Abstract
Background
Endometriosis is a multifactorial disease that mainly affects women of reproductive age. The exact pathogenesis of this disease is still debatable. The role of bacterial endotoxin (lipopolysaccharide, LPS) and Toll‐like receptor 4 (TLR4) in endometriosis were investigated and the possible source of endotoxin in the pelvic environment was examined.
Methods
The limulus amoebocyte lysate test was used to measure the endotoxin levels in the menstrual fluid and peritoneal fluid and their potential role in the growth of endometriosis was investigated. Menstrual blood and endometrial samples were cultured for the presence of microbes. The effect of gonadotrophin‐releasing hormone agonist (GnRHa) treatment on intrauterine microbial colonization (IUMC) and the occurrence of endometritis was investigated.
Main findings (Results)
Lipopolysaccharide regulates the pro‐inflammatory response in the pelvis and growth of endometriosis via the LPS/TLR4 cascade. The menstrual blood was highly contaminated with Escherichea coli and the endometrial samples were colonized with other microbes. A cross‐talk between inflammation and ovarian steroids or the stress reaction also was observed in the pelvis. Treatment with GnRHa further worsens intrauterine microbial colonization, with the consequent occurrence of endometritis in women with endometriosis.
Conclusion
For the first time, a new concept called the “bacterial contamination hypothesis” is proposed in endometriosis. This study's findings of IUMC in women with endometriosis could hold new therapeutic potential in addition to the conventional estrogen‐suppressing agent.
Keywords: bacterial endotoxin, endometriosis, gonadotrophin‐releasing hormone agonist, menstrual blood, Toll‐like receptor
1. INTRODUCTION
Endometriosis is a multifactorial disease that mostly affects women of reproductive age and is associated with chronic pelvic pain and infertility. There are some established hypotheses and regulatory factors that support the development or maintenance of this disease.1, 2, 3, 4 However, it is difficult to uniformly explain the pathogenesis of endometriosis by a single factor. Even after a long 300 years, most of the literature still claims that the pathogenesis and/or pathophysiology of endometriosis is unclear. Studies have shown that the growth and progression of endometriosis continue even in an ovariectomized animal. This indicates that besides ovarian steroid hormones, the growth of endometriosis can be regulated by the innate immune system in the pelvic environment. As a component of the innate immune system, increased infiltration of macrophages (Mφ) has been described in the intact tissue and peritoneal fluid (PF) of women with endometriosis.5
The understanding of the innate immune system is a result, in large part, from the pioneering studies of Charles Janeway, who demonstrated that innate immunity covers many areas of host defense against pathogenic microbes.6 During the last decade, investigations of the innate immune system have shown that microbial pathogens are recognized by Toll‐like receptors (TLRs) that, in turn, regulate the activation of both innate and adaptive immunity.7 Mammalian innate immune cells, such as Mφ and dendritic cells, can be activated by microbial components (non‐self), such as endotoxin or lipopolysaccharide (LPS) from Gram‐negative bacteria. The pattern recognition receptors (PRRs) of the host that recognize pathogen‐associated molecular patterns in the female reproductive tract are expressed on the cells of the innate immune system. Toll‐like receptors are one group of PRRs that are expressed on Mφ, dendritic cells, and as more recently shown, on neutrophils, natural killer cells, and on epithelial cells.8, 9, 10
Originally described over 300 years ago, endometriosis is classically defined by the presence of endometrial glands and stroma in extrauterine locations.1 Endometriosis is an estrogen‐dependent disease that mostly affects women of reproductive age. Recently, it has been demonstrated that besides hormonal regulation, both secondary (cytokines/growth factors) and initial inflammatory (LPS) mediators are known to be involved in the growth of endometriosis.2, 3, 10 Some of the literature, including the authors', has demonstrated the expression of TLRs in Mφ and other dendritic cells.11, 12, 13, 14 This review article will discuss: (1) a fundamental concept of the TLR system for easy understanding by the readers, (2) the biological function of the initial inflammatory mediator, bacterial endotoxin or LPS, in the eutopic and ectopic endometria, (3) confirmation of the presence of LPS in the pelvic environment, (4) the source of LPS in menstrual blood, (5) the mechanistic basis of bacterial contamination in the intrauterine environment, (6) cross‐talk between inflammation and the stress reaction in endometriosis, (7) cross‐talk between ovarian steroids and inflammation in endometriosis, (8) the cause–effect of endometriosis on bacterial contamination, (9) the association of intrauterine microbial colonization (IUMC) in women treated and untreated with an estrogen‐suppressing agent, (10) intrauterine microbial colonization after treatment with an estrogen‐suppressing agent, and (11) the possible association of intrauterine bacterial contamination with the reproductive outcome.
2. TOLL‐LIKE RECEPTOR SYSTEM
2.1. Toll‐like receptor family
Until recently, 11 different members have been reported in the family of mammalian TLRs. Among them, TLRs1‐9 are conserved between the human and the mouse.14, 15 TLR10 appears to be functional in the human, but non‐functional in the mouse. On the contrary, mouse TLR11 appears to be functional, but non‐functional in the human.16 Initially, a potential role of TLR4 was established in the recognition of the microbial component.17 However, subsequent studies revealed important roles of the individual TLRs in recognizing specific microbial components that are derived from pathogens, including bacteria, fungi, protozoa, and viruses. A detailed description of the different TLRs, their respective ligands, and their intracellular mode of action already have been reported.9, 18, 19 Here, we focus on the role of TLR4 in endometriosis.
2.2. Ligands of Toll‐like receptor 4
There are two types of ligands, exogenous and endogenous, for TLR4.20 Bacterial endotoxin or LPS, F protein from the respiratory syncytial virus, chlamydial heat shock protein (Hsp)60, and taxol, a plant‐derived anticancer reagent, all belong to the exogenous ligands of TLR4. The action of taxol mimics the action of LPS in mice but not in humans.20 The endogenous ligands of TLR4 comprise fibrinogen, fibronectin, heparin sulphate, hyaluronic acid, and Hsps60 and 70. Although a minimal concentration of LPS has the ability to activate TLR4, all the endogenous ligands need very high concentrations to activate TLR4.20, 21, 22, 23
2.3. Signaling pathways that are triggered by Toll‐like receptor 4
The detailed signaling pathways of TLR4 in response to LPS are described elsewhere.24, 25 It seems that all the TLR signaling pathways are similar and elicit similar biological responses, except TLR3. Lipopolysaccharide is immediately captured by LPS‐binding protein that delivers LPS to TLR4 or CD14 soon after its release in bodily fluids. Lipopolysaccharide is a potent activator of Mφ and other dendritic cells. Different monocyte markers, including CD14, lack a trans‐membrane domain and thus are incapable of transducing signals.26 Once LPS/TLR4 binds with TLR4, it stimulates a cascade of intracellular adopter molecules and triggers the gene expression of a number of target molecules (cytokines/chemokines/growth factors) by inducing nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐kB) activation.4
2.4. Toll‐like receptor system in eutopic and ectopic endometria
It is commonly understood that the upper genital tract is vulnerable to the spread of microorganisms from the lower genital tract, resulting in the development of infectious diseases, such as endometritis and salpingitis. In fact, an enormous number of Gram‐negative and Gram‐positive microbes is present in the vaginal cavity. All these microbes reside in the vaginal cavity as normal vaginal flora and can cause genitourinary infections on ascending migration.27 Escherichia coli (E. coli) are the most commonly isolated pathogenic bacteria in the bovine and also in the human vaginal cavity.28, 29
It is speculated that the ascending migration of E. coli towards the endometrial cavity is possible and may cause contamination of the endometrium, based on the phases of the menstrual cycle. Nine TLRs already are identified at the protein and messenger (m)RNA level, including TLR4, in the human endometrium.12, 30, 31 As a component of the innate immune system, an increase in the infiltration of Mφ has been found in the normal endometrium and also in the endometrium of women with different reproductive diseases.32, 33 A number of recent studies reported the expression of TLR4 mRNA and protein in Mφ, endometrial and endometriotic epithelial cells, and stromal cells.11, 12, 31 The distributing pattern of TLR4 in Mφ, endometrial cells, and endometriotic cells was found to be identical during the proliferative phase. However, this expression pattern appeared to be higher in the secretory phase of the menstrual cycle.11, 12, 13 This was observed equally for women with and without endometriosis.
3. BIOLOGICAL FUNCTION OF LIPOPOLYSACCHARIDE IN ENDOMETRIOSIS
The function of LPS in Mφ, endometrial, and endometriotic cells has been observed. The exposure of peritoneal Mφ to LPS significantly increased the production of a number of macromolecules, such as hepatocyte growth factor (HGF), vascular endothelial cell growth factor (VEGF), interleukin (IL)‐6, IL‐8, and tumor necrosis factor‐alpha (TNF‐α) in a dose‐dependent fashion.12, 34, 35, 36, 37 This effect of LPS was abrogated by the pretreatment of cells with neutralizing antibodies for TLR4 and also by a LPS antagonist, polymyxin B.11 This cellular specificity indicates that Mφ respond to LPS through TLR4. The authors also found that both eutopic and ectopic endometrial stromal cells (ESCs) and endometrial epithelial cells (EECs) were able to significantly proliferate in response to LPS and that this growth‐promoting effect of LPS decreased after treatment with anti‐TLR4 antibody.4, 10, 11 It is presumed that the blocking of TLR4 is effective in order to suppress the inflammatory response in the pelvic environment and cell growth. Another study indicated that the TLR4 system might represent local immunity in the human endometrium, with different modes of TLR4 actions between the ESCs and the EECs.31
As a secondary inflammatory mediator, different macromolecules (cytokines/chemokines/growth factors) in the pelvic environment are believed to enhance the growth of endometriosis. The authors reported that as an initial inflammatory mediator, bacterial endotoxin (LPS) could be a potential inflammatory mediator of Mφ stimulation and the consequent production of HGF, VEGF, IL‐6, and TNF‐α in the pelvic environment.11, 36 This LPS, together with LPS‐induced secondary inflammatory mediators, are possibly involved in the growth of endometriosis in an autocrine or paracrine mechanism.36, 37
In addition to TLR4 blocking, the stimulating effect of LPS can be abrogated after treatment with NF‐kB inhibitor.37 This was confirmed in another experiment using ESCs that had been derived from chocolate cyst linings of the ovary. The authors demonstrated that NF‐kB inhibitor was able to significantly suppress LPS‐stimulated TNF‐α and IL‐8 production by ESCs, as well as ESC proliferation. This indicates that as an initial inflammatory mediator, the functional activity of LPS is regulated by both TLR4 at the receptor level on the cell surface and by NF‐kB at the nucleus. It is presumed that a substantial amount of endotoxin in the menstrual fluid (MF) and PF could be involved in pelvic inflammation and could promote the TLR4/NF‐kB‐mediated growth of endometriosis.
4. PRESENCE OF LIPOPOLYSACCHARIDE IN THE INTRAUTERINE AND PELVIC ENVIRONMENT
The authors examined the endotoxin concentration for the first time in the MF and PF of women with or without endometriosis. The limulus amoebocyte lysate test was used to measure the endotoxin levels in the PF that had been collected from women with and without endometriosis. With informed consent and a strict aseptic measure, menstrual blood was collected from a proportion of these women. It was found that the endotoxin (LPS) concentration in the MF was four‐to‐sixfold, and significantly higher, in the women with endometriosis than that in those without endometriosis.11 Regarding the distributed endotoxin concentration in the PF, based on the phases of the menstrual cycle, a maximum concentration during the menstrual phase and a modest concentration in either the proliferative or the secretory phase of the menstrual cycle was found.11
5. SOURCE OF LIPOPOLYSACCHARIDE IN THE MENSTRUAL BLOOD
The residual accumulation of bacterial endotoxin in the pelvic environment can be explained by two possible mechanisms: (1) the translocation of E. coli or endotoxin from the gut through enterocytes and their entry into the pelvic cavity, as demonstrated in one study,38 and (2) contamination of the menstrual blood by E. coli after ascending migration from the vagina. The authors confirmed, by the bacterial culture method, that the menstrual blood of women with endometriosis was highly contaminated with E. coli, compared to that in the control women.11 These findings suggested that the contamination of menstrual blood with E. coli in women with endometriosis could be a constant source of bacterial endotoxin in the PF because of a periodic retrograde menstrual flow and that this cyclic event could initiate the TLR4‐mediated growth of endometriosis.
6. MECHANISTIC BASIS OF BACTERIAL CONTAMINATION IN THE MENSTRUAL BLOOD
The authors have proposed two mechanisms that were involved in the E. coli contamination of the menstrual blood: (1) higher prostaglandin E2 (PGE2) levels in the MF and PF of women with endometriosis was involved in the bacterial growth of E. coli in a bacterial culture system39 and that this effect of PGE2 on bacteria might be contributed to by its direct growth‐promoting effect on E. coli or by its indirect immunosuppression effect on peripheral blood lymphocytes39 and (2) the decreased expression of antimicrobial peptides, such as human β‐defensin (HBD) and/or secretory leukocyte protease inhibitor (SLPI), in the endometrium. Usually, HBD and SLPI are expressed by the epithelial layers of the vagina, ectocervix, endocervix, endometrium, and fallopian tubes.40 These antimicrobial peptides are normally regulated by cyclic estrogen40 and their expression pattern might be down‐regulated after estradiol (E2)/progesterone withdrawal during menstruation. The decreased expression of the antimicrobial peptides in the intrauterine or intravaginal luminal epithelium during the menstrual phase could be involved in the bacterial contamination of menstrual blood in women with endometriosis.
7. CROSS‐TALK BETWEEN INFLAMMATION AND THE STRESS REACTION IN ENDOMETRIOSIS
Endometrial tissue degradation during menstruation, its backflow, and consequent attachment or invasion into the pelvis conferred a physical or chemical tissue stress reaction in women who suffered from endometriosis. Other stressful stimuli, such as heat shock, UV radiation, viral or bacterial infections, and pelvic inflammation, induced an increase in the intracellular synthesis of stress‐induced proteins, such as Hsps.41, 42, 43, 44, 45 The so‐called “danger theory” states that antigen‐presenting cells can be activated by endogenous substances that are released by damaged or stressful tissues46 and that this effect of Hsps has been reported to be mediated by TLR4, either alone or in combination with LPS.36
The authors' recent study demonstrated the release of a variable amount of endogenous Hsp70 by the different peritoneal lesions and eutopic endometria of women with endometriosis. It was found that Hsp70 induced TLR4‐mediated inflammation and the growth of endometriosis.36 Although polymyxin B was unable to suppress the combined LPS‐ and Hsp70‐mediated growth of endometriosis, the growth‐promoting effect of combined LPS and Hsp70 was significantly suppressed when the biological function of TLR4 was blocked with anti‐TLR4 antibody.36 This indicates that the LPS‐ and Hsp70‐mediated inflammatory reaction and growth of endometriosis could be mediated by TLR4 in the pelvic environment.
Oxidative stress is another form of tissue stress reaction that results from excessive iron accumulation in the endometriotic fluid. Endometriotic lesions, including chocolate cysts and blood‐filled opaque red lesions, are hemorrhagic during menstruation.46, 47 Oxidative stress is related to atherosclerosis, neurodegeneration, cancer, and aging.48, 49, 50 Excessive reactive oxygen species (ROS) production or oxidative stress might be associated with endometriosis. Recently, it has been demonstrated that in addition to the effects of endogenous danger signals via TLRs, tissue oxidative stress itself can promote the NF‐kB‐ or TLR4‐mediated growth of endometriosis.51 In fact, LPS itself has the capacity to produce ROS by Mφ. These findings are consistent with the understanding that LPS, endogenous danger signals, and oxidative stress could promote the onset and progression of endometriosis after the activation of TLRs and/or NF‐kB signaling.
8. CROSS‐TALK BETWEEN OVARIAN STEROIDS AND INFLAMMATION IN ENDOMETRIOSIS
The effect of estrogen, either alone or in combination with initial or secondary inflammatory mediators, on the growth regulation of endometriosis has been reported.52, 53, 54, 55 The authors previously demonstrated the Mφ‐mediated production of HGF, VEGF, IL‐6, and TNF‐α in response to ovarian steroids, which was further enhanced after treatment with LPS.52, 54 An additive effect was observed between E2 and LPS on the Mφ‐mediated secretion of macromolecules and on the proliferation of eutopic and ectopic endometrial stromal cells, when compared with their single treatment.53, 54, 55 This effect of E2 plus LPS on cell growth was markedly abrogated after pretreatment of the cells with anti‐TLR4 antibody and intracervical insemination, an estrogen antagonist.53, 54 These findings suggested that E2 exhibited a pro‐inflammatory response and that an immune–endocrine cross‐talk between estrogen and endotoxin in the pelvic environment could be involved as an additive inflammatory response in the pelvic environment and growth of endometriosis.
9. CAUSE–EFFECT OF ENDOMETRIOSIS ON BACTERIAL CONTAMINATION
9.1. Scattering of endometrial cells and increased cell motility or invasion by lipopolysaccharide‐induced hepatocyte growth factor
The most critical question may arise now: “Is this bacterial contamination the effect of endometriosis or the cause of endometriosis?” Based on the authors' serial experiments, the conclusion is that bacterial contamination in menstrual blood could be the effect of endometriosis and, at the same time, also might develop endometriosis. It was demonstrated that LPS regulates the expression of HGF and its receptor, c‐Met, in the PF, ESCs, and EECs.3, 11, 34, 36 Compared to the control women, a higher concentration of HGF in the PF and MF of women with endometriosis caused the scattering of the EECs and ESCs in a dose‐dependent fashion. Boyden's chamber assay/matrigel in vitro assay indicated that HGF significantly increased the migration and invasion of ESCs.34, 36, 52 These findings suggested that LPS‐induced HGF might be involved in the retrograde passage of cells during menstruation, with consequent cellular invasion once the endometrial cells came into contact with the peritoneal mesothelium.
9.2. Lipopolysaccharide‐induced expression of cell–cell adhesion molecules in the endometrium and pelvic peritoneum
Bacterial endotoxin (LPS) has been reported to regulate a number of cell–cell adhesion molecules (intercellular adhesion molecule 1/vascular cell adhesion molecule 1/fibronectin/laminin) and their receptors (integrin α3 and integrin α6).56, 57, 58 Recently, the authors collected endometrium and its corresponding peritoneum from the same patients during the menstrual phase in order to examine the expression pattern of cell–cell contact molecules. The immunohistochemistry study indicated that two cell–cell adhesion molecules, fibronectin and laminin, were highly expressed in both gland cells and stromal cells. Interestingly, the flat mesothelial cells of the peritoneum that had been derived from these patients displayed a higher expression of integrin α3, a receptor for fibronectin, and integrin α6, a receptor for laminin (Figure 1). These findings suggest that LPS‐induced fibronectin and laminin might help in the cellular attachment to the peritoneal mesothelium after binding with their corresponding receptors once degraded functional endometrial cells appear in the pelvis during the period of menstruation.
Figure 1.
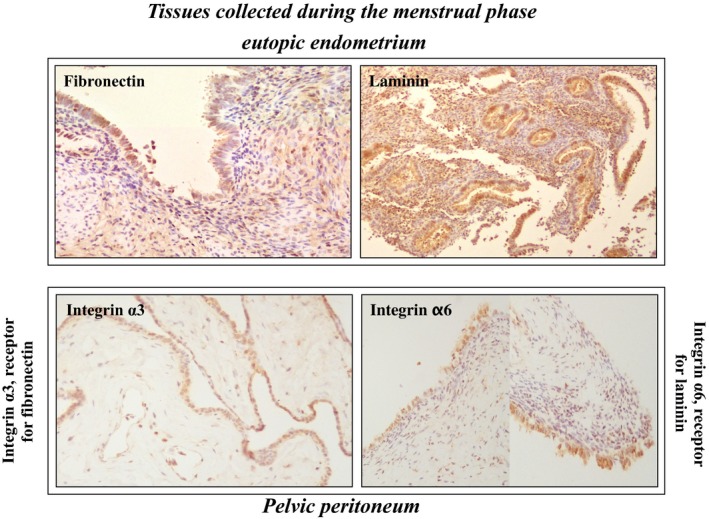
Immunohistochemical expressions of the cell–cell adhesion molecules, fibronectin and laminin, in the eutopic endometria (upper panel) and their corresponding receptors, integrin α3 and integrin α6, in the pelvic peritoneum (lower panel). All these tissue samples were collected from women with endometriosis during the menstrual phase. Fibronectin and laminin were highly expressed in the gland cells and stromal cells of the eutopic endometria. Similarly, integrin α3 and integrin α6 were expressed in the mesothelial cells of the pelvic peritoneum that was derived from the same patient
All these findings of HGF and fibronectin and laminin in the endometrial and mesothelial cells could suggest that, after bacterial contamination, as an initial inflammatory mediator, LPS could have an indirect effect on the development and progression of endometriosis.
10. INTRAUTERINE MICROBIAL COLONIZATION AFTER TREATMENT WITH AN ESTROGEN‐SUPPRESSING AGENT
10.1. Intrauterine microbial colonization in the gonadotrophin‐releasing hormone agonist‐treated women
Information regarding the existence of a subclinical vaginal infection or subclinical uterine infection in women with and without endometriosis is limited. Recently, the authors examined the intravaginal pH of women with and without endometriosis, with the concept that although an acidic intravaginal environment protects from infection, a shifting of the vaginal pH towards an alkaline environment increases the risk of microbial colonization.59 There was found a significant shifting of the intravaginal pH to ≥4.5 in those women with endometriosis, compared to the control women (79.3% vs 58.4%, P < 0.03, X 2‐test). Compared to the untreated women, the use of gonadotrophin‐releasing hormone agonist (GnRHa) therapy also shifted the vaginal pH to ≥4.5 in both the control women (P = 0.004, X 2‐test)) and in the women with endometriosis (P = 0.03, X 2‐test). There was not found any difference in the intravaginal pH (<4.5 vs ≥4.5) among the phases of the menstrual cycle in the women with and without endometriosis or between the revised American Society for Reproductive Medicine stages I‐II and III‐IV in the women with endometriosis. The authors investigated the bacteriological evidence of bacterial vaginosis in vaginal smears, based on a modified scoring system of Nugent's criteria.60 A higher risk was found in increasing the intermediate flora (total score, 4‐6) (P = 0.05, X 2‐test) and in decreasing the normal vaginal flora (total score, 0‐3) (P = 0.007, X 2‐test) in the GnRHa‐treated women with endometriosis, compared to the GnRHa‐untreated women.59
The authors examined the pattern of bacterial growth in the endometrial samples that had been derived from the GnRHa‐treated and ‐untreated women with and without endometriosis, using the bacterial culture method. Among the colony formations of nine different microbial species, according to the treatment status of GnRHa, a significantly increased colony formation was found of Gardnerella and E. coli (P < 0.05 for each) in the GnRHa‐treated control women and Gardnerella, Enterococci, and E. coli (P < 0.05 for each) was found in the GnRHa‐treated women with endometriosis, compared to the GnRHa‐untreated women.59 A Kruskal–Wallis test still indicated a higher growth of these microbial species after GnRHa treatment than other microbes. The microbial growth of lactic acid‐producing protective bacteria (Lactobacillus spp.) was decreased in the endometrial samples that had been derived from the women with endometriosis and after GnRHa treatment. Most recently, the authors confirmed the bacterial culture‐based findings by the molecular method.61
10.2. Occurrence of endometritis in the gonadotrophin‐releasing hormone agonist‐treated women
The authors' finding of IUMC was consequently associated with the occurrence of both acute and chronic endometritis in the women with endometriosis. The occurrence of endometritis was significantly higher in the GnRHa‐treated women than in the GnRHa‐untreated women, with and without endometriosis (control, 68.4% vs 26.5%, P = 0.003; endometriosis, 85.7% vs 37.2%, P = 0.001, both by X 2‐test).59 From these recent findings, it is presumed that a worsening of IUMC and a higher occurrence of endometritis could occur in women with endometriosis after GnRHa treatment. These findings of the association between endometriosis and chronic endometriosis were supported by two recently published reports.62, 63
11. ASSOCIATION OF BACTERIAL CONTAMINATION OR ENDOMETRITIS WITH THE REPRODUCTIVE OUTCOME
The common problems of women who suffer from endometriosis are an impairment in the quality of life and producing a state of subfertility or infertility. A number of mechanisms have been proposed to support these adverse effects of endometriosis.64, 65, 66, 67, 68, 69 As endometriosis is a chronic inflammatory disease, a moderate‐to‐severe inflammatory reaction in the pelvic environment of women with endometriosis leads to the formation of tubo‐ovarian adhesion or peri‐tubal adhesion, finally resulting in the narrowing or occlusion of the Fallopian tubes.66, 67, 68 On the contrary, bacterial endotoxin (LPS) that has been derived from Gram‐negative bacteria can directly cause endometrial or tubal damage. Endotoxin has been found to be deleterious to pre‐implantation stage embryos.70 The presence of endotoxin in in vitro fertilization (IVF) culture media results in a high rate of polyspermy, decreased embryo cleavage rate, and blastocyst formation in human and bovine species.70 A recent assisted reproductive technology clinical trial demonstrated that the pregnancy rate after IVF–embryo transfer was significantly higher in those women with an endotoxin level of <200 pg/mL in the MF than in the women with an endotoxin level of >200 pg/mL.69 All these accumulated findings indicate that, in addition to an inflammatory reaction, different ligands that are derived from different microbes in the intrauterine environment can induce a variable detrimental effect on the reproductive outcome and that this adverse effect also could occur in women with endometriosis.
Endotoxin also possesses the capacity to induce the apoptosis of cells by impairing sperm motility and inducing spermicidal activity.70 A recent study demonstrated the expression of TLR4 and TLR2 in human and mouse sperm and measured suboptimal concentrations of endotoxin and peptidoglycan in human semen.70 It was found that the addition of LPS (the ligand of TLR4) and peptidoglycan (the ligand of TLR2) in the absence of leukocytes directly and significantly reduced the motility and increased the apoptotic rate of both human and mouse sperm and suppressed fertilization by sperm both in vivo and in vitro.70 These findings further strengthened the detrimental effect of bacterial endotoxin on the reproductive outcome.
Recently, an elegant effort was made to find the evidence that the endometrial microbiota has an effect on implantation success or failure. The molecular detection of microbiota in the endometrial fluid demonstrated the existence of an endometrial microbiota that is highly stable during the acquisition of endometrial receptivity.71 When bacterial communities from paired endometrial fluid and vaginal aspirate samples within the same women were analyzed, different bacterial communities were identified between the uterine cavity and the vagina of some participants. A similar study also found that the presence of a range of microbial candidates in a receptive endometrium was associated with significant decreases in implantations, pregnancies, ongoing pregnancies, and live birth rates in infertile women who were undergoing IVF.71 These findings further added an association between intrauterine microbial colonization and an adverse reproductive outcome.
Chronic endometritis (CE) was found to be a frequent finding in the women with recurrent implantation failure (RIF) after IVF. A CE was identified in 30% of patients with RIF and those women with biopsy‐proven CEs had lower implantation rates than those with absent CE.72, 73 A subsequent study proposed antibiotic therapy in patients with CE. The implantation rate and ongoing pregnancy rate were significantly increased after the antibiotic treatment of the women with hysteroscopy‐proven CE.74 Two recent studies further documented that CE that is associated with infection with common bacteria is associated with RIF and emphasized the importance of an antibiotic treatment in improving the reproductive outcome at a subsequent IVF cycle.74, 75 Based on these findings, it is presumed that CE secondary to intrauterine microbial colonization could have a potential detrimental effect on the reproductive outcome and could warrant the early diagnosis of CE and necessary therapeutic management of patients who are suffering from repeated implantation or pregnancy failure.
12. CONCLUSION
The authors proposed for the first time a new concept called the “bacterial contamination hypothesis” in endometriosis and the involvement of the LPS/TLR4 cascade in the growth regulation of endometriosis. This study's results suggest that a substantial amount of endotoxin in PF related to the reflux of menstrual blood is involved in pelvic inflammation and could promote the TLR4‐mediated growth and progression of endometriosis. A diagrammatic representation of this new concept on endometriosis is shown in Figure 2. As endometriosis is a multifactorial disease, it should be remembered that a persistent cross‐talk between inflammation and ovarian steroids and the stress reaction occurs in the pelvic environment that can further induce the growth and progression of endometriosis. Although different literature until now claimed that targeting bacterial endotoxin or TLR4 and NF‐kB could be useful as a therapeutic strategy to suppress pelvic inflammation and the growth of endometriosis, their clinical application is questionable. The authors have learned from their continuous study for the last 15 years that, as an inflammatory mediator, LPS could be the initial trigger and bacterial contamination its source in the intrauterine environment that could be the primary cause in the growth regulation of endometriosis, either alone or in combination with ovarian steroids or a tissue stress reaction in the pelvis. The authors' most recent studies further indicated the occurrence of subclinical uterine infection and endometritis in women with endometriosis after GnRHa treatment. These findings could have some epidemiological and biological impact in better understanding the pathogenesis of endometriosis and its related disease burden. The worsening of intrauterine microbial colonization and a higher occurrence of endometritis in GnRHa‐treated women with endometriosis may hint to some future therapeutic potential in their management, as well as the prevention of the recurrence of endometriosis. If prevention is still better than cure, the intravaginal or oral application of some probiotics and/or antibiotics could offer some protection against subclinical vaginal or uterine infection, with a consequent improvement in the quality of life and fertility outcome of women who suffer from this enigmatic disease. Further studies are required to strengthen this study's proposed concept in the management of endometriosis.
Figure 2.
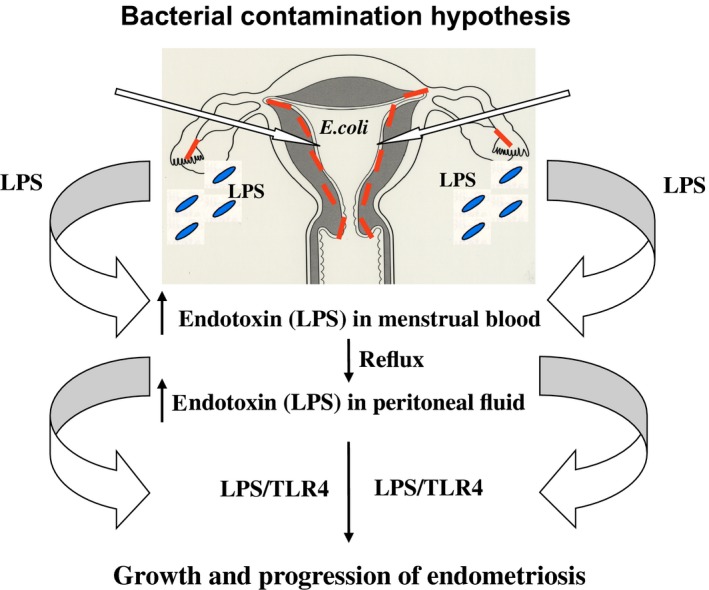
Diagrammatic presentation of the lipopolysaccharide (LPS)/Toll‐like receptor (TLR)4 cascade in the bacterial contamination hypothesis of endometriosis. The constant release of LPS from Escherichia coli (E. coli) contamination of the menstrual blood causes a higher endotoxin (LPS) concentration in the menstrual blood and consequently higher levels of endotoxin in the PF due to the retrograde flow of menstrual blood into the pelvis. Peritoneal macrophages, eutopic and ectopic endometrial epithelial cells, and epithelial stromal cells express TLR4, a receptor for LPS. The LPS/TLR4 complex induces pelvic inflammation and promotes the growth and progression of endometriosis via intracellular adaptor molecules and nuclear factor kappa‐light‐chain‐enhancer of activated B cells activation
CONFLICT OF INTEREST
The authors declare no conflict of interest. Human Rights Statement and Informed Consent: Approval from the Institutional Review Board of Nagasaki University, Nagasaki, Japan, was obtained for this study. Animal Studies: This article does not contain any study with animal participants that was performed by any of the authors.
ACKNOWLEDGEMENTS
We thank Kazumi Hayashida and Kyoko Ishida, Department of Obstetrics and Gynecology, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan, for their excellent technical assistance. The authors also thank all the doctors of Saiseikai Nagasaki Hospital, Nagasaki, Japan, for their kind assistance in collecting samples for this study.
Khan KN, Fujishita A, Hiraki K, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125–133. https://doi.org/10.1002/rmb2.12083
Funding information
This work was supported by Grants‐in‐Aid for Scientific Research Grant No. 24592474 and 15K10675 from the Japan Society for the Promotion of Science (to K. N. Khan)
REFERENCES
- 1. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49‐56. [DOI] [PubMed] [Google Scholar]
- 3. Khan KN, Kitajima M, Hiraki K, et al. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60:383‐404. [DOI] [PubMed] [Google Scholar]
- 4. Khan KN, Kitajima M, Hiraki K, et al. Toll‐like receptors in innate immunity: role of bacterial endotoxin and toll‐like receptor 4 (TLR4) in endometrium and endometriosis. Gynecol Obstet Invest. 2009;68:40‐52. [DOI] [PubMed] [Google Scholar]
- 5. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril. 2004;81:652‐661. [DOI] [PubMed] [Google Scholar]
- 6. Janeway CAJ. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1‐13. [DOI] [PubMed] [Google Scholar]
- 7. Janeway CAJ, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197‐216. [DOI] [PubMed] [Google Scholar]
- 8. Reichhart JM. TLR5 takes aim at bacterial propeller. Nat Immunol. 2003;4:1159‐1160. [DOI] [PubMed] [Google Scholar]
- 9. Takeda K, Akira S. Toll‐like receptors in innate immunity. Int Immunol. 2005;17:1‐14. [DOI] [PubMed] [Google Scholar]
- 10. Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Reviews. 2005;206:306‐335. [DOI] [PubMed] [Google Scholar]
- 11. Khan KN, Kitajima M, Hiraki K, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 2010;94:2860‐2863. [DOI] [PubMed] [Google Scholar]
- 12. Fazeli A, Bruce C, Anumba DO. Characterization of Toll‐like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372‐1378. [DOI] [PubMed] [Google Scholar]
- 13. Aflatoonian R, Tuckerman E, Elliot SL, et al. Menstrual cycle‐dependent changes of toll‐like receptors in endometrium. Hum Reprod. 2007;22:586‐593. [DOI] [PubMed] [Google Scholar]
- 14. Hirata T, Osuga Y, Hamasaki K, et al. Expression of Toll‐like receptor 2, 3, 4, and 9 in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74:53‐60. [DOI] [PubMed] [Google Scholar]
- 15. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci. 1998;95:588‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang D, Zhang G, Hayden MS, et al. A toll‐like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522‐1526. [DOI] [PubMed] [Google Scholar]
- 17. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutation in Tlr4 gene. Science. 1998;282:2085‐2088. [DOI] [PubMed] [Google Scholar]
- 18. Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram‐negative and gram‐positive bacterial cell wall components. Immunity. 1999;11:443‐451. [DOI] [PubMed] [Google Scholar]
- 19. Nasu K, Itoh H, Yugae A, Nishida M, Narahara H. Human oviductal epithelial cells express Toll‐like receptor 3 and respond to double‐stranded RNA: fallopian tube‐specific mucosal immunity against viral infection. Hum Reprod. 2007;22:356‐361. [DOI] [PubMed] [Google Scholar]
- 20. Kiechl S, Lorenz E, Reindl M, et al. Toll‐like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185‐192. [DOI] [PubMed] [Google Scholar]
- 21. Akira S, Takeda K. Toll‐like receptor signaling. Nat Rev Immunol. 2004;4:499‐511. [DOI] [PubMed] [Google Scholar]
- 22. Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll‐like receptor 4 (TLR4)‐deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749‐3752. [PubMed] [Google Scholar]
- 23. Wallin RPA, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren H‐G. Heat‐shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130‐135. [DOI] [PubMed] [Google Scholar]
- 24. McCoy CE, O'Neill LAJ. The role of Toll‐like receptors in macrophages. Front Biosci. 2008;13:62‐70. [DOI] [PubMed] [Google Scholar]
- 25. Parker LC, Prince LR, Sabroe I. Translational mini‐review series on Toll‐like receptors: networks regulated by Toll‐like receptors mediate innate and adaptive immunity . Clin Exp Immunol. 2007;147:199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akira S. Mammalian Toll‐like receptors. Curr Opin Immunol. 2003;15:5‐11. [DOI] [PubMed] [Google Scholar]
- 27. Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002;123:837‐845. [PubMed] [Google Scholar]
- 28. Larsen S, Galask RP. Vaginal microbial flora: practical and theoretic relevance. Obstet Gynecol. 1980;55:100S‐113S. [DOI] [PubMed] [Google Scholar]
- 29. Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL. TLRs mediate IFN‐γ production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219‐6224. [DOI] [PubMed] [Google Scholar]
- 30. Young SL, Lyddon TD, Jorgenson RL, Misfeldt ML. Expression of Toll‐like receptors in human endometrial epithelial cells and cell lines. Am J Reprod Immunol. 2004;52:67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirata T, Osuga Y, Hirota Y, et al. Evidence for the presence of Toll‐like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548‐556. [DOI] [PubMed] [Google Scholar]
- 32. Khan KN, Kitajima M, Hiraki K, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010;25:642‐653. [DOI] [PubMed] [Google Scholar]
- 33. Miura S, Khan KN, Kitajima M, et al. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum Reprod. 2006;21:2545‐2554. [DOI] [PubMed] [Google Scholar]
- 34. Khan KN, Masuzaki H, Fujishita A, et al. Regulation of hepatocyte growth factor by basal and stimulated‐macrophages in women with endometriosis. Hum Reprod. 2005;20:49‐60. [DOI] [PubMed] [Google Scholar]
- 35. Khan KN, Kitajima M, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Multifunctional role of hepatocyte growth factor (HGF) in the development of pelvic endometriosis. Am J Reprod Immunol. 2008;60:383‐404. [DOI] [PubMed] [Google Scholar]
- 36. Khan KN, Kitajima M, Imamura T, et al. Toll‐like receptor 4 (TLR4)‐mediated growth of endometriosis by human heat shock protein 70 (Hsp70). Hum Reprod. 2008;23:2210‐2219. [DOI] [PubMed] [Google Scholar]
- 37. Iba Y, Harada T, Horie S, Deura I, Iwabe T, Terakawa N. Lipopolysaccharide‐promoted proliferation of endometriotic stromal cells via induction of tumor necrosis factor α and interleukin‐8 expression. Fertil Steril. 2004;82(Suppl 3):1036‐1042. [DOI] [PubMed] [Google Scholar]
- 38. Alexander JW, Boyce ST, Babcock GF, et al. The process of microbial translocation. Ann Surg. 1990;212:496‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan KN, Kitajima M, Yamaguchi N, et al. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Hum Reprod. 2012;27:3417‐3424. [DOI] [PubMed] [Google Scholar]
- 40. Valore EV, Park CH, Quayle AJ, Wilee KR, McCray PB Jr, Ganz T. Human β‐defensin‐1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asea A, Krafft SK, Kurt‐Jones EA, et al. HSP70 stimulates cytokine production through a CD14‐dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435‐442. [DOI] [PubMed] [Google Scholar]
- 42. Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll‐like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028‐15034. [DOI] [PubMed] [Google Scholar]
- 43. Zugel U, Kaufmann SH. Immune response against heat‐shock proteins in infectious diseases. Immunobiology. 1999;201:22‐35. [DOI] [PubMed] [Google Scholar]
- 44. Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399‐415. [DOI] [PubMed] [Google Scholar]
- 45. Inoue T, Khan KN, Kitajima M, Hiraki H, Fujishita A, Masuzaki H. Cross‐talk between inflammation and stress reaction in Toll‐like receptor 4‐mediated growth of endometriosis. In: Proceedings of the 11th World Congress on Endometriosis. 2011:204.
- 46. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Higher activity by opaque endometriotic lesions than non‐opaque lesions in women with endometriosis. Acta Obstet Gynecol Scand. 2004;83:375‐382. [DOI] [PubMed] [Google Scholar]
- 47. Burney RO, Lathi RB. Menstrual bleeding from an endometriotic bleeding. Fertil Steril. 2009;91:1926‐1927. [DOI] [PubMed] [Google Scholar]
- 48. Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310‐318. [DOI] [PubMed] [Google Scholar]
- 49. Kajihara H, Yamada Y, Kanayama S, et al. New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signals. Gynecol Endocrinol. 2011;27:73‐79. [DOI] [PubMed] [Google Scholar]
- 50. Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones estradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13‐22. [DOI] [PubMed] [Google Scholar]
- 51. Herath S, Fischer FD, Werling D, et al. Expression and function of Toll‐like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147:562‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan KN, Masuzaki H, Fujishita A, et al. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20:2004‐2013. [DOI] [PubMed] [Google Scholar]
- 53. Khan KN, Kitajima M, Inoue T, Fujishita A, Nakashima M, Masuzaki H. 17β‐estradiol and lipopolysaccharide additively promote pelvic inflammation and growth of endometriosis. Reprod Sci. 2015;22:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan KN, Kitajima M, Hiraki K, et al. Combined effect of estrogen and endotoxin on pelvic inflammation and growth of endometriosis. In: 64th Proceedings of Acta Obstretica et Gynecologica Japonica 2012:813.
- 55. Cakmak H, Guzeloglu‐Kayisli O, Kayisli UA, Arici A. Immune–endocrine interactions in endometriosis. Front Biosci. 2009;E1:429‐443. [DOI] [PubMed] [Google Scholar]
- 56. Guijarro‐Munoz I, Comte M, Alvarez‐Cienfuegos A, Alvarez‐Vallina L, Sanz L. Lipopolysaccharide activates Toll‐like receptor4 (TLR4)‐mediated NF‐kB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289:2457‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang JH, Manning BJ, Wu QD, Blankson S, Bouchier‐Hayes D, Redmond HP. Endotoxin/lipopolysaccharide activates NF‐kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin‐dependent mechanism. J Immunol. 2003;170:795‐804. [DOI] [PubMed] [Google Scholar]
- 58. Rai V, Hopkisson J, Kennedy S, Bergqvist A, Barlow DH, Mardon HJ. Integrins alpha 3 and alpha 6 are differentially expressed in endometrium and endometriosis. J Pathol. 1996;180:181‐187. [DOI] [PubMed] [Google Scholar]
- 59. Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra‐uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum Reprod. 2014;29:2446‐2456. [DOI] [PubMed] [Google Scholar]
- 60. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan KN, Fujishita A, Masumoto H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69‐75. [DOI] [PubMed] [Google Scholar]
- 62. Takebayashi A, Kimura F, Kishi Y, et al. The association between endometriosis and chronic endometriosis. PLoS ONE. 2014;9:e88354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cicinelli E, Trojano G, Mastromauro M, et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil Steril. 2017;108:289‐295. [DOI] [PubMed] [Google Scholar]
- 64. D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Sem Reprod Med. 2003;21:243‐254. [DOI] [PubMed] [Google Scholar]
- 65. Tomassetti C, Meuleman C, Pexsters A, et al. Endometriosis, recurrent miscarriage and implantation failure: is there an immunological link? Reprod BioMed Online. 2006;13:58‐64. [DOI] [PubMed] [Google Scholar]
- 66. Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adamson GD, Pasta DJ. Surgical treatment of endometriosis‐associated infertility: meta‐analysis compared with survival analysis. Am J Obstet Gynecol. 1994;171:1488‐1504. [DOI] [PubMed] [Google Scholar]
- 68. Deb K, Chatturvedi MM, Jaiswal YK. Gram‐negative bacterial endotoxin‐induced infertility: a birds eye view. Gynecol Obstet Invest. 2004;57:224‐232. [DOI] [PubMed] [Google Scholar]
- 69. Kamiyama S, Teruya Y, Nohara M, Kanzawa K. Impact of detection of bacterial endotoxin in menstrual effluent on the pregnancy rate in in vitro fertilization and embryo transfer. Fertil Steril. 2004;82:788‐792. [DOI] [PubMed] [Google Scholar]
- 70. Fujita Y, Mihara T, Okazaki T, et al. Toll‐like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26:2799‐2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moreno I, Codoner FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684‐703. [DOI] [PubMed] [Google Scholar]
- 72. Johnston‐MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93:437‐441. [DOI] [PubMed] [Google Scholar]
- 73. Yang R, Du X, Wang Y, Song X, Yang Y, Qiao J. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet. 2014;289:1363‐1369. [DOI] [PubMed] [Google Scholar]
- 74. Cicinelli E, Matteo M, Tinelli R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30:323‐330. [DOI] [PubMed] [Google Scholar]
- 75. Bouet PE, El Hachem H, Monceau E, Gariepy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105:106‐110. [DOI] [PubMed] [Google Scholar]


