scholar]
- Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61:544–549. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–144. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


