Abstract
Accumulating evidence has shown that lymphocytes modulate behavior and cognition by direct interactions with the central nervous system. Studies have shown that reconstitution by adoptive transfer of lymphocytes from wild type into immune deficient mice restores a number of neurobehavioral deficits observed in these models. Moreover, it has been shown that these effects are mostly mediated by T lymphocytes. Studies of adoptive transfer thus far have employed adult mice, but whether lymphocytes may also modulate behavior during development remains unknown. In the present study, neonate lymphocyte deficient Rag2−/− mice were reconstituted within 48 hours after birth with lymphoid cells from transgenic donors expressing green fluorescent protein, allowing for their identification in various tissues in recipient mice while retaining all functional aspects. Adolescent Rag2−/− and reconstituted Rag2−/− along with C57BL/6J WT mice underwent a series of behavioral tests, including open field, social interaction and sucrose preference tests. At 12 weeks they were evaluated in the Morris Water Maze (MWM). Reconstituted mice showed changes in almost all aspects of behavior that were assessed, with a remarkable complete rescue of impaired social behavior displayed by adolescent Rag2−/− mice. Consistent with previous reports in adult mice, neonatal reconstitution in Rag2−/− mice restored spatial memory in the MWM. The presence of donor lymphocytes in the brain of neonatally reconstituted Rag2−/− mice was confirmed at various developmental points. These findings provide evidence that lymphocytes colonize the brain during postnatal development and modulate behavior across the lifespan supporting a role for adaptive immunity during brain maturation.
Keywords: T cells, adoptive transfer, development, cognition, neuroimmune
Graphical Abstract
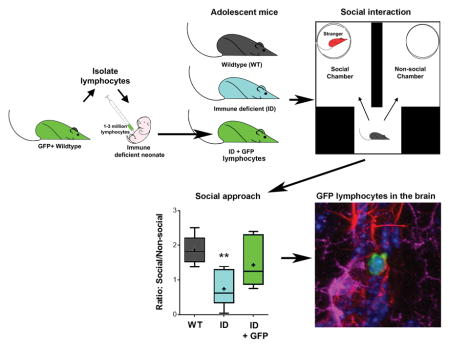
Neonatal immune deficient mice received lymphocytes from transgenic GFP expressing mice and were tested for social behavior during adolescence. Neonatal transfer of lymphocytes rescued social approach behavior in adolescent immune deficient mice. GFP expressing lymphocytes were found across brain regions in adolescent and adult immune deficient mice that received GFP lymphocytes as neonates.
Introduction
Lymphocytes, the cells of the adaptive immune system, are found in almost every tissue and organ, including the brain (Kipnis et al., 2012). Accumulating evidence shows that lymphocytes influence behavior by mediating direct or indirect production of cytokines and growth factors in specific compartments of the brain (Filiano et al., 2017; Herkenham & Kigar, 2017). A role for lymphocytes, mainly mediated by T lymphocytes, has been shown in memory (Ziv et al., 2006; Brynskikh et al., 2008; Wolf et al., 2009; Radjavi et al., 2014), emotional behaviors (Beurel et al., 2013; Rattazzi et al., 2013; Clark et al., 2014a) and stress responsiveness (Cohen et al., 2006; Lewitus et al., 2008; Clark et al., 2014b; Clark et al., 2016). Various mouse models of lymphocyte deficiency display behavioral abnormalities that can be restored by the transfer of these cells from immune competent mice, a process known as reconstitution by adoptive transfer (Rattazzi et al., 2013; Radjavi et al., 2014; Clark et al., 2016). During this process, lymphocytes expand and migrate to different tissues and organs including the brain (Song et al., 2016), governed by mechanisms involving competitive clonal expansion that limits proliferation once the population of cells has reached homeostatic levels similar to those of wild type animals (Troy & Shen, 2003; Min & Paul, 2005). It is believed that this mechanism in adult immune deficient mice recapitulates the natural process of development and maturation of the adaptive immune system during postnatal life (Min et al., 2002; Min et al., 2003; Min et al., 2004; Min & Paul, 2005). Development of a functional T cell repertoire mainly occurs during the postnatal period in mice and humans (Charles A Janeway, 2001), and a study in mice with functional T cell deficiency by deletions in the alpha and gamma chain of the T cell receptor documents brain and behavioral abnormalities with respect to WT mice (Rilett et al., 2015). These studies strongly suggest that lymphocytes may also affect brain function and behavior during the normal concomitant development of the central nervous and adaptive immune systems. However, whether behavioral abnormalities in models of immune deficiency emerge early in life and can be normalized by restoring lymphocyte function during postnatal development has not been investigated.
The T cell repertoire of neonates is very limited (van Ewijk et al., 2000; Schonland et al., 2003) and, as mentioned above, the neonatal lymphoid system has been shown to constitute a physiological lymphopenic environment that follows the same rules of expansion and proliferation observed in adult lymphopenia (Min et al., 2002; Min et al., 2003). In mice, during the first 3 weeks of life, lymphocytes and T cells undergo a series of functional changes including expansion, development of tolerance and the switch to antimicrobial properties that become stable by about 4 weeks of life (Penit & Vasseur, 1989; Dominguez-Gerpe & Rey-Mendez, 2003). In the periphery, CD4+ T cell populations reach mature levels by postnatal day (PND) 7 in the lymph nodes (LNs) and PND 15 in the spleen (Garcia et al., 2000). Studies of adoptive transfer in neonates demonstrate that these processes are recapitulated by this approach in peripheral tissues and organs with optimal efficacy when the transfer is made soon after birth and from young donors of about 2 weeks of age (Min et al., 2002; Min et al., 2003). However, whether neonatal transfer of lymphocytes results in colonization of the brain by this approach is currently unknown.
The Rag2−/− mouse model of immune deficiency has been broadly employed to study the development and function of lymphocytes by means of adoptive transfer (Min & Paul, 2005; Min et al., 2005). In these mice, functional T and B cell deficiency is produced by deletion of the recombination activation gene 2 (RAG2) necessary for the V[D]J re-arrangement process of the T and B cell receptor (Shinkai et al., 1992). Due to the restricted expression of the RAG2 gene to peripheral immune cells (Chun et al., 1991; Clark et al., 2014b), they have been widely employed to study the role of lymphocytes, and T cells in particular, on brain function and behavior (Wolf et al., 2009; McGowan et al., 2011; Rattazzi et al., 2013; Clark et al., 2014a; Clark et al., 2014b; Radjavi et al., 2014; Brachman et al., 2015; Clark et al., 2016; Song et al., 2016). The present study evaluated the effects of lymphocytes on adolescent and adult behavior by means of neonatal adoptive transfer of lymphoid cells from green fluorescent protein (GFP+) transgenic mice into Rag2−/− mice allowing for the identification and localization of these cells in the brain. Our studies revealed a profound effect of lymphocytes on adolescent behavior and the presence of these cells in different compartments of the brain during postnatal development, providing supportive evidence that neuroimmune interactions between the CNS and the adaptive immune system are part of naturally occurring physiological processes during postnatal development.
Methods
Animals and treatments
Breeder pairs for wild type (WT) C57Bl/6J, Rag2−/− on a C57Bl/6 genetic background (B6(Cg)-Rag2tm1.1Cgn/J) and transgenic green fluorescent protein (GFP+) expressing mice on a C57Bl/6 background (C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice were bred in our animal facility in microisolator cages with food and water ad libitum under a constant 12 h light-dark cycle. On PND 2, male offspring (n = 22) from three independent Rag2−/− breeding pairs received an intraperitoneal injection containing 1–3 x 106 lymphoid cells (100 μl in sterile saline) isolated from two-week old male transgenic GFP+ mice. Control littermates received sterile saline. Male age matched C57Bl/6 mice (n = 6) were used as WT controls. Lymphoid cells used for adoptive transfer were prepared as previously described (Song et al., 2016). Briefly, GFP+ mice were euthanized with an overdose of vaporized isoflurane (≥ 4.5% in O2) followed by cervical dislocation. Cervical LNs were rapidly dissected and homogenized in 5 ml DMEM and centrifuged at 400 g (1500 rpm) for 5 min. Red blood cells were removed with ACK lysis buffer (Quality Biological INC, Gaithersburg, MD, USA). The pellet was resuspended in 5 ml PBS and passed through a 40 μm cell strainer to prepare a single cell suspension. The proportion of live and dead cells was determined by Trypan Blue staining (Corning Cellgro). Cells were brought to the concentration of 1–3 x 106 cells/100 μl in sterile physiological saline solution. After adoptive transfer, mice were left undisturbed in their cages until weaned at PND 21. Rag2−/− mice reconstituted with GFP+ lymphocytes (Recon) and control Rag2−/− mice were co-housed and handled by the experimenter in the week leading up to behavioral assays (see Fig. 1A for timeline). No differences in mortality or growth were observed. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine.
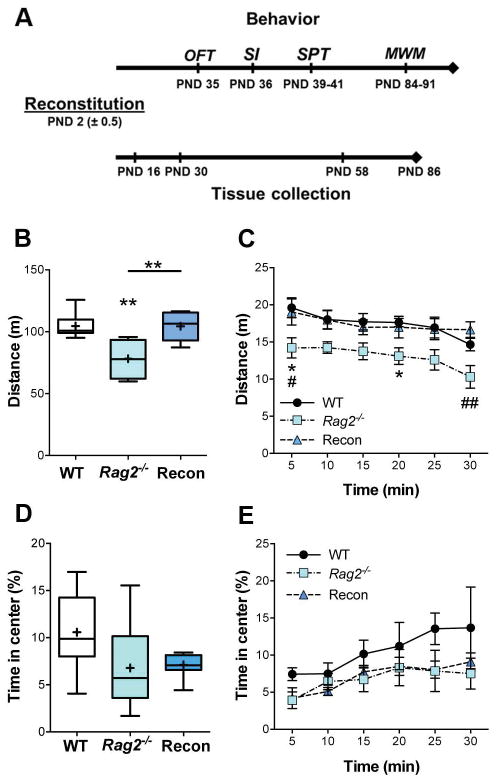
Fig. 1. Open field test.
A) Timeline of experiments. The Open field test (OFT), Social Interaction (SI), and Sucrose Preference Test (SPT) were conducted during the adolescent period (PND 35 – 41). The Morris Water Maze (MWM) took place when mice had reached adulthood (PND 84). In parallel, expansion and colonization of the brain by GFP+ lymphocytes was assessed at 2, 4, 8 and 12 weeks of age in mice not behaviorally tested. B) Total distance traveled (30 min). ANOVA, p = 0.001. C) Distance traveled over the course of the test (5 min bins). 2-way RM ANOVA; time: p = 0.003, immune status: p = 0.001, interaction: ns. D and E) Time in the center of the arena showed no significant difference in anxiety-like behavior. Tukey post hoc test for all analyses. WT: n = 6, Rag2−/−: n = 7, Recon: n = 8. * p≤ 0.05, ** p ≤ 0.01.
Open Field Test (OFT)
On PND 35 mice underwent a 30 min OFT in a 50 x 50 cm arena under low light (30 lux) conditions with background white noise (~68 dB) while being recorded overhead by a digital recording system. Total locomotion and time spent in the center of the arena were analyzed with TopScan (Cleversys Systems, Reston, VA, USA).
Social Interaction Test (SI)
Sociability was assessed on PND 36 using a modified social interaction arena based upon the three-chambered design (Moy et al., 2004). A mouse conditioned-place preference arena of 40 x 40 cm (Stoelting Co., Wood Dale, IL, USA) was divided into 2 equal sized areas (18 x 20 cm) connected at their base by a smaller traversing area (11 x 20 cm) (see Fig. 2). On the day prior to the test, conspecific WT male mice were habituated for 7 minutes to the chamber inside a small wire holding cage (Galaxy Cup; Spectrum Diversified Designs, Inc., Streetsboro OH, USA). On the day of the test, the experimental mouse was placed into the empty chamber and allowed to habituate for 5 min. A conspecific mouse in a holding cage and an empty holding cage were then placed in the far corners of the 2 opposing larger areas. The experimental mouse was then allowed to freely explore for another 5 min while being recorded with an overhead camera. Total locomotion and time spent in each area and in close proximity to the conspecific mouse was analyzed with TopScan (Cleversys Sys.). A social interaction score was calculated by determining the ratio of time spent in the social arena with the conspecific to the time in the non-social arena with the empty cage.
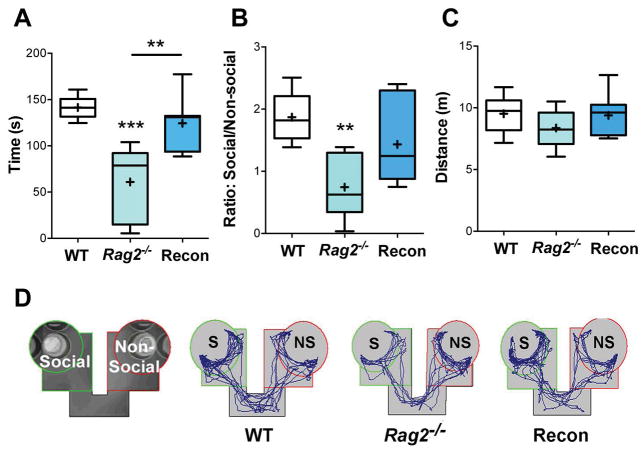
Fig. 2. Social interaction.
A) Time spent in close proximity (green circle) to the conspecific mouse, p < 0.001. B) Social interaction score (ratio of time in the social arena to the non-social arena), p = 0.004. C) Distance traveled during the test showed no significant difference. D) Representative images of the social interaction test. ANOVA with Tukey post hoc test for all analyses. WT: n = 6, Rag2−/−: n = 8, Recon: n = 7. ** p ≤ 0.01, *** p ≤ 0.001.
Sucrose Preference Test (SPT)
The SPT was conducted over 3 days starting on PND 39. In brief, starting on Day 1, mice were single housed in cages with water bottles and food available ad libitum on opposite sides of the cage top. After 24 h the total amount of water consumed from each bottle was measured to determine side preference. One water bottle was then replaced with another containing a 1% sucrose solution. To avoid a side preference bias, sucrose was first provided on the preferred side and then switched to the non-preferred side 24 h later. Total water and sucrose consumption was measured every 24 h and the total sucrose solution and liquid consumed over the 2 days used to calculate sucrose preference. Preference was calculated using [(total sucrose solution consumed)/(total sucrose + total water consumed) x 100]. At the conclusion of the test mice were returned to their home cages with their original cage mates.
Morris Water Maze (MWM)
Beginning at 12 weeks of age (PND 84) mice were assessed for spatial learning and memory in the MWM. The pool is 127 cm in diameter and 60 cm deep and divided into four equal quadrants, with each of the poles of labeled north, south, east, or west. A platform (10 cm in diameter and 46 cm high) sits in the middle of one quadrant of the pool; when hidden it is approximately 2 cm below water level and remains in this position for the entire experiment. The water is made opaque by adding non-toxic white tempura paint, and the temperature is kept at approximately 20° C. During cued trials large, black and white graphics are placed around the room as visual cues. A camera is positioned above the pool to record all the trials.
On Day 1 all groups underwent a visible platform test to examine possible differences in visual acuity, motor abilities and general sensorimotor abilities, such as motivation to escape the water and ability to climb on the platform positioned 1.5 cm above the surface of the water. A small flag was placed on the platform as a visual cue so that the platform could be located. Each mouse underwent 4 trials, alternating trials between cage mates, so that each trial was separated by 5 min. At the beginning of each trial the mouse was placed at a random start point on the opposite side of the pool, facing the wall of the tank. The mouse had 60 s to find the platform; if unsuccessful the mouse was guided to the platform by the observer. Mice remained on the platform for at least 30 s before being removed and returned to their cage.
The following day mice began acquisition training with visual cues posted around the room. During these sessions the platform was submerged just below the surface of the water in the center of one quadrant and the mice underwent 4 trials each as previously described. The mice underwent a total of 5 days of acquisition training before being tested for recall during a single probe trial on Day 7. For this trial, the platform was removed and mice allowed to explore for 60 s. Accurate recall was assessed by the time each mouse spent in the target quadrant and the number of crossings made over the region of the missing platform. Finally, on days 8 and 9, the mice were tested for reversal learning to assess how quickly they learned the new position of the platform, which was relocated to the opposite quadrant with starting points moved to the opposite side of the pool. Mice underwent 4 trials each and the average time to find the platform averaged across trials for each day.
Localization of GFP+ lymphocytes in the brain
Brains of Recon mice were examined at approximately 4 (PND 30; n = 6), 8 (PND 58; n = 5), and 12 (PND 86; n = 4) weeks after neonatal reconstitution for the presence of GFP+ lymphocytes as previously described (Song et al., 2016). Two additional Recon mice were examined 2 weeks after neonatal reconstitution. In brief, mice were anesthetized with vaporized isoflurane (~ 3.5% in O2) and then perfused with 4% paraformaldehyde (PFA) in phosphate buffered saline. The brains were extracted and post-fixed for 24 h in 4% PFA at 4° C and then equilibrated in 30% sucrose. The brains were then frozen and coronal sections (30 μm) were collected along the entire rostral-caudal axis using a cryostat. The brains collected at 10–12 weeks were cut in horizontal sections. Sections were stored in cryprotectant at −80° C until processed for labeling of myelin. After washing in PBS with 0.5% Triton-X 100 (3 x 20 min washes), sections were incubated in FluoroMyelin™ Red (1:300 in PBS; ThermoFisher Scientific, Walthman, MA, USA) for 30 min at room temperature, thoroughly washed with PBS and counterstained with the nuclear maker Hoechst (1:100,000) for 30 min. After a final wash with PBS, sections were mounted onto slides and coverslipped with Fluorosave (Invitrogen, Carlsbad, CA, USA) for microscopic analyses. Fluorescent microscopy was carried out with a Zeiss Axioscop with a Zeiss AxioCam MRc camera and the ZEN 2011 microimaging software (Carl Zeiss AG, Oberkochen, Germany). GFP and Texas Red specific filters were used to separate the GFP and FluoroMyelin™ signals and to identify autofluorescence artifacts. Total number of GFP+ cells were manually counted at PND 30 and 58 in 3 sections per animal at the level of the dorsal (~ Bregma −1.94 mm), medial (~ Bregma −2.54 mm) and ventral (~ Bregma −3.28 mm) hippocampus and averaged to produce a single number per section per animal. Images were acquired in single channels and merged with the ZEN software. Only brightness and contrast were adjusted for figure presentation. For Figure 6, autofluorescence artifacts in the GFP channel were manually eliminated using Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA). Composite figures were prepared with Adobe Photoshop.
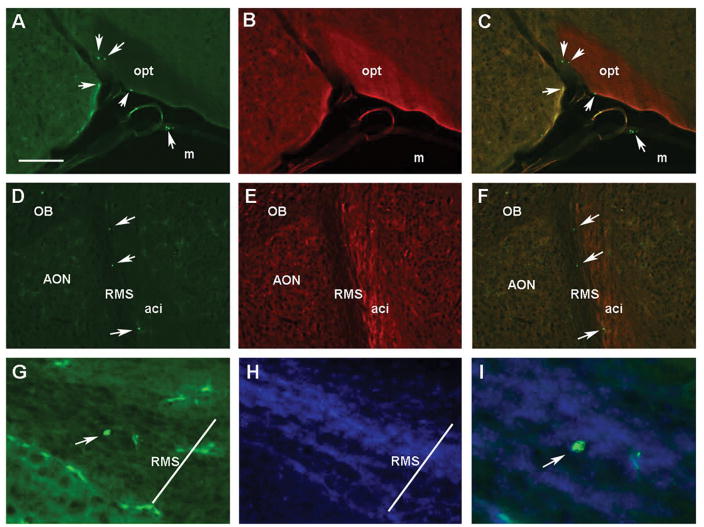
Fig. 6. GFP+ lymphocytes in the brain of adult Recon Rag2−/− mice.
Fluorescent microscopy images of GFP+ cells (green) and FluoroMyelin Red stained coronal (A–C) and horizontal (D–F) brain sections of adult Rag2−/− mice neonatally reconstituted with GFP+ lymphoid cells. Panels A–C show a coronal section showing the optic tract (opt) where GFP+ cells can be found the meninges (m), as well as within the brain. Images show the green (A), red (B) and C) merged channels, respectively. Panels D–I correspond to horizontal sections shown at the level of the anterior olfactory nucleus (AON); D: GFP, E: FluoroMyelin Red, F: merged. GFP+ cells are localized in the rostral migratory stream (RMS) next to the anterior commissure, intrabulbar (aci). G) A GFP+ cell is seen inside the boundaries of the RMS at higher magnification (200X); H) Hoechst nuclear counterstain. I) A merged image showing the GFP+ cell at 400X magnification. Arrows point to identified GFP+ cells (all panels). Scale bar in A = 250 μm for panels A–F. Transverse bar in G & H = 70 μm denoting the approximated width of the RMS. OB: Olfactory bulbs.
Analysis of peripheral T cells by flow cytometry in adult Rag2−/− mice reconstituted as neonates
At the completion of behavioral testing, lymphocytes from the lymph nodes of WT and Recon mice were processed for flow cytometry as previously described (Song et al., 2016). Briefly, mice were euthanized with an overdose of vaporized isoflurane (≥ 4.5% in O2) followed by cervical dislocation and cervical lymph nodes processed into single-cell suspensions as previously described for adoptive transfer of lymphocytes from GFP mice. Cells were resuspended in 50 μl of staining buffer (PBS+0.5% FBS) containing combinations of fluorescence conjugated anti-mouse antibodies (1:100 dilutions) including: anti-CD3-eFluor 450 (ThermoFisher, cat. #48-0032-82), anti-CD8-PerCP-Cy5.5, anti-CD4-PE (BD Biosciences, cat. #553730), and anti-CD16/CD32 (Fc blocker, BD Biosciences, cat. #553142). Cells were incubated at 4° C in the dark for 30 min, washed three times and resuspended at a final volume of 200 μl in staining buffer. Single-cell suspensions were analyzed on a BDTM LSR II flow cytometer (BD Biosciences) and the data processed using FlowJo version 10 software (Tree Star; Ashland, OR, USA).
Statistical Analysis
Behavioral analyses were conducted using GraphPad Prism (v. 6.07; San Diego, CA, USA). Multiple variate comparisons across trials or days were analyzed with a two-way repeated measures ANOVA followed by a Bonferroni or Tukey post hoc test. Data are shown as mean ± SEM across trials or days. Group comparisons were made using a one-way ANOVA. Post hoc analyses were completed using the Tukey test. Data are represented as box and whisker plots with median (line), mean (plus sign) and min/max values. Results with p ≤ 0.05 are reported as significant.
Results
Neonatal lymphocyte reconstitution restores locomotor activity in the OFT in adolescent mice
At PND 35 mice were assessed in the OFT. Horizontal locomotor activity, as determined by the total distance traveled in the arena, was significantly reduced in Rag2−/− mice compared to Recon and WT mice, which showed no difference in locomotion [F(2, 18) = 10.48, p = 0.001] (Fig. 1B). This difference in behavior was consistent throughout the test, and all groups of mice displayed similar habituation to the arena [Time: F(5, 90) = 3.99, p = 0.003; Immune status: F(2, 18) = 10.43, p = 0.001; interaction n.s.] (Fig.1C). No statistical difference among groups was found for the total time in the center of the arena (p = 0.16), a measure of anxiety (Fig. 1D), or in the change in center time over the course of the test [Time: F(5, 90) = 4.525; p = 0.001; immune status and interaction: n.s.] (Fig. 1E). These findings suggest that while reconstitution of Rag2−/− mice with lymphocytes was sufficient to rescue locomotor deficits in the OFT, there was no impact on adolescent anxiety-like behavior in this test.
Neonatal lymphocyte reconstitution rescues deficits in sociability during adolescence
One day after the OFT, mice were assessed in the social interaction test. Rag2−/− mice displayed impairments in sociability, which were absent in Recon mice (Fig. 2). Overall, Rag2−/− mice spent significantly less time near a conspecific mouse than WT and Recon mice [F(2, 18) = 13.73, p < 0.001; Fig. 2A] and had a statistically lower average social interaction score (ratio of time in the social arena to non-social arena) than WT mice [F(2, 28) = 7.71, p = 0.004; Fig. 2B], while Recon mice did not significantly differ from either group. No differences were detected in total activity in the social interaction chamber (p = 0.35) (Fig. 2C) indicating that the effects of neonatal lymphocyte reconstitution in adolescent Rag2−/− mice are specific for social behavior and are not dependent upon locomotor activity.
Neonatal lymphocyte reconstitution increases sucrose preference during adolescence
Two days after the SI test, mice underwent a sucrose preference test. Recon mice showed an increase in sucrose preference compared to Rag2−/− and WT mice [F(2, 19) = 10.31, p = 0.001] (Fig. 3A) with no significant difference among groups in total liquid consumption (p = 0.38; Fig. 3B) or adjusted for individual body weight (p = 0.69; Fig. 3C).
Fig. 3. Sucrose preference.
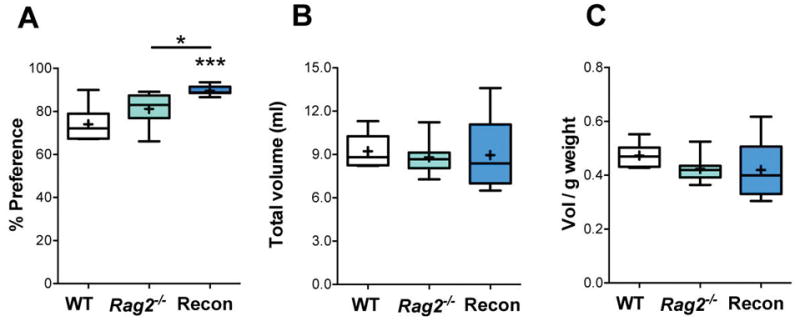
A) Preference for sucrose consumption over the 2-day test calculated with respect to total volume of liquid intake. Total volume of liquid (ml) (B) and total volume of liquid consumed per gram of body weight (C) showed no significant differences between groups. ANOVA with Tukey post hoc test for all analyses. WT: n = 6, Rag2−/−: n = 8, Recon: n = 8. * p ≤ 0.05, *** p ≤ 0.001.
Neonatal lymphocyte reconstitution rescues impairments in spatial learning during adulthood
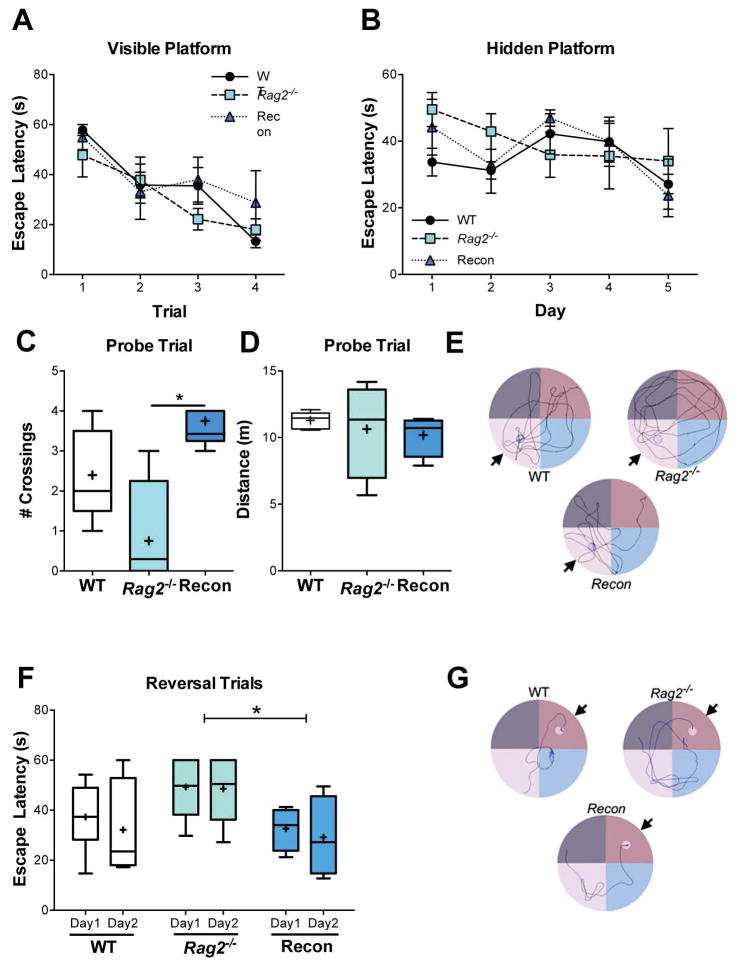
Spatial learning and memory were assessed in the MWM when mice reached 12 weeks of age. No differences in visual acuity or learning were observed when mice were presented with visible platform marked with a flag, with all mice showing a decrease in latency to find the platform as the test progressed [Time: F(3, 39) = 16.22, p < 0.0001, immune status and interaction: n.s.] (Fig. 4A). Tukey post hoc analysis during visible platform trials indicates significant differences between trial 1 (53.54 ± 2.99 s) and trial 2 (35.61 ± 1.33 s; p = 0.003), trial 3 (32.13 ± 4.90 s, p = 0.0004) and trial 4 (20.04 ± 4.58 s, p < 0.0001). Additionally, all groups performed comparably during cued acquisition learning, with a hidden platform [Time: F(4, 52) = 4.53, p = 0.003, immune status and interaction: n.s.] (Fig. 4B). While no one group performed better than any other, post hoc analysis does indicate that there was a significant reduction in escape latency from Day 1 (42.47 ± 4.64 s) to Day 5 (28.26 ± 3.03 s, p = 0.006). However, Rag2−/− mice showed impairments in memory recall during the probe trial one day after completion of acquisition training (Fig. 4C–E). Recon mice had a significantly greater number of crossings over the removed platform compared to Rag2−/− mice [F(2, 10) = 7.114, p = 0.012], although the total amount of time spent in the target quadrant was not statistically different. Additionally, no differences in the total distance traveled (p = 0.752) were detected during the probe trial. Finally, Recon mice displayed a shorter escape latency in the reversal task compared to Rag2−/− mice [Immune status: F(2, 24) = 4.15, p = 0.028, Time: and interaction: n.s.] (Fig. 4F and G). These results indicate that reconstituting lymphocytes in Rag2−/− neonates is sufficient to rescue deficits in the MWM as adults.
Fig. 4. Morris water maze.
A) Performance with a visible, marked platform on test Day 1 (Time: p < 0.0001, immune status and interaction: n.s.) and B) a hidden platform during cued acquisition training over 5 days (Time: p = 0.003, immune status and interaction: n.s.) show similar results for WT, Rag2−/− and Recon mice. 2-way RM ANOVA. C) Number of crossings over area of removed platform during the probe trial, p = 0.012. ANOVA, Tukey post hoc. D) No difference was detected in distance traveled during the probe trial. E) Representative tracings of the probe trials. Arrow marks target quadrant; small circle indicates where the platform had been previously placed. F) Average escape latency during reversal task trials (hidden platform moved to opposite quadrant) over two days (4 trials/day). Immune status: p = 0.028, time and interaction: n.s. 2-way RM ANOVA, Tukey post hoc. G) Representative tracings of the swim paths during Day 2 of reversal trials. Arrow points to the target quadrant; small circle marks the platform placement. WT: n = 6, Rag2−/−: n = 5, Recon: n = 5. * p≤ 0.05, ** p ≤ 0.01.
The postnatal brain supports colonization of T cells
Colonization and localization of GFP+ cells in the brain was studied by fluorescent microscopy. The two animals examined at two weeks of age had very few GFP+ cells detected sporadically in the meningeal spaces of some sections across the rostrocaudal axis from the olfactory bulbs to the end of the cerebellum (data not shown). Four weeks after reconstitution numerous GFP+ cells were found throughout the brain following the same pattern of distribution described previously in adult mice (Song et al., 2016). Lymphocytes were consistently found in circumventricular organs (CVOs) (Fig. 5 A–F) and the choroid plexus (ChP). Similar to adult reconstitution, they were also located in the brain parenchyma in both the grey and white matter with no consistent anatomical pattern across individual mice. Groups of cells were consistently found in white matter structures including the fimbria of the hippocampus, corpus callosum, internal capsule and striatum (Fig. 5) as previously described (Song et al., 2016). In the grey matter, scattered cells localized in different regions of the hippocampal formation, cerebral cortex and hypothalamus (data not shown). The same distribution was found in mice examined at 8 and 12 weeks after reconstitution (Fig. 6 A–C). Quantification of total number of cells per section showed no differences between 4 and 8 weeks after reconstitution (21.5 ± 3 and 18.5 ± 2.7 respectively; p = 0.54 n.s) indicating that the number of cells remain stable during these time points. In horizontal sections (12 weeks), numerous GFP+ cells were found in the parenchyma of the olfactory bulbs as described (Song et al., 2016), but also in the rostral migratory stream (Fig. 6 D–I). In the RMS, GFP+ cells were detected at different locations within this structure (Fig. 6 D–I). These data confirm our previous studies and indicate that neonatal reconstitution with lymphoid cells results in colonization of the adolescent brain following a similar temporal pattern of expansion (4 weeks) as in the adult lymphopenic brain.
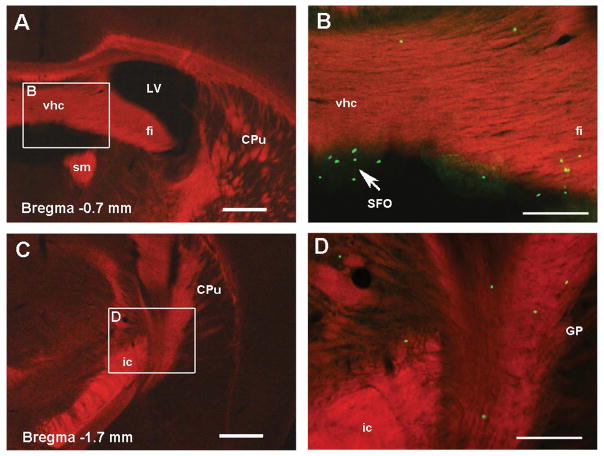
Fig 5. GFP+ cells in the white matter of adolescent Recon Rag2−/− mice.
Fluorescent microscopy images of coronal brain sections of adolescent Rag2−/− mice neonatally reconstituted with GFP+ lymphoid cells. Panels A and C show white matter structures labeled with FluoroMyelin Red (100X magnification). Higher magnification (200X) of the delineated regions are merged with the green channel in Panels B and D to show the localization of GFP+ cells in the white matter and circumventricular organs (arrow). Bregma coordinates correspond to the adult mouse atlas from Franklin and Paxinos, Third ed. 2007. CPu: caudate putamen, fi: fimbria of the hippocampus, GP: globus pallidus, ic: internal capsule, LV: lateral ventricle, SFO: subfornical organ, sm: stria medullaris thalamus, vhc: ventral hippocampal commissure. Scale bar in A, C: 400 μm, B, D: 200 μm.
Proportion of peripheral T cells in adult Rag2−/− mice reconstituted as neonates
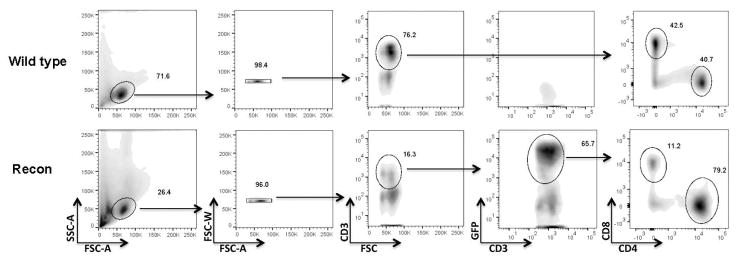
Phenotypic identification of T cells from the whole lymphocyte population was performed in cells from the LNs of WT and Recon mice at the completion of the behavioral testing. Gating and population demarcation was done simultaneously for WT and Recon mice. Similar to our previous study in adult animals, the CD4/CD8 ratio in WT was about 1:1, while Recon mice had a greater ratio (~ 4:1; Fig. 7). The CD4 bias in neonatally reconstituted Rag2−/− mice is consistent with our previous study in adults and may account for the behavioral results in the present experiments (Song et al., 2016). However, the composition of the donor’s lymphoid cells was not determined in the present study and may have influenced these proportions.
Fig 7. T cell composition in the lymph nodes of WT and Recon Rag2−/− mice.
Representative flow cytometry density plots of lymphoid cells isolated from wild type C57BL/6 and Recon Rag2−/− mice at the end of behavioral experiments. As expected, wild type mice are negative for GFP+ cells and have a 1:1 ratio of CD4/CD8 CD3+ T cells. In contrast, the ratio of functional CD4/CD8 T cells in Recon mice (CD3+/GFP+) is ≥ 4:1 (see results section).
Discussion
The present results confirm previous studies showing that neonates support lymphocyte proliferation after adoptive transfer and reveal that this process during postnatal development also involves the brain. This further supports the notion that lymphocytes and T cells play an important role in the normal development and function of the brain and expression of behavior (Filiano et al., 2015; Rilett et al., 2015). These concepts are supported by the display of significant deficits in social behavior during adolescent period in immune deficient Rag2−/− mice that are restored by neonatal adoptive transfer of lymphocytes. Notably, neonatal adoptive transfer results in a stable population of lymphocytes in adult animals that also modulates specific aspects of hippocampal dependent function including recall and reversal learning. Together, these studies contribute to the emerging literature demonstrating a role for lymphocytes and T cells in particular aspects of the development of the brain and the manifestation of behavioral and cognitive phenotypes.
The process of lymphocyte proliferation and expansion after adoptive transfer in neonates is believed to be equivalent to the natural process of maturation of lymphoid organs, leading to a fully functional adaptive immune response in adulthood (Min et al., 2002; Min et al., 2003). It has been shown that transferred cells undergo an initial exponential expansion with a cell division per day during the first week after transfer (Min & Paul, 2005). Our previous study revealed that more than 90% of the lymphocyte population in the brain, which arose from the initial transferred lymphoid population, corresponds to CD3+ T lymphocytes (Song et al., 2016). Most of these cells acquire characteristics of memory cells in the absence of foreign antigenic activation believed to reflect the process of developing tolerance to self-antigens (Goldrath et al., 2000; Troy & Shen, 2003; Min et al., 2004; Min & Paul, 2005). As early as 4 weeks of age, the distribution of neonatally transferred cells in the brain was similar to that found after adoptive transfer in adult animals (Song et al., 2016). GFP+ cells were observed primarily in the meninges and choroid plexus, which have been recently defined as structures that are part of the brain lymphatic system (Aspelund et al., 2015; Louveau et al., 2015). They were also found in the circumventricular organs, which are devoid of blood brain barrier. Consistent with our previous study, GFP+ cells were also found randomly scattered in the brain parenchyma with no defined anatomical pattern. Nevertheless, grouping of cells were often located in areas rich in white matter content, including the fimbria of the hippocampus, the corpus callosum and the internal capsule. While antibody based methods have not been able to detect these cells in the brain parenchyma in WT mice, our approach using transgenic cells have consistently revealed their presence, in particular in structures with high myelin content. Interestingly, early reports on beneficial effects of T cells on brain function and behavior employed immunization with a modified peptide sequence of myelin basic protein (Lewitus et al., 2008; Lewitus & Schwartz, 2009; Lewitus et al., 2009). While speculative at this point, is possible that beneficial effects of T cells are mediated, at least in part, by a role on white matter function.
Reports in the literature on the consequences of adaptive immune deficiency on social behavior suggest an effect for lymphocytes that seems to be dependent on the genetic deletion causing the deficiency and the specific aspect of social behavior evaluated. A study comparing Rag1−/− vs Rag2−/− deficient mice revealed impairments in Rag1−/−, but not Rag2−/− mice, on social recognition memory (McGowan et al., 2011). Both genes are necessary for the variable (diverse) V[D]J recombination process of the immunoglobulin and T and B cell receptors and deletion of either gene causes functional deficiency of lymphocytes (Chun et al., 1991; Shinkai et al., 1992). However, the RAG1 gene is constitutively expressed in the hippocampus, while RAG2 gene expression has not been found in the CNS and is restricted to immature, developing lymphocytes (Chun et al., 1991; Shinkai et al., 1992; Cushman et al., 2003; Clark et al., 2014b). Thus, the authors concluded that the presumed function of the RAG1 gene in the hippocampus, and not the lack of functional lymphocytes, explained the deficits in social memory in Rag1−/− deficient mice. Nevertheless, the present study clearly showed pronounced deficits on sociability in adolescent Rag2−/− mice that were restored by neonatal adoptive transfer of lymphocytes (Fig. 2). The discrepancies between studies may be related to the different developmental stage in which social behavior was evaluated, but is better explained by the different aspects of social behavior analyzed on each study. In the former, the authors focused on the memory component of social interactions, revealing no deficits between Rag2−/− and wild types on recognition memory (McGowan et al., 2011). The present study assessed the social approach component of social behavior evaluated by the three-chamber test (Moy et al., 2004). In this paradigm the conditions set by the chamber and the use of age and sex matched conspecifics maximize the analysis of social approach and investigation behaviors, while minimizing other components such as aggression and mating behavior (Moy et al., 2004). The modification of the apparatus employed here with a small transition chamber at the bottom of the arena maximizes the expression of a choice and avoids non-specific preference sites (Zanos et al., 2016; Terrillion et al., 2017). Thus, the study of McGowan, et al. and the present study analyzed different endophenotypes of social behavior, with the main differences involving social memory vs sociability respectively. In support for a role of lymphocytes in social approach behavior, a recent study employing severe combined immune deficient (SCID) mice reported similar results using the classical version of the three-chamber test (Filiano et al., 2016). Adult SCID mice showed the same social approach deficit described in our study, which was restored by adolescent reconstitution with lymphocytes. These effects were shown dependent on the cytokine interferon-gamma (IFN-γ), likely secreted by meningeal T cells (Filiano et al., 2016). While SCID and the Rag2−/− models differ in the genetic deletion causing immune deficiency, they consistently display social approach deficits supporting a role for adaptive immunity in social behavior. Our study also reveals that these differences, which can be restored by neonatal adoptive transfer, are already established by the pubertal period. Of note, improvements in social interaction in reconstituted adult Rag2−/− mice have been previously reported (Brachman et al., 2015). In this study sociability was assessed using a larger, aggressive CD-1 mouse for social interaction and the urine scent test for social approach. However, the strategy for reconstitution and the time of behavioral testing differ largely from our study. Nevertheless, taken altogether, these studies support a role for lymphocytes in social behavior and the interpretation of social cues.
Another relevant and consistent finding across studies using immune deficient mice relates to the deficits in learning and memory in the MWM. A number of studies have shown impairments in this test in different mouse models of immune deficiency including SCID, Rag1−/−, Rag2−/− and CD4+ T cell deficient mice on different genetic backgrounds, including C57BL/6 and BALB/c (Kipnis et al., 2004; Ziv et al., 2006; Brynskikh et al., 2008; Wolf et al., 2009; Radjavi et al., 2014). These studies also showed that these deficits are restored by transfer of functional lymphocytes and are dependent on CD4+ T cells. Our findings using neonatal adoptive transfer are consistent with these studies and indicate that lymphocytes have a long lasting influence on behavior. The effects of both neonatal and adult adoptive transfer suggest that a functional adaptive immune system is required for normal learning processes, which is further supported by the detrimental effects of CD4+ T cell depletion in adult wild type mice (Wolf et al., 2009). These findings support the proposition that a decline in T cell function may underlie impaired hippocampal dependent learning and memory processes in various physiological and disease states, including HIV and aging (Schwartz et al., 2013).
To our knowledge, the effects of neonatal adoptive transfer of lymphocytes on adolescent hedonic behavior reported here have not been studied thus far in any mouse model of immune deficiency, at any developmental stage. These results, which reveal an enhancement of hedonic behavior in the absence of a deficit with respect to immune competent mice, suggest a unique effect of lymphocytes on the reward system. The literature on the relationship between lymphocytes and behaviors engaging the reward system is very sparse. One study showed that activating dopaminergic neurons in the ventral tegmental area resulted in enhanced anti-bacterial responses for both the innate and adaptive immune systems (Ben-Shaanan et al., 2016). However, whether lymphocytes and T cells feed back into reward brain structures is unknown. Based on the present results, it is tempting to speculate that a positive reward mechanism exists in which behaviors that improve immune function are rewarded by the effects of an enhanced adaptive immune response. Nevertheless, other factors such as an increased metabolic need due to the maintenance of an adaptive immune system, which may have influenced these results, have not been addressed in this study. Moreover, interpreting these effects in immune deficient mice in the absence of a deficit with respect to WT deserves further consideration. Our main premise is that the “direction” of change should be consistent with those corresponding of restoring deficits observed in other tests. In our previous work (Clark et al, 2016), CD4 cells restored deficits on behavioral despair in the forced swim test, thus increases in center time in the OFT and open arms in the EPM in Rag2−/− reconstituted with CD4 cells, in the absence of a deficit compared to WT, were consistent with an improvement in emotionally salient behaviors and were interpreted as anxiolytic effects. Similarly, neonatal reconstitution with lymphocytes stimulated sucrose intake in Rag2−/− mice, which is consistent with an overall improvement of behavior in neonatal reconstituted Rag2−/− mice. However, whether cells of the adaptive immune system rescue deficits or merely improve Rag2−/− behavioral performance is difficult to establish in the absence of further testing and studies.
A limitation of the present study is the focus on male behavior and the effects described here may not extend across sexes. This is relevant in light of a report showing the contribution of T cells on the emergence of sexual differences in brain development and behavior (Rilett et al., 2015). While our previous study showed that the expansion of brain T cells was comparable between male and female adult Rag2−/− mice, specific behavioral sex differences, which usually develop during the adolescent period, may differ between male and female animals. Moreover, the present results should be interpreted with caution as they are based on reconstituting immune deficient mice generated by targeted deletion of the Rag2−/− gene. Future studies using wild type neonates, who support lymphocyte proliferation from allogenic donors, should further our understanding on the effects of diverse lymphocyte subtypes during postnatal development. Finally, while the WT comparison group was matched for age and was obtained from the same breeding colony, they were not litter mates. In summary, the present study provides supportive evidence for a role of lymphocytes on the modulation of behavior during postnatal development, further supporting a role for adaptive immune processes in postnatal brain maturation and adult functions.
Acknowledgments
This work was supported by NIMH grant Silvio O. Conte Centers for Basic Neuroscience or Translational Mental Health Research P50 MH103222.
Abbreviations
- GFP
green fluorescent protein
- LNs
lymph nodes
- MWM
Morris water maze
- OFT
open field test
- PND
postnatal day
- Rag2
recombination activation gene 2
- SCID
severe combined immune deficiency
- SI
social interaction
- SPT
sucrose preference test
- V[D]J
variable, diversity, joining segments
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest
Author’s contributions
LHT designed the study. CNV, XL and JAS coordinated colony breeding and timing of donors and recipients for adoptive transfer. XL and SMC performed the adoptive transfers and flow cytometry. CNV, JAS and SMC performed behavioral studies and data analyses. SMC and LHT performed tissue processing and fluorescent microscopy analyses. SMC and LHT wrote the manuscript.
References
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of Experimental Medicine. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, Schiller M, Green NL, Admon Y, Hakim F, Shen-Orr SS, Rolls A. Activation of the reward system boosts innate and adaptive immunity. Nature Medicine. 2016;22:940–944. doi: 10.1038/nm.4133. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biological Psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. The Journal of Neuroscience. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behavior and Immunity. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Janeway Charles A, Paul Travers J, Walport Mark, Shlomchik Mark J. Immunobiology. 5. Garland Publishing; New York: 2001. [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, Tonelli LH. Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2 mice. Behavioural Brain Research. 2014a;278C:74–82. doi: 10.1016/j.bbr.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, Kusnecov A, Gould TD, Tonelli LH. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behavior and Immunity. 2014b;38:192–201. doi: 10.1016/j.bbi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Soroka JA, Song C, Li X, Tonelli LH. CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(−/−) mice. Stress. 2016;19:303–311. doi: 10.1080/10253890.2016.1191466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. Journal of Neurobiology. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clinical and Vaccine Immunology. 2003;10:13–18. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. Evolution of the thymus size in response to physiological and random events throughout life. Microscopy Research and Technique. 2003;62:464–476. doi: 10.1002/jemt.10408. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Research. 2015;1617:18–27. doi: 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nature Reviews Neuroscience. 2017;18:375–384. doi: 10.1038/nrn.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunologic Research. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. The Journal of Experimental Medicine. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Kigar SL. Contributions of the adaptive immune system to mood regulation: Mechanisms and pathways of neuroimmune interactions. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2017;79:49–57. doi: 10.1016/j.pnpbp.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proceedings of the National Academy of Sciences. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nature Reviews Immunology. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behavior and immunity. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Molecular Psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biological Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Research. 2011;1383:187–195. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proceedings of the National Academy of Sciences. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- Min B, Paul WE. Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Seminars in Immunology. 2005;17:201–207. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Min B, Sempowski GD, Paul WE. Neonates support “homeostatic” proliferation. Advances in Experimental Medicine and Biology. 2002;512:91–95. [PubMed] [Google Scholar]
- Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. Journal of Immunology. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain, and Behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Penit C, Vasseur F. Cell proliferation and differentiation in the fetal and early postnatal mouse thymus. Journal of Immunology. 1989;142:3369–3377. [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behavior and Immunity. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational Psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilett KC, Friedel M, Ellegood J, MacKenzie RN, Lerch JP, Foster JA. Loss of T cells influences sex differences in behavior and brain structure. Brain Behavior and Immunity. 2015;46:249–260. doi: 10.1016/j.bbi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? The Journal of Neuroscience. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH. Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2−/− mice. Brain Behavior and Immunity. 2016 doi: 10.1016/j.bbi.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillion CE, Francis TC, Puche AC, Lobo MK, Gould TD. Decreased nucleus accumbens expression of psychiatric disorder risk gene Cacna1c promotes susceptibility to social stress. International Journal of Neuropsychopharmacology. 2017;20:428–433. doi: 10.1093/ijnp/pyw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. Journal of Immunology. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–1591. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of Immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]