Abstract
Computers are increasingly used to improve prescribing decisions in the management of long-term conditions however the effects on asthma prescribing remain unclear. We aimed to synthesise the evidence for the use of computerised alerts that identify excessive prescribing of short-acting beta2-agonists (SABAs) to improve asthma management for people with asthma. MEDLINE, CINAHL, Embase, Cochrane and Scopus databases (1990–2016) were searched for randomised controlled trials using electronic alerts to identify excessive prescribing of SABAs for people with asthma in primary care. Inclusion eligibility, quality appraisal (Cochrane risk of bias tool) and data extraction were performed by two independent reviewers. Findings were synthesised narratively. A total of 2035 articles were screened and four trials were eligible. Three studies had low risk of bias: one reported a positive effect on our primary outcome of interest, excessive SABA prescribing; another reported positive effects on the ratio of inhaled corticosteroid (ICS)-SABA prescribing, and asthma control; a third reported no effect on outcomes of interest. One study at high risk of bias reported a reduction in exacerbations and primary care consultations. There is some evidence that electronic alerts reduce excessive prescribing of SABAs, when delivered as part of a multicomponent intervention in an integrated health care system. However due to the variation in health care systems, intervention design and outcomes measured, further research is required to establish optimal design of alerting and intervening systems.
Introduction
Asthma affects an estimated 300 million individuals worldwide and almost 30 million people below 45 years of age in Europe.1 With a prevalence of 6% in 2016–20172 and an estimated 5.4 million people receiving treatment,3 asthma is the most common long-term condition in the United Kingdom (UK).4 In 2015-2016 there were approximately 1.4 million hospital admissions for asthma in England and Wales5 and whilst the number of asthma deaths has fallen by five percent from 2015 to 2016, this remains higher than the 15-year average.6
The National Review of Asthma Deaths (NRAD) identified that, of 195 deaths from asthma between 2012 and 2013, 39% of those who died were prescribed more than 12 short-acting beta2-agonist inhalers (SABAs) in the previous year, with 4% prescribed more than 50 SABAs in the same time period.7
Frequent use of SABAs is an internationally recognised marker of poor control8 and a potentially modifiable warning sign of impending serious asthma attacks9–12 and asthma death.13–17 Asthma control is defined as the extent to which the manifestations of asthma, commonly wheeze, shortness of breath, chest tightness, cough and variable expiratory airflow limitation, can be observed in the patient, or have been reduced or removed by treatment.18,19 Control can be assessed by current symptoms and future risk of adverse outcomes;8 patients with good asthma control have less need for SABAs and require no emergency visits.20 Following the National Review of Asthma Deaths, the electronic surveillance of prescription refill frequency was recommended to alert clinicians to people with asthma prescribed excessive quantities of SABAs.7
General practice computer systems increasingly use reminders and alerts for preventative care and disease management21,22 including asthma.23,24 Computer decision support systems (CDSSs), defined as ‘active knowledge systems which use two or more items of patient data to generate case-specific advice,’25 have the potential to influence prescribing behaviour. Efforts to automate reminder systems and improve efficiency in both prevention and chronic disease management have yielded some improvements when assessed using randomised trials.26 However, evaluations suggest CDSSs do not consistently improve prescribing behaviour and clinical outcomes27 and the role of electronic alerts to identify and reduce excessive SABA prescribing remains unclear. This review aims to provide a systematic overview of the extent to which electronic alerts in primary care computer systems can identify excessive prescribing of SABAs, and assess the impact of these interventions on SABA prescribing, asthma management and asthma control.
Results
Study selection
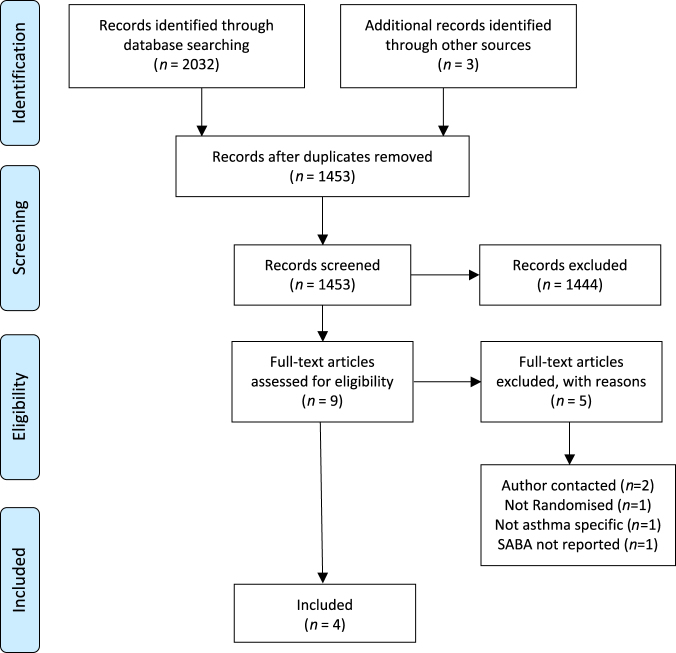
Fig. 1 details the systematic search and eligibility assessment. From 2035 titles, four studies were selected as eligible.28–31 No ongoing or unpublished trials were identified. Risk of bias is reported in Table 1.
Fig. 1.
PRISMA flow chart
Table 1.
Risk of bias
| Study | Selection bias | Allocation concealment bias | Performance bias | Detection bias | Attrition bias | Selective reporting | Other bias | Overall risk |
|---|---|---|---|---|---|---|---|---|
| McCowan et al.28 | No | No | No | Unclear: blinding of outcome assessors not detailed | Yes: attrition rate variation was not fully explained. No intention-to-treat analysis | Unclear: no protocol | No | C-High |
| Eccles et al.29 | No | No | No | No | No | No | No | A-Low |
| Zeiger et al.30 | No | No | No | No | No | No | No | A-Low |
| Tamblyn et al.31 | No | No | No | No | No | No | No | A-Low |
Study characteristics
RCTs were conducted between 2001 and 2015; two recent studies (published in 2014 and 2015) were carried out within integrated healthcare systems in the United States30 and Canada31 respectively, whilst two older studies were from the United Kingdom (published in 2001 and 2002).28,29 The features of interventions are summarised in Table 2. A detailed description of the interventions can be found in Supplementary Appendix 1. Methods of alerting included computerised prompts,28,29 an electronic message to physicians30 and a dashboard alert.31 Three of the four studies included people with asthma under-18 years of age, of which the lower age range for inclusion was 5 years of age,31 12 years of age30 and one not reported.28 No studies stratified findings by age range. The characteristics of studies are presented in Table 3.
Table 2.
Summary of intervention features
Table 3.
Characteristics of included studies
| Author (Country) | Study design | Participant and setting | Age (years) | Time scale | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|
| McCowan et al.28 (2001, UK) | Cluster RCT | 46 clusters: 46 practice, 447 patients | All; Avg yrs Inv: 32.6 Ctl: 37.4 | 6 Months, No baseline data | All ages, on asthma register | Not specified |
| Eccles et al.29 (2002, UK) | Cluster RCT with 2 × 2 incomplete block design | 62 clusters: 62 primary care practices; 5139 patients | > = 18 years | 24 Months; 12 monthsbaseline, 12 months intervention | General practices in north east of England; 50% of doctors using EMIS or AAH Meditel system to view clinical data/issue prescriptions during consultations | Single-handed practices |
| Zeiger et al.30 (2014, USA) | Randomised stratified block design | Managed care organisation; 1999 patients | 12-56 years; Avg yrs Inv: 36.2 Ctl: 36.1 | 20 Months; 8 months intervention; 12 months follow-up | 12– 56 years physician diagnosed asthma; (ICD code: 493) in previous 3 years, > = 7 SABAs dispensed, continuous health-plan membership and pharmacy benefit in the prior year, > = 1 ICS canister dispensed in prior 6 months | Excluded co-morbidities coded in the prior year; COPD, emphysema, CF, chronic bronchitis, bronchiectasis, Churg Strauss syndrome, Wegener granulomatosis, Sarcoidosis, pulmonary hypertension, steroid-dependant asthma. Omalizumab in prior 3 months, required an interpreter |
| Tamblyn et al.31 (2015, Canada) | Cluster RCT | Primary care practices. 81 physician clusters; 4447 patients | > 5 years (8.2% aged 5–18 years) | 33 Months | > 5 years, asthma diagnosis (ICD9 code: 493), insured through provincial drug plan | COPD diagnosis (ICD9: 491,492, 494,496) |
RCT randomised control trial, COPD chronic obstructive pulmonary disease, ICD international classification of diseases, SABAs short-acting beta2-agonists, ICS inhaled corticosteroid, CF cystic fibrosis, INV intervention, CTL control, Avg yrs average years of age
Primary outcome
A brief summary of findings is presented in Table 4. A detailed description of findings can be found in Table 5.
Table 4.
Summary of findings
| Study | Study-defined excessive SABA prescribing | SABA prescribing | ICS prescribing | ICS-SABA prescribing ratio | ICS-LABA prescribing | Asthma reviews | Asthma Exacerbations | Asthma Exacerbation requiring oral steroids | Unscheduled primary care consultations for asthma | Unscheduled secondary care consultations for asthma | Asthma control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| McCowan et al.28 | +/− | +/− | + | +/− | + | +/− | |||||
| Eccles et al.29 | +/− | +/− | +/− | +/− | |||||||
| Zeiger et al.30 | + | + | +/− | + | +/− | +/− | |||||
| Tamblyn et al.31 | + | + |
Key: + positive effect, +/− no effect
SABA short-acting beta2- agonist, ICS inhaled corticosteroid, LABA long-acting beta2-agonist, ED emergency department visit
Table 5.
Detailed description of findings
| Study | Risk of bias | CDSS use | Process outcomes of interest | Clinical outcomes of interest | Interpretation |
|---|---|---|---|---|---|
| McCowan et al.28 | High | Not reported | No between-group difference in number of patients prescribed maintenance therapy based on British asthma guidelines step; p = 0.51. No between-group difference in the number of patients attending practice initiated asthma reviews OR 0.69 (CI 0.21–2.21). |
Fewer exacerbations were reported in the intervention group; 12/147 (8%) in comparison to the control group 57/330 (17%); OR 0.43 (CI 0.21–0.85). Fewer patients were prescribed oral steroids for an exacerbation; 7/147 (5%) of the intervention group compared to 35/330 (11%) of the control group OR 0.42 (CI 0.14–1.29). Fewer primary care consultations were initiated by patients; 22% intervention group compared to 34% control group, OR 0.59 (0.37–0.95). No between-group difference in hospital admissions; OR 0 (CI 0–3.44); or emergency department visits; OR 0 (CI 0–9.16). |
Of the 46 practices registered to participate, 21 were randomised but only 5 completed the trial due to software problems. Patients treated with CDSS initiated less asthma consultations and were less likely to experience an exacerbation. However it was not clear how exacerbation was defined. The CDSS was not integrated and usage rate was not captured. |
| Eccles et al. 29 | Low | Median number of active interactions between groups was zero. | No between-group difference in numbers of SABA prescribed; OR 1.04 (CI 0.83–1.31).No between-group difference in numbers of ICS prescribed; OR 0.95 (CI 0.78–1.16). | No between-group difference in number of consultations for asthma OR 0.94 (CI 0.81–1.08). No between-group difference in number of patients prescribed oral steroids before and after OR 1.0 (CI 0.82–1.22). |
This cluster study design with practices as the unit of randomisation, consisted of two arms, asthma and angina each acting as control for the opposite arm e.g., CDSS care for angina acted as control data for asthma CDSS care. Data analysed 12 months before and after. A high number of practices participated (62); prescribing data was obtained from 1139 patients treated with the intervention and 1385 controls. Process of care data was obtained from 1200 patients treated with the intervention and 1163 controls. The intervention had no effect on process or clinical outcomes. Median intervention usage was zero. Data was analysed on an intention to treat basis. |
| Zeiger et al.30 | Low | Not reported |
aLess patients in the intervention group dispensed excessive SABA: 50.7% vs 57.1% control group; RR 0.89, p = 0.007 (CI 0.82-0.97) and increased time to be dispensed SABA excessively; HR 0.80; p = < 0.001 (CI 0.71–0.91). Greatest effects seen in those with no prior asthma specialist care. Reduction in SABA inhalers dispensed to intervention group at 3 months p = 0.002, 6 months p = < 0.001, 12 months p = < 0.001. Increase in ICS-LABA inhalers dispensed to intervention patients without prior asthma specialist care; 3 months p = 0.004, 6 months p = < 0.001, 12 months p = 0.03. |
No between group difference in number of patients with an exacerbation requiring oral steroids; p = 0.71, either with or without prior specialist asthma care; p = 0.38 vs. p = 0.83. No between group difference in number of patients with an asthma exacerbation requiring > = two oral steroid courses; p = 0.55, either with or without prior specialist asthma care; p = 0.89 vs. p = 0.50. No between group difference in number of asthma ED visits and/or hospitalisation; p = 0.96, either with or without prior specialist asthma care; p = 0.55 vs p = 0.66. |
Real-time outreach intervention in the Kaiser Permanente Southern California (KPSC) managed healthcare system. Usual care included KSPC extensive integrated asthma care management. The intervention reduced excessive SABA use, and ICS/LABA use. Greatest effect were seen in the subgroup of patients without prior asthma specialist care. Physician engagement was not captured as electronic message presented automatically and did not require physician action. Multicomponent intervention included a clinician message, patient letter and allergy referral. |
| Tamblyn et al.31 | Low | Physicians did not use the CDSS intervention ‘Asthma Decision Support’ in 60.5% of consultations for patients with out-of-control asthma | Increased ICS-SABAb mean ratio in the intervention group; mean difference = 0.27 p = 0.034 (CI 0.02–0.51); | Reduction in out of control asthma events in the intervention group rate difference −8.7/100 PY; p = 0.29 (CI −24.7, 7.3). Greatest effects were seen in the sub-group of patients with out-of-control asthma when beginning the study. Rate difference: −28.4, p = 0.04 (CI −55.6,−1.2); The greatest reduction was seen in the subgroup with out-of-control asthma at beginning of the study when treated with CDS alone had Rate difference: −36.9/100 PY; p = 0.01. |
81 physicians were randomised to ‘asthma decision support;’ 2273 patients treated with the intervention and 2174 controls. This intervention increased the mean ratio ICS-SABA use and reduced the rate of out-of-control asthma episodes. Greatest effect were seen in the subgroup of patients with out-of-control asthma at study entry as treated by CDS alone. In 60% of out-of-control visits the decision support was not accessed by clinicians. No data was available on use over time. |
CI 95% confidence intervals, SABA short acting beta2-agonist, ICS inhaled corticosteroid, LABA long acting beta2-agoinst, RCT randomised control trial, I intervention, C Control, OR Odds ratio, RR risk ratio, ED emergency department, PY patients per year, CDSS computer decision support system
aprimary outcome of interest
b Reported as fast-acting b-agonist (FABA)
Study-defined excessive SABA prescribing
Zeiger et al.30 reported a reduction in the number of patients being dispensed excessive SABAs (p = 0.007) and an increase in length of time between SABA prescriptions (p = < 0.001). These effects were noted in the subgroup of patients without prior asthma specialist care who received the intervention (p = < 0.001). Tamblyn et al.31 reported excessive SABA (expressed as fast-acting b-agonist) dispensing as a composite primary outcome – the rate of out-of-control asthma episodes–which included emergency department (ED) attendances and hospitalisations. It was therefore not possible to determine the effect of the intervention on SABAs alone.
Secondary outcomes
SABA prescribing
Zeiger et al.30 reported a reduction in the number of SABAs dispensed at 3 months (p = < 0.001), 6 months (p = < 0.001) and 12 months (p = < 0.001) in the subgroup of patients without prior specialist asthma care. Eccles et al.29 reported no significant effect of a computerised decision support system on SABA prescription in the 12 months before and after the intervention (odds ratio (OR) 1.04, 95% CI 0.83–1.31).
ICS prescribing
Zeiger et al.30 reported no difference in the number of patients dispensed ICS (not as a combination inhaler), whilst Eccles et al.29 reported no difference in the number of patients prescribed ICS before and after the intervention. McCowan et al.28 reported no between-group difference in maintenance prescribing patterns and no difference in the proportion of patients classified by management step.
Ratio of ICS-SABA prescribed
Tamblyn et al.31 reported an increase in the ratio of ICS-SABAs dispensed (mean difference (MD) 0.27, p = 0.03; 95% CI 0.02–0.51) with higher ratios reported in both subgroups of patients whose asthma was controlled and out of control at the start of the study. Zeiger et al.30 reported a controller (ICS) to total medication ratio of greater or equal to 0.5 at 3, 6 and 12 months, in particular for those without prior asthma specialist care. As the ICS to total medication ratio was calculated by the number of ICS canisters or 30-day supplies of oral controller medications dispensed, divided by the total number of controller units and SABA inhalers, it was not possible to determine the ICS-SABA ratio specifically.
ICS/LABA prescribing
Zeiger et al.30 reported an increase in the number of patients in the subgroup without prior asthma specialist care dispensed an ICS-LABA inhaler at 3 months (p = 0.004), 6 months (p = < 0.001) and 12 months (p = 0.03).
Asthma reviews
McCowan et al.28 reported no reduction in the number of patients attending practice-initiated asthma reviews.
Study-defined asthma exacerbations
McCowan et al.28 observed a reduction in asthma exacerbations, with 8% of patients who received the intervention reporting an acute asthma exacerbation compared to 17% in the control group (OR 0.42; 95% CI 0.21-0.85). However, there was no difference in the use of oral steroids to manage these attacks in the intervention and control group. Zeiger et al.30 reported no difference in the numbers of patients prescribed oral steroids for an exacerbation irrespective of prior asthma specialist care status. Neither McCowan et al.28 nor Zeiger et al.30 explicitly defined an asthma exacerbation. Eccles et al.29 reported no difference in the numbers of patients prescribed oral steroids before and after the intervention but did not specifically report asthma exacerbations.
Unscheduled consultations for asthma
Eccles et al.29 found no between-group reduction in the number of primary care asthma consultations, whilst McCowan et al.28 reported that patients who received the intervention initiated fewer primary care consultations (OR 0.59; 95% CI 0.37–0.95). However neither study clarified whether consultations were scheduled or unscheduled. Both McCowan et al.28 and Zeiger et al.30 reported no effect of the intervention on ED attendances or hospitalisations for asthma. Tamblyn et al.31 reported ED visits and hospitalisations for asthma as a composite outcome defined as ‘rate of out-of-control asthma episodes,’ therefore secondary care consultations for asthma could not be specifically determined.
Asthma control
Tamblyn et al.31 reported a reduction in the rate of out-of-control asthma events, defined as a composite outcome of excessive SABA use, ED attendance and hospitalisations for asthma, in the sub-group of patients whose asthma was out-of-control at the beginning of the study (MD −28.4, p = 0.04; 95% CI −55.6, −1.2). When stratified by intervention component, the rate of out-of-control asthma events further reduced when patients were treated with CDSS alone (rate difference (RD) −36.9/100 per year, p = 0.01) in comparison to those threated with both CDSS and the asthma home care monitoring programme (RD -28.4, p = 0.04; 95% CI −55.6,−1.2).
Discussion
Main findings
Given the few studies identified, the evidence to support the use of alerts to reduce excessive SABA prescribing in primary care is limited but promising. This review found that electronic alerts, when delivered as a multicomponent intervention in an integrated health care system, have the potential to successfully identify and reduce excessive SABA prescribing. The greatest effect on our outcomes of interest occurred when an alert, delivered in an integrated health care system, flagged excessive SABA prescribing to clinicians and prompted/facilitated intervening actions including referral to an allergy specialist and a patient information letter.30 None of the studies included used a SABA alert as a sole intervention.
Interpretations in relation to published literature
Our findings support previous research on the use of computer decision support for long-term conditions including asthma, chronic obstructive pulmonary disease, diabetes and osteoporosis which found that interventions consisting of multiple components are associated with greater improvement in outcomes than single-target interventions with fewer components.24,32,33
There is however no consensus definition on excessive SABA use in the literature. Definitions of excessive SABA use vary from three or more SABAs per quarter34 to 12 or more SABAs a year.7 Of the two included studies in which excessive SABA use was reported, definitions varied from greater or equal to seven canisters per year (at least four puffs per day per year)30 to greater than 250 doses of SABA in the past 3 months.31
The identification and reduction of excessive SABA use in Zeiger et al.30 study was facilitated by alerts that were not restricted to point-of-care presentation. Such methods of alerting may offer a solution to the dilemma that automatic provision of decision support at point of decision making neither guarantees clinician uptake or engagement27 nor predicts improvements in process of care or patient outcomes.35 The two studies that showed greatest effects on our outcomes of interest were those carried out recently (in the past three years), in which decision support was integrated with electronic health record (EHRs).30,31 Although research has indicated that advice presented within EHRs is less likely to improve care or outcomes than stand-alone programmes,35 our findings support the evidence that computer decision support integrated with clinician workflow is associated with improved outcomes.22,32 Zeiger et al’s finding of a reduction of excessive SABA use supports the evidence that electronic health records and electronic messaging in an integrated health care system increases clinician adherence to evidence-based guidelines.36
In one study, users failed to engage with decision support29 whilst in another, clinicians failed to interact with the CDSS in approximately 60% of cases.31 However it is not clear whether levels of engagement were consistent between clinicians and whether clinician interaction declined over time. There may be valid reasons to account for the variability in decision support engagement which include technical design of the CDSS, the setting in which the system is deployed and the characteristics of users and the patients treated.27 The higher user engagement in Tamblyn et al.31 is likely due to the increased ease of use associated with more recent, sophisticated decision support integrated within a comprehensive EHR system that accesses pharmacy, as well as primary and secondary care data. This is in comparison to older interventions, such as that of Eccles et al.29 where the intervention was not integrated with the EHR, and in which pharmacy and secondary care data was not captured.
Alerts integrated within EHRs may interrupt clinician workflow and result in “alert fatigue” with up to 96% of alerts over ridden or ignored in one study.37 Following user feedback, Eccles et al.29 altered decision support to trigger when a clinician entered a relevant morbidity code rather than being automatically activated upon entering a patient’s medical record. Whilst this may have been an attempt to minimise alert fatigue it did not improve CDSS user interaction. It is likely that very low CDSS interactions reflected clinical guidelines being located in a separate system not supported within clinician workflow.
Qualitative research used in conjunction with RCTs has the potential to beneficially influence intervention design and delivery38 however none of the included studies reported using qualitative methods to complement intervention design. Such methods may optimise alert design, improve clinician interaction with decision support and aid the interpretation of results.
Strength and limitations
As interventions to improve prescribing volumes/rates do not necessarily result in more ‘appropriate’ prescribing or improved patient outcomes27 both process and clinical outcomes were assessed in this review. However few studies met our inclusion criteria, with only one study reporting our primary outcome of interest. Due to the limited number of published reports of randomised controlled trials in our analyses, there may be possibility of publication bias or selective reporting. Interventions in the two older studies28,29 were poorly described which may have limited our interpretation of the findings. We were unable to conduct a meta-analysis due to heterogeneity in intervention design and outcomes evaluated. Due to a lack of reporting no conclusions could be made on health economics.
Implications for clinical care and future research
There is an increased focus on the digitalisation of the NHS in an attempt to improve safety and quality of care.39 Recommendations have called for the national use of electronic alerts to identify excessive prescribing of SABAs in the UK.7,40 Due to the few studies identified in this review, the role of alerts to reduce excessive SABA prescribing in the UK’s publically funded national health service (NHS) remains unclear. Integrated care can take many forms involving collaboration between policy providers and commissioners and between service providers, however benefits arise primarily when clinical teams and services are brought together and incentives are aligned to support service improvement.36 It is likely that a combination of design, technical capabilities and variety of intervention components, when delivered in an integrated health care system, facilitated the improvements to SABA prescribing and asthma management identified in recent studies. In a publicly funded health care system such as the NHS it remains challenging to deliver such improvements. However this review identifies a number of areas where potential exists and where further research is recommended.
In the UK, 78% of bronchodilators are issued on repeat prescription41 yet research fails to address the use of alerts at this point in the prescribing process. Furthermore, two studies from the UK, carried out over a decade ago, did not integrate interventions within EHRs, in contrast to more recent studies from North America. Future research should consider novel ways to deliver SABA alerts as a sole intervention and/or as part of a multicomponent intervention in primary care. Furthermore, the point in the prescribing process at which a SABA alert will have greatest impact should be explored. Interventions should be trialled both in and outside of the consultation to target clinicians and people with asthma.
Standardised methods for the design and reporting of CDSS interventions are recommended to enable a thorough evaluation of process and clinical outcomes. We support previous recommendations that studies use a taxonomy or framework such as Kawamoto et al.21 and Berlin et al.42 to theoretically underpin the design and reporting of interventions.25,43 An explicitly defined outcome set that includes more standardised endpoints, e.g., excessive SABA prescribing and asthma exacerbations, may help the translation of research findings into clinical practice. We recommend that future studies report on both the implementation process and health economics outcomes associated with CDSS-based alerts. Third party external validation of CDSSs is recommended.35 Systems evaluation involving academic-commercial collaborations and user testing should be explored to aid the translational research process. End-users should be involved in the design of alerts to optimise interventions and trial design. Future studies should consider mixed methods designs that incorporate qualitative methods before, during and/or after an RCT. Such methods may help determine the barriers and facilitators to alert usage in practice, as well as assisting in the development of alerts that are transferable to the real-world clinical setting.
Conclusion
There is some evidence that electronic alerts integrated with EHRs and delivered as part of a multicomponent intervention reduce excessive SABA prescribing. Due to variations in health care systems, intervention designs and outcomes measured, further research is required to determine the effects of alerts on excessive SABA prescribing in a publically funded health system. Future research should determine the point at which novel alerts will most effectively reduce excessive SABA prescribing and be accepted by users.
Materials and methods
The study design was a systematic review, performed following PRISMA-guidelines.44 Methods of analysis and inclusion criteria were specified in advance and documented in a protocol45 and registered on PROSPERO (International Prospective Register of Systematic Reviews; www.crd.york.ac.uk/PROSPERO/) with identifier CRD 42016035633.
Selection criteria
Studies were considered for inclusion in this systematic review according to the following criteria.
Participants
Studies that delivered care to adults and/or children with asthma, in a primary care setting. Primary care was defined as healthcare delivered in a community setting, most commonly in general practice, by a clinician, nurse or pharmacist. Non-clinical staff for example administrators and/or receptionists were also included.
Intervention
CDSSs were included if they incorporated an alert initiated by the excessive prescribing or dispensing of SABAs for asthma. Alerts used in secondary or tertiary care, for other respiratory conditions that were not asthma were excluded.
Comparison
The comparator was ‘usual care.’
Outcomes
Our primary outcome of interest was excessive SABA prescribing. Excessive prescribing of SABA was assessed on a study-defined basis. Secondary outcomes of interest included additional measures of prescribing and process of care (future SABA and ICS prescribing, ICS/SABA prescribing ratio, ICS/long-acting beta2-agonist prescribing (LABA), asthma reviews), and clinical outcomes (asthma exacerbations with/without oral steroids, unscheduled primary and secondary care asthma consultations, asthma control).
Study design
Only randomised controlled trials (RCTs), in any language, were included as they are considered the most rigorous way to evaluate intervention effectiveness.46
Search strategy
We searched Medline, Embase, Cinahl, Scopus and Cochrane Library databases from 1990 to 2016 with the search terms listed in Supplementary Appendix 2. We contacted the authors of included studies to clarify intervention design and outcomes measured where necessary. Ongoing and unpublished trials were searched for using the following websites: https://www.isrctn.com/ and https://clinicaltrials.gov/.
Two authors (SM and CG) independently screened titles and abstracts, assessing them against the inclusion criteria. Both authors reviewed the full text of each potentially eligible paper to determine suitability for inclusion. Disagreements were resolved by discussion and, if necessary, arbitration of a third researcher (ADS, AB, MT).
Data extraction and quality appraisal
Using a piloted data extraction form, SM and ADS independently extracted the following data from included trials: country, setting, funding, study design, healthcare professional and patient population, features of the CDSS intervention, description of the control group, outcome measures, results and risk of bias assessment. SM and ADS compared data extraction, and disagreements were arbitrated by a third researcher (CG) if necessary.
We assessed the risk of bias in each trial using the seven-criteria approach described in section eight of the Cochrane Handbook for Systematic Reviews of Interventions.47
Data analysis
Due to heterogeneity in the CDSS interventions used and in outcomes measured, we undertook a narrative synthesis.
Data availability
Authors confirm that all relevant data are included in the paper and/or its supplementary information files.
Electronic supplementary material
Detailed description of intervention characteristics
Acknowledgements
The authors wish to thank Asthma UK and Queen Mary University London for funding this work as part of a PhD studentship carried out by S.M. A.D.S. is funded by a NIHR Academic Clinical Lectureship. A.B. is a National Institute for Health Research (NIHR) Senior Investigator and additionally was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. M.T. is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Wessex, NIHR School of Primary Care Research and NIHR Southampton Biomedical Research Centre. C.G. is supported by the NIHR CLAHRC North Thames at Bart’s Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This work is funded by Asthma UK and Queen Mary University of London.
Author contributions
This work forms part of an Asthma UK Centre for Applied Centre PhD Studentship being undertaken by S.M. C.G. had the original idea for the study. S.M., A.B., M.T. and C.G. contributed to the plan and/or design of the study. S.M. and C.G. completed study screening. S.M. and A.D.S. performed data extraction. S.M. drafted the manuscript and A.D.S., A.B., M.T. and C.G. commented on each draft version. All authors read and approved the final manuscript.
Competing interests
C.G. is an assistant editor of npj Primary Care Respiratory Medicine and M.T. is an associate editor of npj Primary Care Respiratory Medicine but were involved in neither the editorial review of, nor any decision to publish or not publish this article.
Footnotes
Electronic supplementary material
Supplementary information accompanies the paper on the npj Primary Care Respiratory Medicine website (10.1038/s41533-018-0080-z).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibson JG, Loddenkemper R, Lundbäck. B, Sibille Y. Respiratory health and disease in Europe: the new European lung white book. Eur. Respir. J. 2013;42:559–563. doi: 10.1183/09031936.00105513. [DOI] [PubMed] [Google Scholar]
- 2.Health and Social Care Information Centre. Quality and Outcomes Framework-Prevalence, Achievements and Exceptions Report, England 2016–2017. https://www.gov.uk/government/statistics/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data-2016-to-2017.
- 3.Asthma U.K. Asthma facts and statistics. https://www.asthma.org.uk/about/media/facts-and-statistics/.
- 4.National Institute for Health and Excellence. Asthma Quality Standard (QS25). https://www.nice.org.uk/guidance/qs25.
- 5.NHS Digital. Hospital Admitted Patient Care Activity, 2015-16. https://www.gov.uk/government/statistics/hospital-admitted-patient-care-activity-2015-to-2016.
- 6.Asthma U.K. Asthma UK calls for action to end preventable asthma deaths. (2017).
- 7.Royal College of Physicians. Why Asthma Still Kills-The National Review of Asthma Deaths (NRAD) Confidential Enquiry report. https://www.rcplondon.ac.uk/projects/national-review-asthma-deaths (2014).
- 8.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ (2017).
- 9.Schatz M, et al. Asthma quality-of-care markers using administrative data. Chest. 2005;128:1968–1973. doi: 10.1378/chest.128.4.1968. [DOI] [PubMed] [Google Scholar]
- 10.Paris J, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann. Allergy Asthma Immunol. 2008;101:482–487. doi: 10.1016/S1081-1206(10)60286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M, et al. Metrics of salbutamol use as predictors of future adverse outcomes in asthma. Clin. Exp. Allergy. 2013;43:1144–1151. doi: 10.1111/cea.12166. [DOI] [PubMed] [Google Scholar]
- 12.British Thoracic Society and Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. (2016).
- 13.Spitzer WO, et al. The use of beta-agonists and the risk of death and near death from asthma. N. Engl. J. Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 14.Suissa S, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am. J. Respir. Crit. Care Med. 1994;149:604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 15.Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur. Respir. J. 1994;7:1602–1609. doi: 10.1183/09031936.94.07091602. [DOI] [PubMed] [Google Scholar]
- 16.Anderson HR, et al. Bronchodilator treatment and deaths from asthma: case-control study. BMJ. 2005;330:117. doi: 10.1136/bmj.38316.729907.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanes SF, Garcia Rodriguez LA, Huerta C. Respiratory medications and risk of asthma death. Thorax. 2002;57:683–686. doi: 10.1136/thorax.57.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddel HK, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DR, et al. A new perspective on concepts of asthma severity and control. Eur. Respir. J. 2008;32:545–554. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 20.Bateman ED, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson SA, et al. Do computerised clinical decision support systems for prescribing change practice? A systematic review of the literature (1990-2007) BMC Health Serv. Res. 2009;9:154. doi: 10.1186/1472-6963-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fathima M, Peiris D, Naik-Panvelkar P, Saini B, Armour CL. Effectiveness of computerized clinical decision support systems for asthma and chronic obstructive pulmonary disease in primary care: a systematic review. BMC Pulm. Med. 2014;14:189. doi: 10.1186/1471-2466-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matui P, Wyatt JC, Pinnock H, Sheikh A, McLean S. Computer decision support systems for asthma: a systematic review. NPJ Prim. Care Respir. Med. 2014;24:14005. doi: 10.1038/npjpcrm.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt, J. & Spiegelhalter, D. Field trials of medical decision-aids: potential problems and solutions. Proc. Annu. Symp. Comput. Appl. Med. Care, 3–7 (1991). http://www.ncbi.nlm.nih.gov/pubmed/1807610. [PMC free article] [PubMed]
- 26.Black AD, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med. 2011;8:e1000387. doi: 10.1371/journal.pmed.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moxey A, et al. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J. Am. Med. Inform. Assoc. 2009;17:25–33. doi: 10.1197/jamia.M3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCowan C, et al. Lessons from a randomized controlled trial designed to evaluate computer decision support software to improve the management of asthma. Med. Inform. Internet Med. 2001;26:191–201. doi: 10.1080/14639230110067890. [DOI] [PubMed] [Google Scholar]
- 29.Eccles M, et al. Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: Cluster randomised controlled trial. BMJ. 2002;325:941–944. doi: 10.1136/bmj.325.7370.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiger RS, et al. Real-time asthma outreach reduces excessive short-acting β2-agonist use: a randomized study. J. Allergy Clin. Immunol. Pract. 2014;2:445–456. doi: 10.1016/j.jaip.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Tamblyn R, et al. Evaluating the impact of an integrated computer-based decision support with person-centered analytics for the management of asthma in primary care: a randomized controlled trial. J. Am. Med. Inform. Assoc. 2015;22:773–783. doi: 10.1093/jamia/ocu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roshanov PS, et al. Computerized clinical decision support systems for chronic disease management: a decision-maker-researcher partnership systematic review. Implement Sci. 2011;6:92. doi: 10.1186/1748-5908-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastner M, Straus SE. Clinical decision support tools for osteoporosis disease management: a systematic review of randomized controlled trials. J. Gen. Intern Med. 2008;23:2095–2105. doi: 10.1007/s11606-008-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver HS, et al. Quarterly assessment of short-acting β2-adrenergic agonist use as a predictor of subsequent health care use for asthmatic patients in the United States. J. Asthma. 2010;47:660–666. doi: 10.3109/02770901003702824. [DOI] [PubMed] [Google Scholar]
- 35.Roshanov PS, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ. 2013;346:f657. doi: 10.1136/bmj.f657. [DOI] [PubMed] [Google Scholar]
- 36.Curry, N. & Ham, C. Clinical and service integration: the route to improved outcomes. The King’s Fund. http://www.kingsfund.org.uk/publications/clinical-and-service-integration (2010).
- 37.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J. Am. Med Inform. Assoc. 2006;13:138–147. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open. 2013;3:e002889. doi: 10.1136/bmjopen-2013-002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brailer JAM, et al. Making IT work-harnessing the power of health information technology to improve care in England. London: Department of Health; 2016. [Google Scholar]
- 40.Asthma U.K. Connected asthma: how technology will transform care. https://www.asthma.org.uk/connectedasthma (2016).
- 41.Petty, D. The repeat prescription report. Pharmacy2U. https://www.pharmacy2u.co.uk/prescription-report.html (2017).
- 42.Berlin A, Sorani M, Sim I. A taxonomic description of computer-based clinical decision support systems. J. Biomed. Inform. 2006;39:656–667. doi: 10.1016/j.jbi.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Car, J. et al. The Impact of eHealth on the Quality and Safety of Healthcare. A Report for the NHS Connecting for Health Evaluation Programme: Extended executive summary (2008).
- 44.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKibben S, Bush A, Thomas M, Griffiths C. The use of electronic alerts in primary care computer systems to identify the over-prescription of short-acting beta2-agonists in people with asthma: a protocol for a systematic review. NPJ Prim. Care Respir. Med. 2017;27:30. doi: 10.1038/s41533-017-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig P, et al. Developing and evaluating complex interventions: the new medical research council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins J. P. T. & Green S. (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. http://www.cochrane-handbook.org (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description of intervention characteristics
Data Availability Statement
Authors confirm that all relevant data are included in the paper and/or its supplementary information files.