Abstract
Dissemination of vector-borne viruses, such as Zika virus (ZIKV), in tropical and sub-tropical regions has a complicated impact on the immunopathogenesis of other endemic viruses such as dengue virus (DENV), chikungunya virus (CHIKV) and human immunodeficiency virus (HIV). The consequences of the possible co-infections with these viruses have specifically shown significant impact on the treatment and vaccination strategies. ZIKV is a mosquito-borne flavivirus from African and Asian lineages that causes neurological complications in infected humans. Many of DENV and CHIKV endemic regions have been experiencing outbreaks of ZIKV infection. Intriguingly, the mosquitoes, Aedes Aegypti and Aedes Albopictus, can simultaneously transmit all the combinations of ZIKV, DENV, and CHIKV to the humans. The co-circulation of these viruses leads to a complicated immune response due to the pre-existence or co-existence of ZIKV infection with DENV and CHIKV infections. The non-vector transmission of ZIKV, especially, via sexual intercourse and placenta represents an additional burden that may hander the treatment strategies of other sexually transmitted diseases such as HIV. Collectively, ZIKV co-circulation and co-infection with other viruses have inevitable impact on the host immune response, diagnosis techniques, and vaccine development strategies for the control of these co-infections.
Keywords: Zika virus, HIV, flavivirus, Dengue, Chikungunya, co-circulation, vaccination, co-infection
1. Introduction
Zika virus (ZIKV), an emerging mosquito-borne flavivirus, has attracted researcher's attention after the 2015–2016 imperative outbreak that has been associated with neurological complications. For years, the scientific research has focused on the most vital flaviviruses that have a significant impact on human health such as Dengue Virus (DENV), Yellow Fever Virus (YFV), Japanese Encephalitis Virus (JEV), and West Nile Virus (WNV) [1]. In February 2016, the World Health Organization has considered ZIKV infection a public health emergency [2]. ZIKV has a widespread distribution mainly in the countries of Africa, South America, and Southeast Asia; however, travelling has contributed to the expansion of ZIKV infection to non-epidemic nations of Europe, North America and Australia [3–5].
ZIKV was first found in macaque monkeys in the Zika forest in Uganda in 1947 and the first ZIKV infection in humans was reported in Nigeria, 1954 [6]. East Africa represents the African ZIKV lineage that was then transferred to Malaysia in Southeast Asia. The Asian lineage originated after virus dissemination to the Pacific Islands and the Americans [7]. The Asian-lineage ZIKV strain is currently circulating in the Western Hemisphere which can be differentiated from African-lineage ZIKV via molecular genomic and proteomic techniques [8].
Currently, ZIKV has spread to at least 33 countries and most recently in territories in the Americas [9, 10]. Importantly, ZIKV infection disseminates in the DENV and Chikungunya virus (CHIKV) epidemic areas raising the possibilities of developing immunological complications and the incidence of viral co-infections. These areas are also endemic with other infections, most importantly HIV. The co-circulation and co-infection of ZIKV with these viruses represent the current biomedical and public health challenge in term of diagnosis, treatment and vaccine development.
In this review, we discuss the present aspects of ZIKV co-circulation and co-infection with DENV and CHIKV, ZIKV infection to HIV patients especially pregnant women, and the biological control of ZIKV and implications for vaccine design.
2. ZIKV co-circulation with other arboviruses
ZIKV is classified as a positive single-stranded RNA virus belonging to the Flaviviridae family, is transmitted by mosquito bite, and exists in two distinct lineages. The single open reading frame (ORF) RNA encodes a premature viral polyprotein. Host cell furin and viral protease cleave the viral polyprotein into three structural proteins (capsid, envelope and membrane precursor) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The non-structural proteins are crucial for virus replication in the host cell [11].
The primary root of ZIKV infection in the epidemic zones is the mosquito bite. The warm tropical and subtropical regions of the world represent the preferred environment of Aedes mosquitos, the primary vector of flavivirus transmission [12, 13]. The two mosquito species, Aedes aegypti and Aedes Albopictus are usually considered as a primary vector for ZIKV, DENV and the alphavirus, CHIKV transmission. However, ZIKV was also isolated from other mosquito species such as Aedes africanus, Aedes apicoargenteus, Aedes luteocephalus, Aedes furciferand, Aedes taylori, and Aedes vittatus [14–17]. Distribution of Aedes Aegypti and Aedes Albopictus in the terrestrial habitat leads to co-circulating of these viruses and creating significant practical difficulties in controlling and diagnosing these arboviruses infections [18, 19]. These two species of mosquitoes can be infected with ZIKV, DENV and CHIKV and can transmit all combinations of these viruses simultaneously [20].
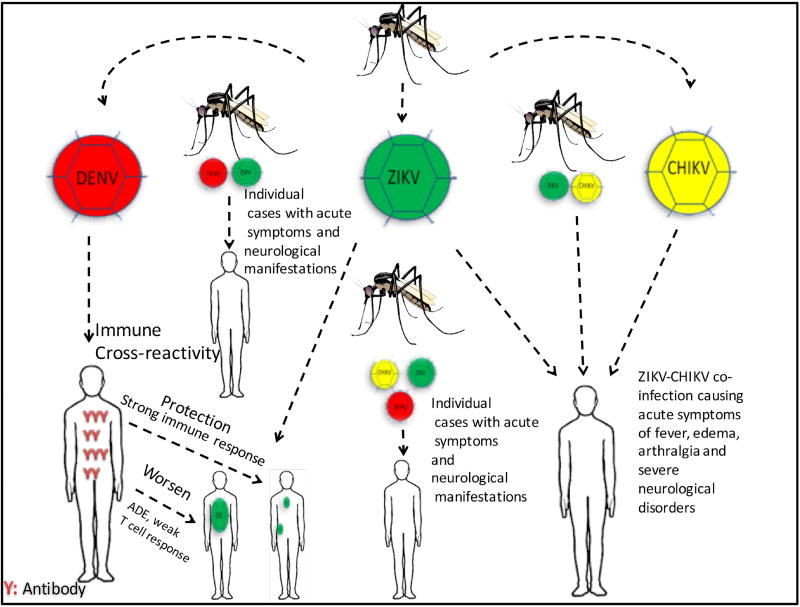
ZIKV, DENV and CHIKV share similar clinical symptoms; therefore, the diagnosis of ZIKV infection is particularly challenging in DENV or CHIKV endemic regions [21, 22]. Patients with CHIKV or DENV portray with leukopenia and thrombocytopenia. Moreover, hepatomegaly and bleeding appear in patients with symptomatic chikungunya can be seen predominantly in DENV infection. Other symptoms like fever, arthralgias, myalgias and some with lymphedema, conjunctivitis, limbedema, could be observed in ZIKV, DENV, or CHIKV infections [23] (Fig. 1). A study by Colombo et al., 2017 showed the most common symptoms in the patients with ZIKV infection were rash (100%), arthralgia (77.1%), fever (74.0%), myalgia (74.0%) and non-purulent conjunctivitis (69.8%). In patients with DENV infections, the most frequently observed symptoms were rash (100%), arthralgia (70.1%), fever (79.1%), myalgia (74.6%) and headache (73.1%). The measure of association between clinical manifestations among ZIKV and DENV infected patients detected a significant difference only in abdominal pain, leukopenia, and thrombocytopenia [24].
Fig. 1. ZIKV co-circulation and co-infection with DENV and CHIKV.
Aedes aegypti and Aedes Albopictus are the primary vector for ZIKV, DENV, and CHIKV, and it can infect humans with all combinations of these viruses simultaneously. These mosquitoes co-circulate ZIKV, DENV, and CHIKV creating significant practical difficulties in controlling and diagnosing these arbovirus infections. Previous infection or vaccination of DENV that trigger strong T cell responses can offer protection against ZIKV infection. However, poor T-cell immunity response against DENV or ZIKV infection could increase the possibility of antibody-dependent enhancement (ADE) that worsen ZIKV infection by facilitating virus uptake by host cell. The pre-existing of ZIKV infection followed by CHIKV infection or ZIKV-CHIKV co-infection increase the incidence Guillain-Barré Syndrome and other neurological complications.
Due to the similarity in infection symptoms of ZIKV, DENV, and CHIKV, the molecular-based techniques represent the optimal diagnostic method, in addition to conventional culture and serological tests. The serological test is inexpensive, simple, and available in resource limited settings. However, serum antibody cross-reactivity of DENV and ZIKV has been observed in the endemic areas [21]. Genomic and proteomic analysis of co-circulating viruses is essential to develop highly sensitive diagnostic tests. The most sophisticated molecular techniques are highly accurate in recognizing co-circulating viruses and even co-infection with viruses with high sequence similarity. For example, a validated real-time reverse transcription PCR assay using specific probes showed an accurate differentiation of ZIKV, CHIKV and the four serotypes of DENV [25–27]. Comparative proteomic analysis of co-circulating arboviruses to find a specific epitope could be an essential step to develop a simple, accurate, and affordable serological based diagnostic assay [28, 29].
Epidemiological studies are required to elucidate the transmission, co-circulation, and infection complications of ZIKV, DENV, and CHIKV at the endemic areas of these viruses. The research consortium "ZIKAction" in Latin America, Europe and the Caribbean has been recently funded by the European Commission. It represents an excellent example of such studies illustrating the epidemiology and vertical transmission of ZIKV, the natural history of the consequences of ZIKV infection during pregnancy on the fetus’s congenital and non-congenital syndromes [30]. The available resources of the recent epidemiological observation could facilitate the worldwide data dissemination of virus co-circulation. The Pacific Public Health Surveillance Network (PPHSN) provides an excellent source of information for communicable diseases, especially the outbreak of arboviruses including ZIKV, DENV and CHIKV [31]. An interactive map is available online [32] providing real-time data of the epidemic and emerging infectious diseases important for precaution preparations of the surrounding countries (Fig. 3).
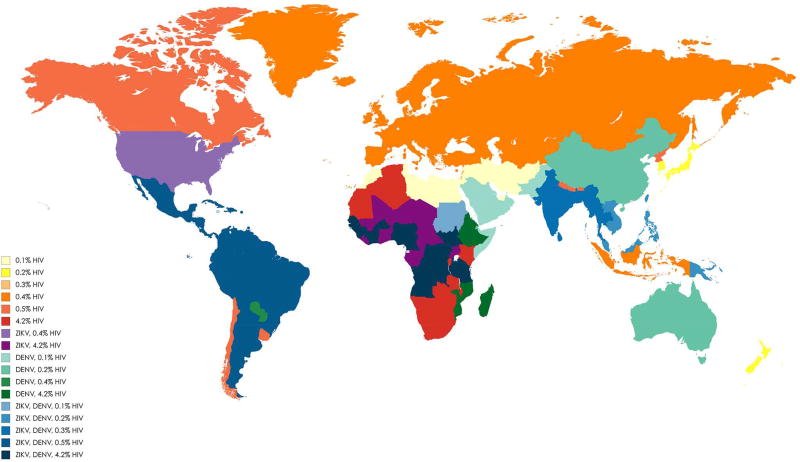
Fig. 3. Geographical distribution and overlapping area for ZIKV, DENV, and various prevalence of HIV [among adults aged 15–49, based on WHO report 2017].
The map was created through the following link: https://mapchart.net/world.html. The areas with only various mean prevalence of HIV and no report of DENV and ZIKV are shown in yellow to red colour rays. Areas with report of ZIKV combined with 0.4% or 4.2% mean prevalence of HIV are shown in light and dark purple color, respectively. Areas with report of only DENV combined with various mean prevalence of HIV are shown in light to dark green colour rays. Areas with report of ZIKV and DENV combined with various mean prevalence of HIV are shown in light to dark blue colour rays. Intensity of the colours increases when the mean prevalence of HIV increases in areas.
3. Preceding ZIKV or DENV infections promote both diseases
3.1. T cells cross-reactivity
Heterologous immunity refers to a cross-reactivity of pathogen-specific memory T cells to a subsequent infection due to high homology of pathogen epitopes that can lead to a decrease or increase in infectivity of the second pathogen. The population of memory T cells could be affected by heterologous immunity creating a considerable alteration in the T cell pool, repertoire, and dominance causing a significant variation in viral infection [33]. This phenomenon has been observed in patients infected with influenza virus followed by acute infection with hepatitis C virus (HCV). Influenza-specific memory CD8+ T cells cross-reacted with HCV epitope and increased HCV infectivity and subsequent liver damage [34]. On the other hand, immunized mice against lymphocytic choriomeningitis virus (LCMV) developed LCMV-specific CD4+ and CD8+ T cells that diminished infection with vaccinia virus and Pichinde virus [35].
Many of DENV endemic regions have been experiencing outbreaks of ZIKV infection. The high homology of DENV and ZIKV structural and non-structural proteins enforce the fact that the pre-existing immunity to DENV might affect the immune response to ZIKV infection [36]. In structural and non-structural proteins of both viruses, the presence of identical epitopes could stimulate T-lymphocyte response to the ZIKV or DENV infections [29]. The CD8+ T cell response to DENV mostly targets non-structural (NS) proteins such as NS3, NS4B and NS5 while ZIKV-specific CD8+ T cell responses are predominantly targeting E, prM, and C structural proteins. Both ZIKV and DENV CD4+ T cell responses are directed in approximately equal proportions against structural and non-structural proteins [37].
Previous infection or the vaccination of DENV triggers CD4+ and CD8+T cell responses that may interfere with ZIKV infection [37, 38]. In mice immunized against DENV, then challenged with ZIKV led to developing a cross-reactive CD8+ T cell against DENV and ZIKV [39]. This fact indicates that the strong T cell response against DENV or ZIKV infection could boost a considerable protection against ZIKV infection. However, identification of identical peptides between ZIKV and neuronal cell proteins could be linked to Guillain–Barré syndrome. Therefore, the strong cross-reactivity of CD8+ T cell responses to ZIKV could attack human neuronal antigens [40–42]. It is essential to consider the observations on DENV vaccine that failed to show significant protection for seronegative individuals attributed to the weak T-cell response [43]. Such poor T-cell response against DENV or ZIKV infection could increase the possibility of antibody-dependent enhancement (ADE).
3.2. Antibody-dependent enhancement (ADE)
The co-circulation of ZIKV and DENV in the endemic areas and their structural similarity could potentially lead to enhance their infections via antibody-dependent enhancement (ADE). The ADE is a phenomenon that occurs due to the cellular uptake of infectious virus-antibody complexes following their interaction with Fc receptors expressed on the host cell [44]. Interestingly, the collected sera from individuals living in a DENV-epidemic area containing DENV antibodies were able to enhance ZIKV infection in human macrophages [45], suggesting that the history of previous exposure to DENV can enhance ZIKV infection.
Similarly, the prior exposure to ZIKV infection can worsen the outcome of DENV infection [46]. Preceding ZIKV infection naturally induced cross-reactive antibodies to DENV-2 virus infection. Collectively, cross-reactive antibodies that amplified due to pre-existing immunity to ZIKV could induce ADE to the four DENV serotypes [46]. Therefore, the immuno-interaction between ZIKV and DENV serotypes might lead to developing ADE that could become an issue to be considered in the development of DENV or ZIKV vaccine in the endemic areas with flaviviruses especially DENV [47].
Neutralizing antibodies to specific envelope domain of DENV showed protective activity against ZIKV while the cross-reactive DENV specific antibodies are mostly enhancing ZIKV infection. Immuno-response of the host is mainly directed to the viral envelope glycoprotein that contains 90 head-to-tail homo-dimers packed tightly to form a smooth surface of the infectious virion. Each monomer consists of a central domain I (DI) that binds to an extended dimerization domain II (DII) from one side and to an immunoglobulin-like domain III (DIII) from another side. The specific envelope dimer epitopes (EDE1 and EDE2) have also been identified [48, 49].
The polyclonal antibody responses against the whole envelope protein are highly cross-reactive among DENV serotypes and ZIKV, while polyclonal antibodies against DIII domain showed no ADE towards ZIKV [50] and vice versa [51]. Antibodies against DIII domain especially DIII C-C loop showed considerable neutralization against ZIKV in vitro and a mouse model of ZIKV infection [52]. Additionally, antibodies that recognize DENV E-dimer epitope (EDE) can neutralize ZIKV infection in cell culture [53–56]. Specifically, antibodies against DENV EDE1 showed higher neutralization activity to ZIKV compared to DENV EDE2 [53, 54]. Whereas, antibodies against linear epitopes such as fusion-loop epitope were able to induce ADE because it showed binding affinity to ZIKV but were incompetent to inactivate the virus [53]. Although the immunity towards DENV infection might enhance infectivity, identification of unique antigenic epitopes of DENV and ZIKV could pave the way for designing and developing an efficient ZIKV vaccine.
4. ZIKV– CHIKV co-infection
Re-emergence and expansion of ZIKV and CHIKV infections worldwide represent a severe public health problem especially for tropical and subtropical regions. The suspected cases of ZIKV infection in the Americans during 2015 and 2016 were more than 360,000. CHIKV infection disseminated across 45 countries and territories in 2013, causing 1.7 million suspected cases [57]. CHIKV is an alphavirus belonging to the Togaviridae family; and like ZIKV, it is a positive-sense single-stranded RNA virus transmitted by Aedes mosquitoes. The two mosquito species, Aedes Aegypti and Aedes Albopictus, transmit ZIKV and CHIKV, and weather temperature primarily influences the transmission competency of Aedes mosquitoes. For example, the highest risk of viral transmission by Aedes Aegypti typically occurs when the temperature is 26 °C to 29 °C [58, 59].
ZIKV and CHIKV are endemic in many countries, and both viruses have a potential neurotropism. It is very likely the incidence of co-infection with both viruses might lead to the development of severe neurological complications. ZIKV causes neurologic complications such as microcephaly in neonates, encephalitis in adults, and Guillain-Barré Syndrome. Similarly, CHIKV infection presents severe encephalitis in neonates and older adults [60–64]. ZIKV and CHIKV infections frequently trend to targeting peripheral nerves like the incidence of Guillain-Barre Syndrome [61, 65–67]. Also, a previous infection with other pathogens such as Mycoplasma pneumoniae increase the risk of developing Guillain-Barré Sydrome in patients infected with Zika virus [68].
It is important to note that Aedes Aegypti is able to transmit both viruses simultaneously without considerable effect on the vector capability; therefore, the incidence of co-infection with ZIKV and CHIKV is most likely [69]. A case report showed a triple co-infection caused by ZIKV, CHIKV, and DENV to a pregnant woman from Colombia led to neurological manifestations [70]. Another case of co-infection describing three cases of ZIKV–CHIKV co-infection showed neurological complications detected at a single centre in Ecuador [71] and Colombia [72].
Patients with ZIKV-CHIKV co-infection experienced acute symptoms of fever, edema, arthralgia and severed neurological disorders. The neurological tests showed asymmetrical muscle weakness on limbs accompanied by absent deep tendon reflexes, pain in the back and extremities, paresthesia in the hands and feet, and mild sensory abnormalities [73]. It is interesting to note that the pre-existing of ZIKV infection followed by CHIKV infection or ZIKV-CHIKV co-infection increase the incidence of Guillain-Barré Syndrome [74, 75] and other neurological complications such as encephalitis, myelitis, meningitis, and microcephaly [72, 76, 77]. The summary of these viruses co-infections are illustrated in Fig. 1.
Only several case reports have described ZIKV-CHIKV co-infection after the recent ZIKV outbreak. Further studies are required to investigate co-infection of neurotropic viruses such as ZIKV and CHIKV in the endemic areas. These studies would support the efforts to better understand neurological complications as a result of simultaneous infections.
5. ZIKV co-infection in pregnant women with human immunodeficiency virus (HIV) infection
Transmission of ZIKV infection from the mother to the fetus via placenta is one of the non-vector ZIKV transmission. This phenomenon could induce an additional risk for the mother and the fetus in endemic areas with other sexually transmitted infections especially, HIV. A case report study described an autochthonous case of Asian clade ZIKV infection in an HIV-infected patient in Rio de Janeiro, Brazil. The patient showed mild symptoms and recovered well without significant abnormalities [78]. In the same ZIKV-epidemic area, another report described a case of ZIKV infection acquired during the first trimester in an HIV-infected pregnant woman that led to multiple fetal malformations and fetal demise [79]. Therefore, investigation of ZIKV tropism in placental tissues may pave the way to understand better how ZIKV infection can induce teratogenic effects in pregnant women with HIV.
The functional placenta vital for growth of the fetus may be affected by the ZIKV infection. The ZIKV infection in pregnant women can have a significant impact on the growth rate of the fetal brain causing a condition termed microcephaly [80, 81]. It is possible that ZIKV transmits from the mother to the fetus via placenta or the placenta may undergo pathological changes that contribute to developing microcephaly [82, 83]. The primary human placental macrophages (Hofbauer cells) present in the chorionic villi and placental villous fibroblasts accommodate ZIKV and permit virus amplification in placental tissues [84]. Additionally, the ZIKV infection induces proliferation and prominent hyperplasia of Hofbauer cells that eventually intensify virus infection [85, 86]. Thus, the rapid virus amplification in the roaming Hofbauer cells and some histiocytes in the intervillous spaces may enhance the transmission of ZIKV from the infected placenta to the fetus brain [84, 87]. ZIKV induces apoptosis in first-trimester trophoblasts and prevents differentiation of these cells [88], causing chronic placentitis [87]. Further studies are required to understand the pathogenesis of ZIKV infection during pregnancy, especially the placental pathology that could provide significant data on vertical transmission of this pathogen.
At present, it is unclear whether HIV infection increases the sensitivity to ZIKV infection and whether ZIKV infection could worsen HIV infection, especially during pregnancy. The placental tissues showed high sensitivity to ZIKV infection and induced teratogenic effects by creating an additional risk for the pregnant women with other infections [88]. Apart from placental dysfunction due to the co-infections, ZIKV infection showed significant impact on host immune response. A recent study showed that CD14+ monocytes are the primary target for ZIKV, especially during pregnancy, inducing inflammatory responses and immunotolerance [89].
The impact of ZIKV infection towards the host immune response could passively impact the status of HIV-infected women during pregnancy and the response to ART (antiretroviral treatment) drug regimen to protect the fetus from HIV infection. In July 2017, the National Institutes of Health (NIH) launched a study to determine whether the ZIKV infection might create additional risks for pregnant mothers infected with HIV. For the next 4 to 6 years, this study will provide information on the consequences of ZIKV-HIV co-infection and whether each virus could increase the infectivity of the other [90]. Further studies are required to better understand the impact of ZIKV-HIV co-infection on the integrity of placental tissues, the immune response, and ART regimen during pregnancy to restrict HIV transmission to the fetus, and the sensitivity to ZIKV infection for HIV-infected patients (Fig. 2).
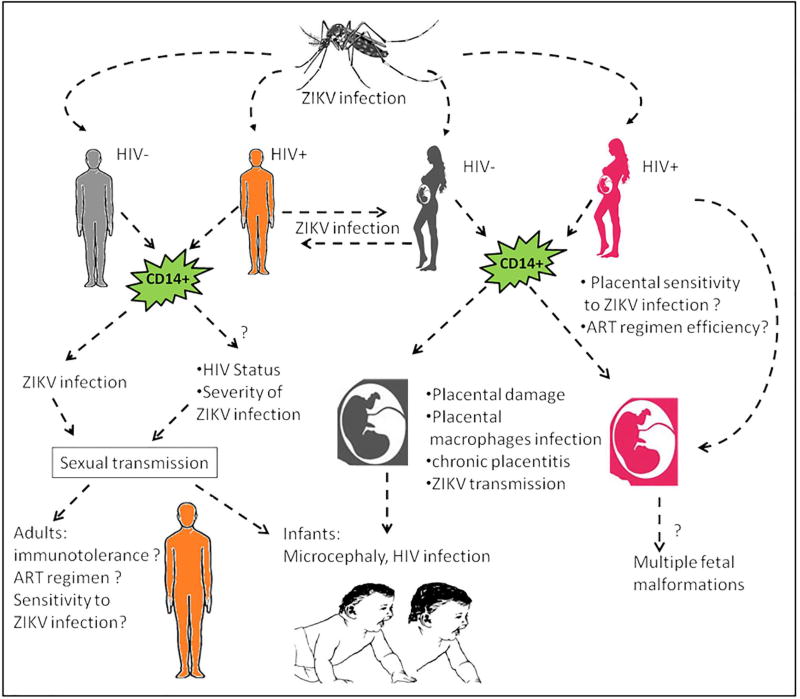
Fig. 2. ZIKV infection to humans with human immunodeficiency virus (HIV) infection.
ZIKV infection can be disseminated via vector or non-vector transmission. ZIKV infection targets CD14+ monocytes leading to increased inflammatory responses and immunotolerance, creating an additional risk for HIV-infected patients. ZIKV infection impacts the efficiency of ART regimen and increases the possibility of HIV transmission to the fetus in addition to the incidence of multiple malformations including microcephaly.
6. Importance of ZIKV vaccination/therapy strategy in co-circulation status with flaviviruses and HIV
Many flaviviruses have expanded in geographic distribution in recent years, with DENV being the most prevalent, infecting approximately 400 million people each year (Fig. 3). Climate change, virus evolution, and social factors may increase the risk of flavivirus infections and may lead to the emergence of flaviviruses in non-endemic areas [91]. The explosive emergence of ZIKV in Latin America in 2014 refocused international attention on this medically important group of viruses. Vaccination is a main stay of public health strategy for combating flavivirus infections. However, numerous challenges exist along the path from development to delivery of a tolerable and effective vaccine.
Various strategies have been taken into account to develop several prophylactic ZIKV vaccine candidates. These strategies include the subunit vaccine [92], RNA-NLP based [93], DNA-based [94], formalin-inactivated [94], live-attenuated virus (LAV) [95, 96], and also chimeras using Dengue [97], adenoviral [98] and alphaviral [99] vectors, and some targeting protection against congenital transmission [96, 100]. High-tech vaccine candidates are in various preclinical and clinical phases for commercialization in the near future.
Within the Flaviviridae family, vaccination against YFV [101] and JEV [102] are largely preventable; whereas, DENV [103] and ZIKV [104] vaccines represent unmet goals. One major concern that has to be taken into account while developing a vaccine for ZIKV is the prospect of using a vaccine in endemic areas where other flaviviruses are also co-circulating (Fig. 3). There are reports that showed the immunization against DENV enhanced ZIKV infection [105–107]. Thus, the role of ADE in the development of congenital and neurological complications associated with ZIKV infections should be well studied before taking further actions for ZIKV vaccination in DENV-endemic areas.
As described in this review, immune responses to Zika vaccination would highly depend on the previous exposure to other flaviviral infections. Co-circulation of multiple serotypes of DENV further complicates vaccine design against DENV and ZIKV [108]. The type of Zika vaccine, timing of vaccination in such areas, and the required post-vaccination immune response would depend on the co-circulation status of other flaviviruses that have cross-immunoreactivity with ZIKV [106–107]. The co-circulation and immune cross-reactivity of flaviviruses in endemic areas may lead to adverse, even life-threatening effects and sequelae upon immunization with flavivirus vaccine [105, 109].
Previous vaccination against other flaviviruses such as YFV and DENV makes the vaccination against ZIKV more complicated. New methods have been identified to develop potential ZIKV-specific vaccine candidates. Mutation in the envelop conserved fusion loop of ZIKV induced an efficient protective immune response and diminished the antibodies that cross-react with DENV [100]. As such, this method could achieve the key goal given concerns of ADE in sequential infection by closely related flaviviruses [105, 109].
The interaction between ZIKV infection and HIV infection has been paid considerable attention. Such interactions may alter the epidemiology, pathogenesis, immunology, and response to therapy of these pathogens [110, 111]. For instance, the co-infecting pathogens can accelerate HIV pathogenesis as well as facilitate its transmission by increasing viral replication efficiency. As the ZIKV infection expands, exposure in immunosuppressed patients may unveil new and more severe clinical manifestations. Lessons from other flaviviruses has shown that most co-infected DENV-HIV patients present mild clinical manifestations of DENV infection. Thus, DENV infection may influence the clinical profile and immune response in HIV patients [112]; DENV-HIV patients could have severe DENV infection outcomes [113]. The recent epidemic of ZIKV in South America has raised new concerns about HIV-ZIKV co-infected patients with life-threatening sequelae [79]. Close surveillance in HIV-positive individuals and in vivo model studies of such co-infection are important because there is large geographical overlap of populations exposed to both ZIKV and HIV infections [114](Fig. 3). Taken together, for HIV patients in ZIKV endemic areas, more in vitro and in vivo studies are required to discover the mechanisms of ZIKV pathogenesis in these immunocompromised patients. Such studies are helpful to set a best strategy for an efficient vaccination against ZIKV infections.
7. Biological control of ZIKV infection
Wolbachia, the endosymbiotic bacteria that infects mosquitoes, can block or reduce a mosquito's competency to carry and disseminate vector-transmitted viruses. It is capable of infecting medicinal-important mosquito species such as Culex quinquefasciatus, Aedes albopictus, Anopheles gambiae and Anopheles coluzzii, Aedes albopictus, and Aedes aegypti [115, 116]. Current research has shown a substantial ability of Wolbachia in inhibiting viral infections including ZIKV, DENV, and CHIKV [117–121]. Thus, Wolbachia represents a broad spectrum antiviral application that could control ZIKV mono-infection or co-infection via controlling mosquito-transmitted viruses that co-circulate in the same epidemic areas.
Wolbachia eliminates virus infection in mosquitoes by a reproductive phenotype called the cytoplasmic incompatibility. Interestingly, mating a Wolbachia-infected male with an uninfected mosquito female produces unviable offspring. While, mating infected or uninfected males with Wolbachia-infected females produce viable offspring [115]. Releasing different strains of Wolbachia-infected males such as wAlbA, wAlbB, wPip, wStri, and wMelPop could induce sterility and reduce mosquito populations [122–127]. Aedes aegypti has been found infected with wMel limited ZIKV in Colombia [118], Brazil [117, 127], and Singapore [120]. Laboratory studies showed an effective inhibition of ZIKV in mosquito cell lines after infection with wAlbB and wStri strains [126].
Wolbachia can manipulate mosquito cellular function greater than viral infection. Commonly, an extensive reprogramming of cellular metabolism has been observed during viral infection [128]. For example, DENV infection activates the glycolytic pathway of glucose metabolism to promote efficient viral replication [129]. Interestingly, Wolbachia infection showed a more significant impact on the cellular metabolic pathways compared to virus infection [130]. Some lipids were reduced in Wolbachia-infected mosquito cells that were abundant in DENV-infected mosquito cells, suggesting that Wolbachia inhibited virus replication in mosquito cells via shifting the lipid profile [131]. Further research on the impact of Wolbachia infection on cellular pathways that inhibit viral replication could open a new avenue for developing antiviral drugs.
Although Wolbachia are efficient to protect mosquitos against a wide range of viral infections, many factors should be implemented for current and new technologies to achieve successful application. The population density of wild mosquitoes considerably reduced Wolbachia proliferation in Aedes aegypti [132]. Additionally, it is important to consider the ecosystem when Wolbachia-infected mosquitoes are introduced into wild population such as avoiding the high weather temperature [133]. The laboratory invasion of Wolbachia is not necessarily reflecting its field capability of infection. Therefore, minimizing the laboratory adaptation is crucial to increase the relevance of experiments to field mosquitoes that can be achieved by applying out-crossing and maintaining the Wolbachia colonies under optimal conditions [134]. New technologies have been implemented to reduce virus infection including genetically modified mosquitoes that carry dominant lethal genes, such as the OX513A, and advancing gene-drive technologies [135]. Current and new technologies are hampered by policy barriers. Therefore, more studies are warranted to overcome these regulatory deadlocks [136].
8. Conclusions
The co-circulation or co-infection of ZIKV with other viruses leads to complicated consequences that might increase the host sensitivity to ZIKV infection or other infections. In this review, we showed that the co-circulation of ZIKV, DENV and CHIKV via Aedes Aegypti and Aedes Albopictus leads to creating significant difficulties in treating, controlling and diagnosing these arboviruses infections. ZIKV and DENV possess high homology proteins that affect the immune response to ZIKV infection due to antibody-dependent enhancement, while ZIKV and CHIKV have a potential neurotropism infectivity. These viruses showed high similarity in the infection symptoms, suggesting that the molecular-based techniques represent the optimal diagnostic method in addition to conventional culture and serological tests.
ZIKV infection could induce an additional risk for HIV-infected patients, especially during the pregnancy period. Future studies on ZIKV co-infection are necessary to: 1) elucidate the transmission, co-circulation and infection complications of ZIKV, DENV and CHIKV at the endemic areas of these viruses; 2) identify unique antigenic epitopes of DENV and ZIKV for designing and developing efficient ZIKV and DENV vaccines; and 3) clarify whether HIV infection increases the sensitivity to ZIKV infection and whether ZIKV infection could worsen HIV infection.
Supplementary Material
Highlights.
Rise of ZIKV co-infection with vector-borne viruses in overlapping endemic areas.
Studying control and prevention strategies for ZIKV, other flaviviruses and HIV.
Consideration of HIV/ZIKV transmission in vaccine development.
Effective control of ZIKV transmission using Wolbachia against Aedes mosquitos.
Development of ZIKV vaccines need to consider of co-infection with human pathogens.
Acknowledgments
We thank Robin Taylor and Lindsey Knight for critical reading of the manuscript.
Funding:
This work is supported in part by the National Institutes of Health [R01AI113883; R21AI114415; and R21MH113455] and Nebraska Neuroscience Alliance Endowed Fund Award to SNB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solomon T, Mallewa M. Dengue and other emerging flaviviruses. J Infect. 2001;42:104–15. doi: 10.1053/jinf.2001.0802. [DOI] [PubMed] [Google Scholar]
- 2.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387:719–21. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tappe D, Rissland J, Gabriel M, Emmerich P, Gunther S, Held G, et al. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.4.20685. [DOI] [PubMed] [Google Scholar]
- 4.Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, et al. Imported zika virus infection from the cook islands into australia, 2014. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca K, Meatherall B, Zarra D, Drebot M, MacDonald J, Pabbaraju K, et al. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg. 2014;91:1035–8. doi: 10.4269/ajtmh.14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–45. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 7.Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97:269–73. doi: 10.1099/jgv.0.000381. [DOI] [PubMed] [Google Scholar]
- 8.Chotiwan N, Brewster CD, Magalhaes T, Weger-Lucarelli J, Duggal NK, Ruckert C, et al. Rapid and specific detection of Asian- and African-lineage Zika viruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–63. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 11.Hamel R, Liegeois F, Wichit S, Pompon J, Diop F, Talignani L, et al. Zika virus: epidemiology, clinical features and host-virus interactions. Microbes Infect. 2016;18:441–9. doi: 10.1016/j.micinf.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–9. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76:552–62. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 16.Monlun E, Zeller H, Le Guenno B, Traore-Lamizana M, Hervy JP, Adam F, et al. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull Soc Pathol Exot. 1993;86:21–8. [PubMed] [Google Scholar]
- 17.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregianini TS, Ranieri T, Favreto C, Nunes ZMA, Tumioto Giannini GL, Sanberg ND, et al. Emerging arboviruses in Rio Grande do Sul, Brazil: Chikungunya and Zika outbreaks, 2014–2016. Rev Med Virol. 2017 doi: 10.1002/rmv.1943. [DOI] [PubMed] [Google Scholar]
- 19.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 20.Ruckert C, Weger-Lucarelli J, Garcia-Luna SM, Young MC, Byas AD, Murrieta RA, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8:15412. doi: 10.1038/ncomms15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabral-Castro MJ, Cavalcanti MG, Peralta RHS, Peralta JM. Molecular and serological techniques to detect co-circulation of DENV, ZIKV and CHIKV in suspected dengue-like syndrome patients. J Clin Virol. 2016;82:108–11. doi: 10.1016/j.jcv.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Fernanda Estofolete C, Terzian AC, Parreira R, Esteves A, Hardman L, Greque GV, et al. Clinical and laboratory profile of Zika virus infection in dengue suspected patients: A case series. J Clin Virol. 2016;81:25–30. doi: 10.1016/j.jcv.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Morales AJ, Villamil-Gomez WE, Franco-Paredes C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med Infect Dis. 2016;14:177–9. doi: 10.1016/j.tmaid.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Colombo TE, Estofolete CF, Reis AFN, da Silva NS, Aguiar ML, Cabrera EMS, et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol. 2017;96:20–5. doi: 10.1016/j.jcv.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Chan K, Weaver SC, Wong PY, Lie S, Wang E, Guerbois M, et al. Rapid, Affordable and Portable Medium-Throughput Molecular Device for Zika Virus. Sci Rep. 2016;6:38223. doi: 10.1038/srep38223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pabbaraju K, Wong S, Gill K, Fonseca K, Tipples GA, Tellier R. Simultaneous detection of Zika, Chikungunya and Dengue viruses by a multiplex real-time RT-PCR assay. J Clin Virol. 2016;83:66–71. doi: 10.1016/j.jcv.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Liu SQ, Li X, Deng CL, Yuan ZM, Zhang B. Development and evaluation of one-step multiplex real-time RT-PCR assay for simultaneous detection of Zika virus and Chikungunya virus. J Med Virol. 2017 doi: 10.1002/jmv.24970. [DOI] [PubMed] [Google Scholar]
- 28.Lee AJ, Bhattacharya R, Scheuermann RH, Pickett BE. Identification of diagnostic peptide regions that distinguish Zika virus from related mosquito-borne Flaviviruses. PLoS One. 2017;12:e0178199. doi: 10.1371/journal.pone.0178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oom AL, Smith D, Akrami K. Identification of putative unique immunogenic ZIKV and DENV1–4 peptides for diagnostic cellular based tests. Sci Rep. 2017;7:6218. doi: 10.1038/s41598-017-05980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christie C, Giaquinto C. Unravelling the Paediatric and Perinatal Zika Virus Epidemic through Population-based Research. West Indian Med J. 2016;65:239–42. doi: 10.7727/wimj.2016.454. [DOI] [PubMed] [Google Scholar]
- 31.Souares Y Pacific Public Health Surveillance N. Telehealth and outbreak prevention and control: the foundations and advances of the Pacific Public Health Surveillance Network. Pac Health Dialog. 2000;7:11–28. [PubMed] [Google Scholar]
- 32.Epidemic and emerging disease alerts in the Pacific region. 2017 [Google Scholar]
- 33.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–66. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, et al. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–80. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–15. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grifoni A, Pham J, Sidney J, O'Rourke PH, Paul S, Peters B, et al. Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol. 2017 doi: 10.1128/JVI.01469-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade DV, Harris E. Recent advances in understanding the adaptive immune response to Zika virus and the effect of previous flavivirus exposure. Virus Res. 2017 doi: 10.1016/j.virusres.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paquin-Proulx D, Leal FE, Terrassani Silveira CG, Maestri A, Brockmeyer C, Kitchen SM, et al. T-cell Responses in Individuals Infected with Zika Virus and in Those Vaccinated Against Dengue Virus. Pathog Immun. 2017;2:274–92. doi: 10.20411/pai.v2i2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, et al. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat Microbiol. 2017;2:17036. doi: 10.1038/nmicrobiol.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucchese G, Kanduc D. Zika virus and autoimmunity: From microcephaly to Guillain-Barre syndrome, and beyond. Autoimmun Rev. 2016;15:801–8. doi: 10.1016/j.autrev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Wiwanitkit V. Zika virus, autoimmunity and microcephaly: Other things for consideration. Autoimmun Rev. 2016;15:855. doi: 10.1016/j.autrev.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Ritter JM, Martines RB, Zaki SR. Zika Virus: Pathology From the Pandemic. Arch Pathol Lab Med. 2017;141:49–59. doi: 10.5858/arpa.2016-0397-SA. [DOI] [PubMed] [Google Scholar]
- 43.Halstead SB. Achieving safe, effective, and durable Zika virus vaccines: lessons from dengue. Lancet Infect Dis. 2017;17:e378–e82. doi: 10.1016/S1473-3099(17)30362-6. [DOI] [PubMed] [Google Scholar]
- 44.Haslwanter D, Blaas D, Heinz FX, Stiasny K. A novel mechanism of antibody-mediated enhancement of flavivirus infection. PLoS Pathog. 2017;13:e1006643. doi: 10.1371/journal.ppat.1006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Londono-Renteria B, Troupin A, Cardenas JC, Hall A, Perez OG, Cardenas L, et al. A relevant in vitro human model for the study of Zika virus antibody-dependent enhancement. J Gen Virol. 2017;98:1702–12. doi: 10.1099/jgv.0.000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George J, Valiant WG, Mattapallil MJ, Walker M, Huang YS, Vanlandingham DL, et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep. 2017;7:10498. doi: 10.1038/s41598-017-10901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagunas-Rangel FA, Viveros-Sandoval ME, Reyes-Sandoval A. Current trends in Zika vaccine development. J Virus Erad. 2017;3:124–7. doi: 10.1016/S2055-6640(20)30330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–8. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 49.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–9. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 50.Slon Campos JL, Poggianella M, Marchese S, Mossenta M, Rana J, Arnoldi F, et al. DNA-immunisation with dengue virus E protein domains I/II, but not domain III, enhances Zika, West Nile and Yellow Fever virus infection. PLoS One. 2017;12:e0181734. doi: 10.1371/journal.pone.0181734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang M, Dent M, Lai H, Sun H, Chen Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine. 2017;35:4287–94. doi: 10.1016/j.vaccine.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Li S, Du L, Wang C, Zou P, Hong B, et al. Neutralization of Zika virus by germline-like human monoclonal antibodies targeting cryptic epitopes on envelope domain III. Emerg Microbes Infect. 2017;6:e89. doi: 10.1038/emi.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinsky A, et al. Corrigendum: A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16:785. doi: 10.1038/ni0715-785a. [DOI] [PubMed] [Google Scholar]
- 54.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol. 2017;18:1261–9. doi: 10.1038/ni.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metz SW, Gallichotte EN, Brackbill A, Premkumar L, Miley MJ, Baric R, et al. In Vitro Assembly and Stabilization of Dengue and Zika Virus Envelope Protein Homo-Dimers. Sci Rep. 2017;7:4524. doi: 10.1038/s41598-017-04767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shragai T, Tesla B, Murdock C, Harrington LC. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann N Y Acad Sci. 2017;1399:61–77. doi: 10.1111/nyas.13306. [DOI] [PubMed] [Google Scholar]
- 58.Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11:e0005568. doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riou J, Poletto C, Boelle PY. A comparative analysis of Chikungunya and Zika transmission. Epidemics. 2017;19:43–52. doi: 10.1016/j.epidem.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, et al. Neurological complications of Chikungunya virus infection. Neurol India. 2009;57:177–80. doi: 10.4103/0028-3886.51289. [DOI] [PubMed] [Google Scholar]
- 61.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Araujo LM, Ferreira ML, Nascimento OJ. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. 2016;74:253–5. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- 63.Styczynski AR, Malta J, Krow-Lucal ER, Percio J, Nobrega ME, Vargas A, et al. Increased rates of Guillain-Barre syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trop Dis. 2017;11:e0005869. doi: 10.1371/journal.pntd.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doughty CT, Yawetz S, Lyons J. Emerging Causes of Arbovirus Encephalitis in North America: Powassan, Chikungunya, and Zika Viruses. Curr Neurol Neurosci Rep. 2017;17:12. doi: 10.1007/s11910-017-0724-3. [DOI] [PubMed] [Google Scholar]
- 65.Wielanek AC, Monredon JD, Amrani ME, Roger JC, Serveaux JP. Guillain-Barre syndrome complicating a Chikungunya virus infection. Neurology. 2007;69:2105–7. doi: 10.1212/01.wnl.0000277267.07220.88. [DOI] [PubMed] [Google Scholar]
- 66.Lebrun G, Chadda K, Reboux AH, Martinet O, Gauzere BA. Guillain-Barre syndrome after chikungunya infection. Emerg Infect Dis. 2009;15:495–6. doi: 10.3201/eid1503.071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villamil-Gomez W, Silvera LA, Paez-Castellanos J, Rodriguez-Morales AJ. Guillain-Barre syndrome after Chikungunya infection: A case in Colombia. Enferm Infecc Microbiol Clin. 2016;34:140–1. doi: 10.1016/j.eimc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Anaya JM, Rodriguez Y, Monsalve DM, Vega D, Ojeda E, Gonzalez-Bravo D, et al. A comprehensive analysis and immunobiology of autoimmune neurological syndromes during the Zika virus outbreak in Cucuta, Colombia. J Autoimmun. 2017;77:123–38. doi: 10.1016/j.jaut.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Goertz GP, Vogels CBF, Geertsema C, Koenraadt CJM, Pijlman GP. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl Trop Dis. 2017;11:e0005654. doi: 10.1371/journal.pntd.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villamil-Gomez WE, Gonzalez-Camargo O, Rodriguez-Ayubi J, Zapata-Serpa D, Rodriguez-Morales AJ. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016;9:684–6. doi: 10.1016/j.jiph.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Zambrano H, Waggoner JJ, Almeida C, Rivera L, Benjamin JQ, Pinsky BA. Zika Virus and Chikungunya Virus CoInfections: A Series of Three Cases from a Single Center in Ecuador. Am J Trop Med Hyg. 2016;95:894–6. doi: 10.4269/ajtmh.16-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherabuddi K, Iovine NM, Shah K, White SK, Paisie T, Salemi M, et al. Zika and Chikungunya virus co-infection in a traveller returning from Colombia, 2016: virus isolation and genetic analysis. JMM Case Rep. 2016;3:e005072. doi: 10.1099/jmmcr.0.005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brito CAA, Azevedo F, Cordeiro MT, Marques ETA, Jr, Franca RFO. Central and peripheral nervous system involvement caused by Zika and chikungunya coinfection. PLoS Negl Trop Dis. 2017;11:e0005583. doi: 10.1371/journal.pntd.0005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oehler E, Fournier E, Leparc-Goffart I, Larre P, Cubizolle S, Sookhareea C, et al. Increase in cases of Guillain-Barre syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveill. 2015;20:30079. doi: 10.2807/1560-7917.ES.2015.20.48.30079. [DOI] [PubMed] [Google Scholar]
- 75.Balavoine S, Pircher M, Hoen B, Herrmann-Storck C, Najioullah F, Madeux B, et al. Guillain-Barre Syndrome and Chikungunya: Description of All Cases Diagnosed during the 2014 Outbreak in the French West Indies. Am J Trop Med Hyg. 2017;97:356–60. doi: 10.4269/ajtmh.15-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puccioni-Sohler M, Roveroni N, Rosadas C, Ferry F, Peralta JM, Tanuri A. Dengue infection in the nervous system: lessons learned for Zika and Chikungunya. Arq Neuropsiquiatr. 2017;75:123–6. doi: 10.1590/0004-282X20160189. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal A, Vibha D, Srivastava AK, Shukla G, Prasad K. Guillain-Barre syndrome complicating chikungunya virus infection. J Neurovirol. 2017;23:504–7. doi: 10.1007/s13365-017-0516-1. [DOI] [PubMed] [Google Scholar]
- 78.Calvet GA, Filippis AM, Mendonca MC, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74:1–3. doi: 10.1016/j.jcv.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Joao EC, Gouvea MI, Teixeira ML, Mendes-Silva W, Esteves JS, Santos EM, et al. Zika Virus Infection Associated With Congenital Birth Defects in a HIV-infected Pregnant Woman. Pediatr Infect Dis J. 2017;36:500–1. doi: 10.1097/INF.0000000000001482. [DOI] [PubMed] [Google Scholar]
- 80.de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Sobral da Silva P, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901. doi: 10.1136/bmj.i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–34. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adibi JJ, Marques ET, Jr, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta. Lancet. 2016;387:1587–90. doi: 10.1016/S0140-6736(16)00650-4. [DOI] [PubMed] [Google Scholar]
- 83.Yuan S, Luo Q, Zhang ZW, Li ZL. Commentary: Teratogenic effects of the Zika virus and the role of the placenta. Front Cell Infect Microbiol. 2017;7:62. doi: 10.3389/fcimb.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch Pathol Lab Med. 2017;141:43–8. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz DA. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch Gynecol Obstet. 2017;295:1361–8. doi: 10.1007/s00404-017-4361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111:287–93. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aldo P, You Y, Szigeti K, Horvath TL, Lindenbach B, Mor G. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am J Reprod Immunol. 2016;76:348–57. doi: 10.1111/aji.12578. [DOI] [PubMed] [Google Scholar]
- 89.Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, et al. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol. 2017;2:1558–70. doi: 10.1038/s41564-017-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.National Institutes of Health (NIH) [Google Scholar]
- 91.Collins MH, Metz SW. Progress and Works in Progress: Update on Flavivirus Vaccine Development. Clin Ther. 2017;39:1519–36. doi: 10.1016/j.clinthera.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–32. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114–25. e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–8. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017;23:763–7. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shan C, Muruato AE, Jagger BW, Richner J, Nunes BTD, Medeiros DBA, et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat Commun. 2017;8:676. doi: 10.1038/s41467-017-00737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie X, Yang Y, Muruato AE, Zou J, Shan C, Nunes BT, et al. Understanding Zika Virus Stability and Developing a Chimeric Vaccine through Functional Analysis. MBio. 2017;8 doi: 10.1128/mBio.02134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim E, Erdos G, Huang S, Kenniston T, Falo LD, Jr, Gambotto A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–20. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, et al. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 2017;7:252. doi: 10.1038/s41598-017-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell. 2017;170:273–83. e12. doi: 10.1016/j.cell.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gotuzzo E, Yactayo S, Cordova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89:434–44. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608–14. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 103.Simmons CP. A Candidate Dengue Vaccine Walks a Tightrope. N Engl J Med. 2015;373:1263–4. doi: 10.1056/NEJMe1509442. [DOI] [PubMed] [Google Scholar]
- 104.Thomas SJ, L'Azou M, Barrett AD, Jackson NA. Fast-Track Zika Vaccine Development - Is It Possible? N Engl J Med. 2016;375:1212–6. doi: 10.1056/NEJMp1609300. [DOI] [PubMed] [Google Scholar]
- 105.Castanha PMS, Nascimento EJM, Braga C, Cordeiro MT, de Carvalho OV, de Mendonca LR, et al. Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J Infect Dis. 2017;215:781–5. doi: 10.1093/infdis/jiw638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hueston L, Ramirez R, Mahalingam S. Enhancement of Zika Infection by Dengue Virus-Specific Antibody Is Associated With Low Levels of Antiviral Factors. J Infect Dis. 2017;216:612–4. doi: 10.1093/infdis/jix344. [DOI] [PubMed] [Google Scholar]
- 107.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–8. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henein S, Swanstrom J, Byers AM, Moser JM, Shaik SF, Bonaparte M, et al. Dissecting Antibodies Induced by a Chimeric Yellow Fever-Dengue, Live-Attenuated, Tetravalent Dengue Vaccine (CYD-TDV) in Naive and Dengue-Exposed Individuals. J Infect Dis. 2017;215:351–8. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–6. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 110.Karp CL, Neva FA. Tropical infectious diseases in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;28:947–63. doi: 10.1086/514745. quiz 64–5. [DOI] [PubMed] [Google Scholar]
- 111.Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis. 2007;45:1214–20. doi: 10.1086/522180. [DOI] [PubMed] [Google Scholar]
- 112.Pang J, Thein TL, Lye DC, Leo YS. Differential clinical outcome of dengue infection among patients with and without HIV infection: a matched case-control study. Am J Trop Med Hyg. 2015;92:1156–62. doi: 10.4269/ajtmh.15-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Torrentes-Carvalho A, Hottz ED, Marinho CF, da Silva JB, Pinto LM, Fialho LG, et al. Characterization of clinical and immunological features in patients coinfected with dengue virus and HIV. Clin Immunol. 2016;164:95–105. doi: 10.1016/j.clim.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 114.World Health Organization (WHO) [Google Scholar]
- 115.Benelli G, Jeffries CL, Walker T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects. 2016;7 doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duchemin JB, Mee PT, Lynch SE, Vedururu R, Trinidad L, Paradkar P. Zika vector transmission risk in temperate Australia: a vector competence study. Virol J. 2017;14:108. doi: 10.1186/s12985-017-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19:771–4. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caragata EP, Dutra HL, O'Neill SL, Moreira LA. Zika control through the bacterium Wolbachia pipientis. Future Microbiol. 2016;11:1499–502. doi: 10.2217/fmb-2016-0177. [DOI] [PubMed] [Google Scholar]
- 120.Tan CH, Wong PJ, Li MI, Yang H, Ng LC, O'Neill SL. wMel limits zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl Trop Dis. 2017;11:e0005496. doi: 10.1371/journal.pntd.0005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dutra HL, Caragata EP, Moreira LA. The re-emerging arboviral threat: Hidden enemies: The emergence of obscure arboviral diseases, and the potential use of Wolbachia in their control. Bioessays. 2017;39 doi: 10.1002/bies.201600175. [DOI] [PubMed] [Google Scholar]
- 122.Brelsfoard CL, Dobson SL. Wolbachia effects on host fitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis (Diptera: Culicidae) J Med Entomol. 2011;48:1008–15. doi: 10.1603/me10202. [DOI] [PubMed] [Google Scholar]
- 123.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–3. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 124.Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A. 2012;109:255–60. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang G, Etebari K, Asgari S. Wolbachia suppresses cell fusing agent virus in mosquito cells. J Gen Virol. 2016;97:3427–32. doi: 10.1099/jgv.0.000653. [DOI] [PubMed] [Google Scholar]
- 126.Schultz MJ, Isern S, Michael SF, Corley RB, Connor JH, Frydman HM. Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures. J Virol. 2017;91 doi: 10.1128/JVI.00339-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caragata EP, Dutra HL, Moreira LA. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb Cell. 2016;3:293–5. doi: 10.15698/mic2016.07.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goodwin CM, Xu S, Munger J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015;23:789–98. doi: 10.1016/j.tim.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fontaine KA, Sanchez EL, Camarda R, Lagunoff M. Dengue virus induces and requires glycolysis for optimal replication. J Virol. 2015;89:2358–66. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saucereau Y, Valiente Moro C, Dieryckx C, Dupuy JW, Tran FH, Girard V, et al. Comprehensive proteome profiling in Aedes albopictus to decipher Wolbachia-arbovirus interference phenomenon. BMC Genomics. 2017;18:635. doi: 10.1186/s12864-017-3985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Molloy JC, Sommer U, Viant MR, Sinkins SP. Wolbachia Modulates Lipid Metabolism in Aedes albopictus Mosquito Cells. Appl Environ Microbiol. 2016;82:3109–20. doi: 10.1128/AEM.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Oliveira S, Villela DAM, Dias FBS, Moreira LA, Maciel de Freitas R. How does competition among wild type mosquitoes influence the performance of Aedes aegypti and dissemination of Wolbachia pipientis? PLoS Negl Trop Dis. 2017;11:e0005947. doi: 10.1371/journal.pntd.0005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ulrich JN, Beier JC, Devine GJ, Hugo LE. Heat Sensitivity of wMel Wolbachia during Aedes aegypti Development. PLoS Negl Trop Dis. 2016;10:e0004873. doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ross PA, Axford JK, Richardson KM, Endersby-Harshman NM, Hoffmann AA. Maintaining Aedes aegypti Mosquitoes Infected with Wolbachia. J Vis Exp. 2017 doi: 10.3791/56124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ritchie SA, Johnson BJ. Advances in Vector Control Science: Rear-and-Release Strategies Show Promise… but Don't Forget the Basics. J Infect Dis. 2017;215:S103–S8. doi: 10.1093/infdis/jiw575. [DOI] [PubMed] [Google Scholar]
- 136.Dickens BL, Yang J, Cook AR, Carrasco LR. Time to Empower Release of Insects Carrying a Dominant Lethal and Wolbachia Against Zika. Open Forum Infect Dis. 2016;3:ofw103. doi: 10.1093/ofid/ofw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.