Abstract
Aims
To document the prevalence of current depressive symptoms and history of depression across the glycaemic spectrum in older adults, and examine if measures of health status and healthcare satisfaction, access and utilization explain differences in the prevalence of current depressive symptoms by diabetes status
Methods
We conducted a cross-sectional study of 6226 participants aged 67–90 years who attended the 2011–2013 visit of the Atherosclerosis Risk in Communities (ARIC) study. Diabetes was based on self-report, medication use and HbA1c. Current depressive symptoms were defined using the Center for Epidemiologic Studies Depression 11-item questionnaire, and history of depression was assessed via self-report. We examined obesity, history of cardiovascular disease, hypertension, kidney disease, cognitive function, and self-reported health compared with others. Prevalence and prevalence ratios were estimated using age-, race-, and sex-adjusted Poisson regression.
Results
The prevalence of current depressive symptoms was 5.4% in people without diabetes and 11.0% in people with diabetes (prevalence ratio 2.04, 95% CI 1.60, 2.48); the prevalence of history of depression was 11% in people without diabetes and 17.7% in people with diabetes (prevalence ratio 1.61, 95% CI 1.28,1.95). Strong correlates of current depressive symptoms were history of depression (prevalence ratio 3.86, 95% CI 3.05, 4.90) and reporting poor health compared with others (prevalence ratio 3.88, 95% CI 2.93, 5.15). No variables had significantly different associations with depressive symptoms across glycaemic categories (P for interaction >0.10).
Conclusions
In older adults, current depressive symptoms were twice as prevalent in people with diabetes compared with those without. Measures of health status and healthcare did not explain differences in depressive symptoms between people with and without diabetes.
Introduction
Diabetes in older adults is associated with reduced cognitive and functional status, higher risk of institutionalization, and higher risk of mortality [1]. A number of studies have also documented the higher prevalence and risk of depression among older individuals with diabetes [2,3], but few have examined prevalence across the full glycaemic range in adults aged ≥70 years. The primary aim of the present study was to document the prevalence of current depressive symptoms and history of depression across the glycaemic spectrum in older adults, including people with prediabetes and undiagnosed diabetes. The secondary aim was to determine if measures of health status and healthcare satisfaction, access and utilization explained differences in the prevalence of current depressive symptoms across glycaemic groups.
Methods
Study participants
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based, prospective cohort of middle-aged adults recruited from four US communities beginning in 1987. Participants were recruited from the suburbs of: Minneapolis, MN; Washington County, MD; Forsyth County, NC; and Jackson, MI. In the present study, we conducted cross-sectional analyses of participants who attended the 2011–2013 visit. People who had missing HbA1c values (n=125) or missing data on covariates of interest (n=187) were excluded, resulting in an analytical sample size of 6226. The institutional review boards from each study site approved the study; all participants gave written informed consent.
Depressive symptoms definition
We defined current depressive symptoms as a score ≥9 on the Center for Epidemiologic Studies Depression (CES-D) 11-item questionnaire. The CES-D is a frequently used and well-validated measure of depressive symptoms [4]. History of depression was available in the subset of participants who were invited for additional in-person assessment (n=2890). Briefly, selection for additional assessment was conducted as part of the ARIC Neurocognitive Study, based on cognitive function, prior magnetic resonance imaging examination, as well as by random sampling [5].
Diabetes definition
Diagnosed diabetes was defined using self-reported diagnosis or glucose-lowering medication use. Among people without a diagnosis of diabetes, we used HbA1c to group participants into three categories: HbA1c <39 mmol/mol (5.7%; no diabetes), HbA1c 39–46 mmol/mol (5.7–6.4%; prediabetes) and HbA1c 48 mmol/mol (≥6.5%; undiagnosed diabetes). Among participants with diagnosed diabetes, we created two groups by dichotomizing HbA1c as <53 mmol/mol (7%) and ≥53 mmol/mol. The American Diabetes Association guidelines define 7.5% as a ‘reasonable HbA1c goal’ among older adults with intact cognitive and physical function [6]. Results were not appreciably different when using 7% (53 mmol/mol), the generalized ‘reasonable HbA1c goal’ for adults.
Measures of health status
Health compared with others was collected via self-report; participants categorized their health compared with others as poor, fair, good or excellent. We compared the poor and fair categories with the combined good/excellent category. BMI was calculated using measured height (m) and weight (kg) as weight divided by height squared; obesity was defined as a BMI ≥30 kg/m2. History of cardiovascular disease was based on adjudicated cardiovascular events (myocardial infarction, stroke, heart failure) and ARIC study visit ECG-identified myocardial infarction. Hypertension was defined as measured systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of blood pressure-lowering medication. Estimated GFR (eGFR) was calculated based on the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [7]. Cognitive function was classified as no cognitive impairment, mild cognitive impairment, or dementia based on expert committee review [5].
Measures of healthcare
Healthcare satisfaction, access and utilization was assessed via self-report. Participants answered questions on difficulty obtaining an appointment at short notice (within 1–2 days, categorized yes/no), how satisfied they were with the quality of care received (categorized as satisfied yes/no), and if they had delayed getting needed care in the past 12 months (yes/no).
Statistical analysis
We estimated prevalence and prevalence ratios (PRs) using Poisson regression, adjusted for age, race and sex, and tested for differences in correlates across diabetes groups using a chi-squared joint test for interaction. Model diagnostics of model fit (Hosmer–Lemeshow goodness-of-fit test), model specification (link test), deviance residuals and leverage indicated this model fit the data well. Using splines (with three to six knots) to model age did not appreciably alter results; the association between depressive symptoms and age was roughly linear (Fig. S1). As a sensitivity analysis, we also fit a fully adjusted model including all covariates of interest. We did not report correlates for people with undiagnosed diabetes because of the small sample size (n=99). Statistical analysis was performed using STATA/SE 14.2 (StataCorp, College Station, TX, USA). P values < 0.05 were taken to indicate statistical significance.
Results
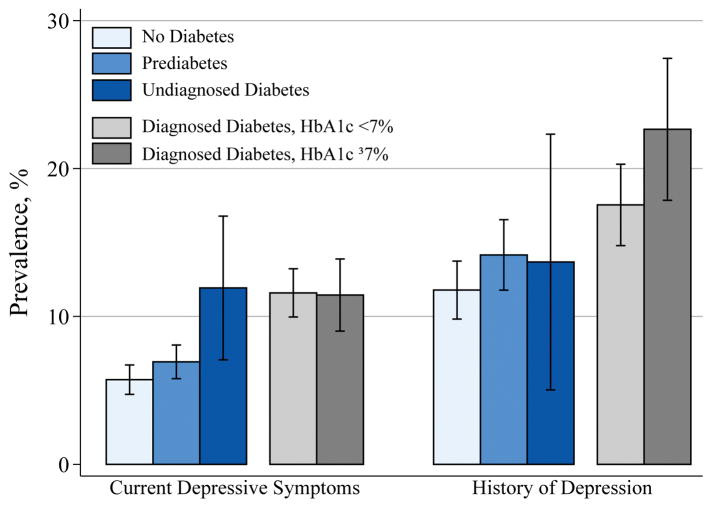
The mean (range;SD) age of the participants was 76 (67–90; 5.3) years, 59% were female, and 23% were black. Participants with diagnosed diabetes vs those without diabetes had a higher prevalence of both current depressive symptoms (11.0% vs 5.4%; PR 2.04, 95% CI 1.60, 2.48) and history of depression (17.7% vs 11.0%; PR 1.61, 95% CI 1.28,1.95). The prevalence of current depressive symptoms in participants with undiagnosed diabetes was 11%, similar to participants with diabetes; the prevalence of history of a depression diagnosis in participants with undiagnosed diabetes was 12%, similar to participants without diabetes (Fig. 1). Participants with prediabetes vs those without diabetes had a marginally higher prevalence of current depressive symptoms (6.6% vs 5.4%; PR 1.22, P =0.106) and of history of depression (13.1% vs 11.0%; PR 1.20, P=0.130)
FIGURE 1.
Prevalence of current depressive symptoms and history of depression, by diabetes status. Current depressive symptoms was defined as a Center for Epidemiologic Studies Depression questionnaire score ≥9. History of depression: self-reported having ever received a diagnosis of depression. Prediabetes was defined as no self-reported diagnosis, no medication use, and an HbA1c of 39–46 mmol/mol. Undiagnosed diabetes was defined as no self-reported diagnosis and an HbA1c of ≥48 mmol/mol. Diagnosed diabetes was defined as a self-reported diagnosis or medication use. Prevalence estimates are from Poisson models adjusted for age, race, and sex.
Strong correlates of current depressive symptoms were: history of depression (PR 3.86); reporting poor (PR 3.88) or fair (PR 2.36) health compared with good or excellent health; dementia (PR 2.72); and reporting not being satisfied with care (PR 2.62) or delaying getting care (PR 2.28). Indicators of current health status had more moderate associations with current depressive symptoms: obesity (PR 1.44), history of cardiovascular disease (PR 1.39), kidney disease (PR 1.39), and hypertension (PR 1.20; Table 1). Associations were similar across diabetes groups; we found no significant difference in associations across diabetes categories (P for interaction >0.10). Results from the fully adjusted model yielded similar associations between correlates and depressive symptoms; glycaemia status was still significantly associated with current depressive symptoms (Table S1).
Table 1.
Prevalence ratios for current depressive symptoms by diabetes status
| PR (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall N=6226 |
Normoglycaemic (HbA1c <39 mmol/mol) N =2241 |
Prediabetes (HbA1c 39–46 mmol/mol) N =1883 |
Diabetes (HbA1c <7.5%) N =1748 |
Diabetes (HbA1c ≥7.5%) N =354 |
P for interaction | |
|
|
||||||
| History of depression | 3.86 (3.05,4.90)* | 4.63 (2.88,7.45)* | 4.57 (2.77,7.55)* | 2.71 (1.83,4.03)* | 3.55 (1.69,7.46)* | 0.210 |
|
| ||||||
| Health indicators | ||||||
| Health compared with others | ||||||
| Fair (reference: good/excellent) | 2.36 (1.97,2.82)* | 2.73 (1.84,4.05)* | 2.63 (1.85,3.75)* | 1.78 (1.35,2.36)* | 1.82 (1.03,3.20)* | 0.171 |
| Poor (reference: good/excellent) | 3.88 (2.93,5.15)* | 2.76 (1.21,6.32)* | 2.18 (0.89,5.35) | 3.88 (2.62,5.73)* | 4.16 (2.38,7.27)* | |
| BMI ≥30 kg/m2 (reference: <30 kg/m2) | 1.44 (1.22,1.71)* | 1.11 (0.74,1.66) | 1.51 (1.07,2.14)* | 1.22 (0.93,1.59) | 1.21 (0.73,2.02) | 0.793 |
| History of CVD (reference: no) | 1.39 (1.15,1.66)* | 1.13 (0.73,1.76) | 1.26 (0.85,1.85) | 1.33 (1.01,1.74)* | 0.99 (0.57,1.73) | 0.902 |
| Hypertension (reference: no) | 1.20 (0.97,1.49) | 1.10 (0.74,1.64) | 0.98 (0.66,1.46) | 0.91 (0.63,1.32) | 2.28 (0.58,8.98) | 0.486 |
| eGFR <60 mL/min/1.73 m2 (reference: ≥60 mL/min/1.73 m2) | 1.39 (1.17,1.65)* | 1.65 (1.16,2.35)* | 1.60 (1.13,2.26)* | 1.13 (0.86,1.49) | 0.72 (0.39,1.36) | 0.124 |
|
| ||||||
| Cognition | ||||||
| Mild cognitive impairment (reference: cognitively normal) | 1.64 (1.37,1.95)* | 1.62 (1.11,2.36)* | 1.73 (1.22,2.44)* | 1.47 (1.12,1.94)* | 1.63 (0.96,2.76) | 0.697 |
| Dementia (reference: cognitively normal) | 2.72 (2.10,3.51)* | 3.56 (2.07,6.14)* | 1.85 (0.98,3.48) | 2.74 (1.83,4.09)* | 2.53 (1.24,5.18)* | |
|
| ||||||
| Healthcare variables | ||||||
| Not satisfied with care (reference: yes) | 2.62 (1.94,3.54)* | 3.78 (2.12,6.73)* | 2.39 (1.35,4.22)* | 2.23 (1.32,3.77)* | 2.99 (1.35,6.65)* | 0.598 |
| Delayed getting care (reference: no) | 2.28 (1.72,3.01)* | 1.97 (0.96,4.03) | 1.92 (1.05,3.50)* | 1.98 (1.26,3.09)* | 3.61 (2.05,6.38)* | 0.169 |
| Trouble getting an appointment at short notice (reference: no) | 1.53 (1.26,1.87)* | 1.49 (0.98,2.26) | 1.56 (1.04,2.33)* | 1.59 (1.16,2.18)* | 1.50 (0.82,2.73) | 0.958 |
CVD, cardiovascular disease; eGFR, estimated GFR; PR, prevalence ratio.
PRs are estimated from Poisson models adjusted for age, race, and sex. Prediabetes was defined as no self-reported diagnosis, no medication use, and an HbA1c of 39–46 mmol/mol. Diagnosed diabetes was defined as a self-reported diagnosis or medication use. People with undiagnosed diabetes are not included in this Table.
P value for interaction is from a chi-squared joint test, testing that all coefficients across diabetes groups are equal.
P <0.05.
Discussion
The association between diabetes and depression has been well documented [8,10,11], but fewer studies have examined associations in older adults and across the glycaemic spectrum. Our results indicate higher prevalence of current depressive symptoms in older adults with diabetes, regardless of glycaemia status, and potentially higher prevalence in people with prediabetes compared with those without diabetes. These differences were not explained by measures of health status or access to care, which may imply that the complications of diabetes, and the difficulty associated with managing a complex disease through access to care, are not primary drivers of the observed differences in this population of highly insured older adults. This is also supported by the similar findings in people with undiagnosed diabetes.
The high prevalence of current depressive symptoms and history of depression in people with diagnosed diabetes in this community-based older adult population was similar to that observed in previous studies [8,9]. History of a depression diagnosis was one of the strongest correlates of recent depressive symptoms, adding support to the American Diabetes Association guidelines for annual screening for depression in older adults, especially those with a history of depression. Objective measures of health status (obesity, history of cardiovascular disease, kidney disease, or hypertension) showed more moderate correlations. We did not observe differences in health access variables or health status that explained the differences in the prevalence of depressive symptoms by diabetes status in this population of older adults. The lack of differences across diabetes categories may be attributable in part to the high level of healthcare coverage: 98% of participants were enrolled in Medicare and 87% of participants had additional healthcare coverage.
There is scant evidence as to whether depressive symptoms in people with prediabetes in their seventh decade of life are associated with a higher risk of progression to diabetes. One study of Canadian adults (mean age ~53 years) found that people with prediabetes were nearly 3 times more likely to progress to a diabetes diagnosis if they also had depressive symptoms [13]. Another study of older English adults (mean age ~66 years) found a 1.5–2 times higher risk of progression to diabetes among people with prediabetes and mild or high depressive symptoms compared with people with prediabetes and no depressive symptoms [14]. It is unclear whether depressive symptoms in older adults with prediabetes and diabetes are associated with subsequent progression to diabetes or worsening glycaemic control and adverse cognitive, functional and cardiovascular outcomes, respectively. Prospective studies examining these questions in older adults are needed.
The present study has several limitations. First, its cross-sectional design limited our ability to determine the temporal ordering between the correlates and depressive symptoms. Second, we used dichotomized measures in some instances, which may not capture the granularity in healthcare satisfaction, access and utilization. Strengths of the study include the large, community-based population of older adults, the use of HbA1c to classify glycaemia status, and the ability to examine numerous correlates of depressive symptoms.
In conclusion, we observed a high prevalence of current depressive symptoms and history of depression among older, well-insured persons with diabetes, which were not explained by health status and healthcare access. Additional studies are needed to identify the correlates of depressive symptoms in this population that can lead to future primary and secondary preventive interventions.
Supplementary Material
Figure S1 Association between age and probability of depressive symptoms.
Table S1 Prevalence ratios (95% CI) for current depressive symptoms by diabetes status: fully adjusted model.
What’s new?
The association between diabetes and depression has been well documented, but few studies have examined associations in adults aged >70 years, across the glycaemic spectrum, and explored health status and healthcare variables that might explain these differences.
We estimated the prevalence of current depressive symptoms and history of depression across the glycaemic spectrum.
Current depressive symptoms were twice as prevalent in people with diabetes than in those without. Measures of health status and healthcare did not explain differences in depressive symptoms according to glycaemia status.
Acknowledgments
Funding sources
The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts: HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. A.M.R. was supported by NIH/NHLBI grant T32HL007024. This research was supported by NIH/NIDDK grant R01DK089174. E.S. was also supported by K24DK106414. B.G.W. was supported by NIH/NIA and NHLBI grants. The funding source was not involved in the data collection, analysis, interpretation of findings, nor the preparation, review, approval, and submission of this short communication for publication.
The authors thank the staff and participants of the ARIC Study for their important contributions.
Footnotes
Competing interests
B.G.W. has previously received reimbursements from ACADIA Pharmaceuticals not related to the focus of this manuscript.
Additional Supporting Information may be found in the online version of this article:
References
- 1.Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 3.Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 5.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimer’s Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of Medical Care in Diabetes–2017. Diabetes Care. 2017;40(Suppl 1):S1–S135. [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy T, Lloyd CE. Epidemiology of depression and diabetes: A systematic review. J Affect Disord. 2012;142:S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 10.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale JR, Williams SM, Bowyer V. What is the effect of peer support on diabetes outcomes in adults? A systematic review. Diabet Med. 2012;29:1361–1377. doi: 10.1111/j.1464-5491.2012.03749.x. [DOI] [PubMed] [Google Scholar]
- 13.Deschênes SS, Burns RJ, Graham E, Schmitz N. Prediabetes, depressive and anxiety symptoms, and risk of type 2 diabetes: A community-based cohort study. J Psychosom Res. 2016;89:85–90. doi: 10.1016/j.jpsychores.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Graham E, Au B, Schmitz N. Depressive symptoms, prediabetes, and incident diabetes in older English adults. Int J Geriatr Psychiatry. 2017;32:1450–1458. doi: 10.1002/gps.4634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Association between age and probability of depressive symptoms.
Table S1 Prevalence ratios (95% CI) for current depressive symptoms by diabetes status: fully adjusted model.