Abstract
Acquired thermotolerance is a complex physiological phenomenon that enables plants to survive normally lethal temperatures. This study characterizes the temperature sensitivity of Arabidopsis using a chlorophyll accumulation bioassay, describes a procedure for selection of acquired thermotolerance mutants, and provides the physiological characterization of one mutant (AtTS02) isolated by this procedure. Exposure of etiolated Arabidopsis seedlings to 48°C or 50°C for 30 min blocks subsequent chlorophyll accumulation and is eventually lethal. Arabidopsis seedlings can be protected against the effects of a 50°C, 30-min challenge by a 4-h pre-incubation at 38°C. By the use of the milder challenge, 44°C for 30 min, and protective pretreatment, mutants lacking components of the acquired thermotolerance system were isolated. Putative mutants isolated by this procedure exhibited chlorophyll accumulation levels (our measure of acquired thermotolerance) ranging from 10% to 98% of control seedling levels following pre-incubation at 38°C and challenge at 50°C. The induction temperatures for maximum acquired thermotolerance prior to a high temperature challenge were the same in AtTS02 and RLD seedlings, although the absolute level of chlorophyll accumulation was reduced in the mutant. Genetic analysis showed that the loss of acquired thermotolerance in AtTS02 was a recessive trait. The pattern of proteins synthesized at 25°C and 38°C in the RLD and AtTS02 revealed the reduction in the level of a 27-kD heat shock protein in AtTS02. Genetic analysis showed that the reduction of this protein level was correlated with the acquired thermotolerance phenotype.
Plants experience high air and soil temperatures during periods of drought and when fields receive limited irrigation. Elevated plant temperatures that occur under these conditions negatively impact plant health and productivity as exemplified by changes in metabolism such as the selective destabilization of secretory protein mRNAs in barley aleurone (Brodl and Ho, 1991, 1992), the disruption of cap and poly(A) tail function during translation (Gallie et al., 1995), induction of shortening of barley primary leaves and coleoptile length (Beator et al., 1992), and elevation of the level of xanthophyll lutein in dark-grown pea plantlets; and by changes in other processes such as induction of circadian rhythmicity and changes in morphogenesis (Otto et al., 1992). Plants, like all organisms, respond to an elevation in temperature by the synthesis of heat shock proteins (HSPs) (for review, see Vierling, 1991). The appearance of plant HSPs is strongly correlated to the development of a condition termed “acquired thermotolerance.” Acquired thermotolerance is induced by pre-exposure to elevated but non-lethal temperatures and leads to enhanced protection of plant cells from subsequent heat-induced injury. Although the correlation between the development of acquired thermotolerance and the appearance of HSPs is strong, a cause-and-effect relationship between the two has been difficult to demonstrate, even with our extensive knowledge of the functions of individual HSPs. To understand the relationship between HSPs and acquired thermotolerance, mutations would be required that result in a coordinate change in the expressions of HSPs. Such mutants would allow for the study of: (a) the signal pathway from heat stress to gene activation; (b) the mechanism of transcriptional regulation of HSP genes; and (c) the role of HSPs in thermotolerance (Schöffl et al., 1998). To date, the mutational analysis of HSPs has been limited to organisms other than higher plants although plant HSPs have been investigated in heterologous systems. The HSP104 gene of yeast has been demonstrated to play an essential role in thermotolerance by virtue of the fact that HSP104-deficient yeast is unable to acquire thermotolerance (Sanchez and Lindquist, 1990). Plant homologs GmHsp101 of soybean (Lee et al., 1994) and AtHsp101 of Arabidopsis (Schirmer et al., 1994) are capable of complementing the HSP104 deficient mutation in yeast providing strong evidence that there may also be a strong link between this HSP and acquired thermotolerance in plants. In a similar study, bacterial thermotolerance was enhanced by the synthesis of a plant 16.9-kD HSP (Yeh et al., 1997). The strongest evidence for a direct link between HSP expression and thermotolerance comes from the demonstration that the constitutive expression of a heat shock transcription factor increased the level of thermotolerance in Arabidopsis without prior exposure to elevated temperatures (Lee et al., 1995; Prändl et al., 1998). Thus, although the importance of different HSPs in acquired thermotolerance may vary between organisms (Parsell et al., 1993), the literature strongly supports the of involvement of HSPs in thermotolerance and underscores the need for mutants (and the likelihood that such mutants will involve HSP genes).
To obtain useful mutants it is necessary to develop a rapid and straight forward screen for alterations in the level of acquired thermotolerance. Plant regrowth, electrolyte leakage, and 2,3,5-triphenyltetrazolium chloride reduction are commonly used procedures for evaluating thermotolerance (Wu and Wallner, 1983) but all three present problems for screening large numbers of plants for mutant identification. Wu and Wallner (1983) in comparing electrolyte leakage, triphenyltetrazolium chloride reduction, and cellular regrowth of pear cell cultures concluded that only regrowth tests were acceptable for assessing heat injury to cultured plant cells. Electrolyte leakage and triphenyltetrazolium chloride reduction detected heat induced injury only at temperatures 6°C to 8°C above temperatures identified as injurious by cell regrowth tests. Although the observations of Wu and Wallner clearly addressed the limitations of these two techniques, their usage continued throughout the past decade due to the lack of acceptable alternatives. Plant regrowth, although a sensitive assay for heat tolerance, is limited by the length of time required to detect an affect and is logistically impractical for mutational analysis and screening of transgenic plants. Burke (1994), however, has developed a simple, species-non-specific, reliable, and accurate protocol for the quantification of thermotolerance. This protocol is based on the inhibition of chlorophyll accumulation in etiolated tissue by challenges at lethal temperatures and the prevention of this inhibition by pre-incubation at a non-lethal elevated temperature; i.e. acquired thermotolerance. The usefulness of this protocol for the detection of acquired thermotolerance was confirmed in a study characterizing acquired thermotolerance in soybean (Burke, 1998), and it is this bioassay that we have chosen to use in the screening for and characterization of Arabidopsis thermotolerance mutants.
We believe that the isolation and characterization of higher plant mutants is needed to advance our understanding of the role of individual HSPs in providing acquired thermotolerance. In this report we detail the temperature characteristics of acquired thermotolerance in Arabidopsis seedlings, the isolation of thermotolerance mutants using the bioassay described by Burke (1994), and, as a means of demonstrating the usefulness of the screen, the detailed characterization of an Arabidopsis mutant (AtTS02) that is deficient in its ability to acquire thermotolerance. We also provide evidence that the loss of acquired thermotolerance in this mutant is correlated with the loss of a 27-kD HSP.
RESULTS
Optimal Conditions to Assay Acquired Thermotolerance in Arabidopsis Seedlings
To develop a thermotolerance screening procedure using the inhibition of chlorophyll accumulation as described by Burke (1994) for Arabidopsis it was necessary to assess the characteristics of acquired thermotolerance in this species.
Determination of the Temperature for Maximum Chlorophyll Accumulation
The temperature that allowed maximum chlorophyll accumulation was determined by exposing etiolated Arabidopsis (Columbia) seedlings to a series of temperatures during a 24-h exposure to continuous light (Fig. 1A). The peak of chlorophyll accumulation occurred at 25°C and similar patterns of chlorophyll accumulation were observed for RLD and C24 ecotypes (data not shown).
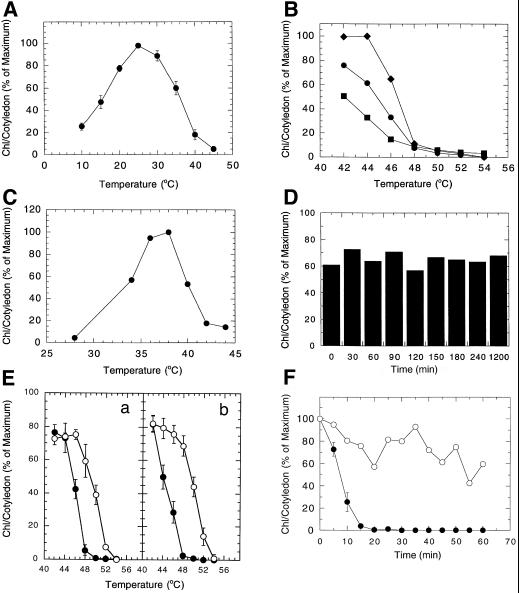
Figure 1.
A, Temperature optimum for chlorophyll accumulation in cotyledons of Arabidopsis (Columbia) seedlings. B, Challenge temperatures for 30 min that inhibit chlorophyll accumulation at 25°C in cotyledons of Arabidopsis (C24) seedlings. Chlorophyll levels determined at 24 h (▪), 48 h (●), and 72 h (♦) following the 30-min temperature challenge. C, Pre-incubation temperatures that induce acquired thermotolerance of chlorophyll accumulation in cotyledons of Arabidopsis (C24) seedlings to a previously lethal 48°C challenge. D, Duration of acquired thermotolerance for chlorophyll accumulation in cotyledons of Arabidopsis seedlings pre-incubated at 38°C for 4 h and challenged at 48°C at specified time periods. E, Effectiveness of 30-min temperature challenges on reducing the level of chlorophyll accumulation in control (●) and 4-h, 38°C pre-incubated (○) cotyledons of RLD (a) and Columbia (b) Arabidopsis seedlings. F, Time course of injury to chlorophyll accumulation in control (●) and 4-h, 38°C pre-incubated (○) cotyledons of Arabidopsis seedlings resulting from a 50°C challenge.
Challenge and Pre-Incubation Temperature Determination
The temperature-induced inhibition of subsequent chlorophyll accumulation at 25°C in Arabidopsis (C24) seedlings was determined by exposing etiolated seedlings to challenge temperatures between 44°C and 56°C in the dark for 30 min prior to the 25°C, 24-h light treatment. Chlorophyll accumulation was prevented by a 30-min challenge at 48°C, or above (Fig. 1B). Light exposures of 24, 48, and 72 h following the temperature challenge revealed significant chlorophyll accumulation at challenge temperatures below 48°C indicating that these temperatures are sublethal, and no accumulation at 48°C or above (lethal temperature challenges). Depending on the severity of the challenge that was desired, temperatures of 48°C or 50°C for 30 min were used in subsequent experiments to inhibit chlorophyll accumulation.
The temperature at which thermotolerance is acquired in Arabidopsis was evaluated by incubating Arabidopsis (C24) seedlings at set temperatures in the dark for 4 h prior to the 30-min, 48°C challenge (Fig. 1C). The maximum levels of chlorophyll accumulation were observed in seedlings pre-incubated at 36°C and 38°C, with the 38°C pre-incubation providing the highest level of chlorophyll accumulation and thus thermal protection. Maximal thermal protection was also obtained with a 38°C, 4-h pre-incubation for RLD and Columbia ecotypes (data not shown).
Duration and Magnitude of Acquired Thermotolerance
The stability of chlorophyll accumulation (namely acquired thermotolerance) in the 4-h, 38°C pre-incubated Arabidopsis seedlings was determined by delaying the 30-min 48°C challenge for increasing lengths of time. Chlorophyll accumulation levels remained constant, at about 60% to 70% of control chlorophyll accumulation, throughout a 20-h evaluation period suggesting a stable acquired thermotolerance level (Fig. 1D). These results differ from those reported for soybean cotyledons where the pattern of chlorophyll accumulation declined by approximately 50% over the 20-h period following the initial pre-incubation (Burke, 1998).
To determine how much protection was afforded by the 38°C pre-incubation, the pattern of chlorophyll accumulation following a 30-min challenge at a range of elevated temperatures was evaluated in RLD and Columbia ecotypes with and without the 4-h, 38°C pre-incubation. Chlorophyll levels were maximum at the minimum challenge temperature of 42°C for both RLD (Fig. 1Ea) and the Columbia (Fig. 1Eb) ecotypes when challenged without the 38°C pre-incubation. Chlorophyll accumulation was significantly inhibited by increased challenge temperatures, reaching minimum accumulation levels following challenges at 48°C to 50°C. Pre-incubation for 4 h at 38°C (white circles) provided increased protection in both ecotypes to approximately the same level. RLD seedlings accumulated chlorophyll levels to approximately 75% of control levels with challenges of 42°C, 44°C, and 46°C. Chlorophyll accumulation declined with increasing challenge temperatures between 48°C and 54°C. Columbia seedlings accumulated chlorophyll to approximately 80% of control levels with challenges of 42°C, 44°C, and 46°C. Chlorophyll accumulation declined to minimal levels with the 54°C challenge in a pattern similar to the RLD seedlings. The magnitude of acquired thermotolerance in 38°C pre-incubated seedlings of both ecotypes provided chlorophyll accumulation at temperatures 2°C to 6°C higher than seedlings that had not received the 38°C pre-incubation.
Additional studies evaluated the effectiveness of the 4-h, 38°C pre-incubation temperature in providing protection against a 50°C challenge temperature over a range of challenge times. The time course for the loss of the chlorophyll accumulation following a 50°C challenge temperature on Arabidopsis (Columbia) seedlings with (white circles) and without (black circles) the 38°C pre-incubation is provided in Figure 1F. The time required to block subsequent chlorophyll accumulation in untreated control seedlings was reduced from 30 min for a 48°C (Fig. 1B) challenge to 20 min for a 50°C challenge (Fig. 1F). The 4-h, 38°C pre-incubation provided a relatively stable protection level up to 1 h (white circles), well beyond the 20 min shown to block chlorophyll accumulation (black circles) in control seedlings.
Correlation between the Induction of Acquired Thermotolerance and HSP Synthesis
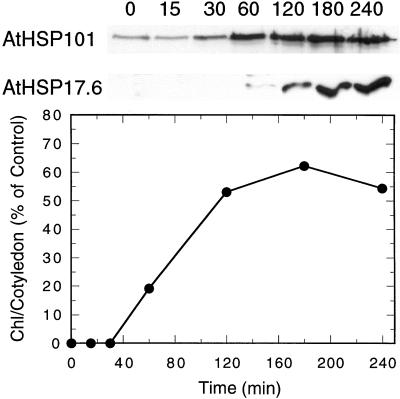
To determine if the pattern of HSP synthesis in Arabidopsis correlated with acquired thermotolerance as measured by the chlorophyll accumulation bioassay we compared chlorophyll accumulation with the accumulation of AtHSP101 and AtHSP17.6 under the temperature regime designed to induce acquired thermotolerance as determined in our previous experiments (Fig. 2). The two individual HSPs were identified with monospecific antibodies kindly provided by Dr. Elizabeth Vierling from the University of Arizona. Chlorophyll, measured after 24 h of light exposure at 25°C, accumulated in seedlings pre-incubated at 38°C for 60 min or longer prior to the 48°C 30-min challenge. The highest levels of chlorophyll were seen in cotyledons that received 120, 180, and 240 min of 38°C pre-incubation. Accumulation of AtHSP101 was low at 0 and 15 min of pre-incubation, increasing after 30 min to high levels. AtHSP17.6 was not apparent until after 60 min of pre-incubation at 38°C and continued to increase to 240 min. The data clearly demonstrate a close correlation between acquired thermotolerance, as measured using the chlorophyll accumulation bioassay, and HSP synthesis in Arabidopsis.
Figure 2.
The time course of induction of the HSPs (AtHSP101 and AtHSP17.6) and acquired thermotolerance in cotyledons of Arabidopsis seedlings during a 38°C pre-incubation. HSP induction was observed by western analysis, whereas acquired thermotolerance induction was measured by the ability to accumulate chlorophyll after a 30-min 48°C challenge.
Screening for Acquired Thermotolerance Mutants
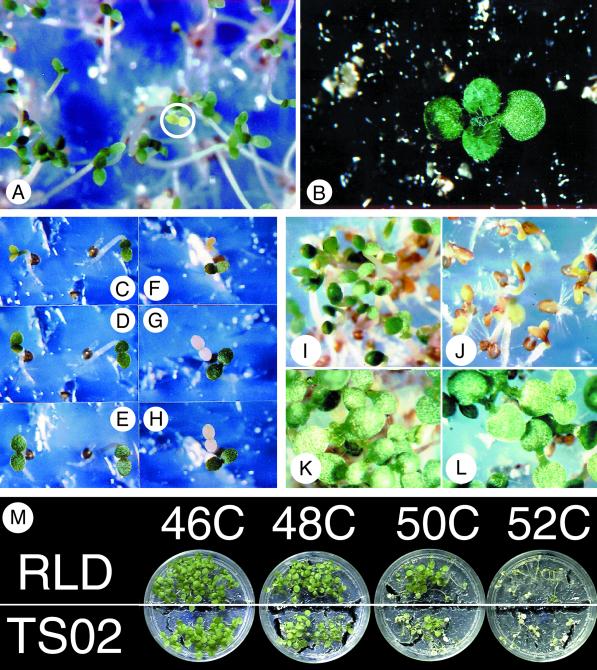
Using the time and temperature parameters established for the chlorophyll accumulation bioassay of wild-type RLD, we screened M2 RLD Arabidopsis seeds from plants derived from ethyl methane sulfonate treated seeds to isolate mutants lacking all or some of the components of the acquired thermotolerance system. To recover putative mutants, a 44°C, 30-min challenge was used in place of the 48°C, 30-min treatment. This reduced challenge temperature still allowed visible detection of thermotolerance mutants due to reduced chlorophyll accumulation (Fig. 1E), but also allowed the chlorophyll levels to recover following 72 h of continuous light (Fig. 1B) aiding the recovery of the mutant for seed production (Fig. 3, A and B). Figure 3, C to H, shows the accumulation of chlorophyll in the cotyledons of RLD mutants selected as putative acquired thermotolerance mutants. Two seedlings are shown in each frame, one identified as a putative mutant, and the second showing the protection of chlorophyll accumulation by the 4-h, 38°C pre-incubation. Figure 3, C to E, shows seedlings after 24, 48, and 72 h of continuous light following a 4-h, 38°C pre-incubation and 30-min, 44°C challenge. The seedling on the right shows normal chlorophyll levels, exhibited by the green cotyledons after 24 h of continuous light. The seedling on the left was light green after 24 h and accumulated chlorophyll to control levels within 72 h. Those mutants that did not have altered components of the acquired thermotolerance pathway but had an altered pigment system were white, yellow, or light green even when exposed to continuous light for 72 h (Fig. 3, F–H) in contrast to a protected seedling exhibiting high chlorophyll levels within 24 h of light exposure shown in the lower right portion of frames Figure 3, F to H.
Figure 3.
Seedlings of EMS treated RLD Arabidopsis during the screening and selection process. A, Putative acquired thermotolerance mutant challenged at 44°C for 30 min identified by reduced chlorophyll accumulation (white circle) despite a 38°C for 4 h pre-incubation and a 16-h light exposure at 25°C. B, Chlorophyll accumulation in the putative acquired thermotolerance mutant shown in A. C, D, and E, A Putative acquired thermotolerance mutant (left) and a thermotolerant seedling (right) after 24, 48, and 72 h of continuous light respectively. F, G, and H, A Pigment mutant (top) and a thermotolerant seedling (bottom) after 24, 48, and 72 h of continuous light respectively. I, RLD seedlings pre-incubated at 38°C for 4 h and challenged at 46°C for 30 min. J, AtTS02 seedlings pre-incubated at 38°C for 4 h and challenged at 46°C for 30 min. RLD (K) and AtTSO2 (L) seedlings pre-incubated at 38°C for 4 h, challenged at 46°C for 30 min, and allowed to accumulate chlorophyll for 6 d. M, Light-grown RLD and AtTS02 seedlings 48 h after a 4-h, 38°C pre-incubation and 30-min challenge at 46°C, 48°C, 50°C, or 52°C.
Putative acquired thermotolerance mutants, those that were yellow or light green after 24 h of light and subsequently accumulated high chlorophyll levels (Fig. 3, C–E), were recovered. Using this screening procedure we isolated 36 putative acquired thermotolerance mutants, 11 of which subsequently died following transplanting to soil. The surviving mutants were allowed to self-fertilize and their seeds were further evaluated for the acquired thermotolerance mutant phenotype.
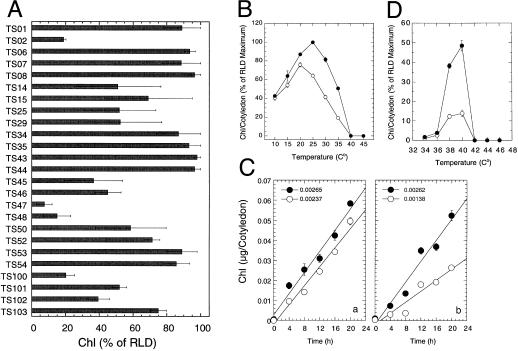
Figure 4A presents a bar graph representation of the results of our initial screen of the seeds produced by our selected lines. Those lines having the greatest expression of the mutant phenotype under the initial screen were advanced for further characterization. On average, based upon our analysis of over 5 g of EMS mutagenized Arabidopsis seeds, we obtained one putative acquired thermotolerance deficient mutant for every 5,000 to 6,000 seeds analyzed.
Figure 4.
A, Levels of acquired thermotolerance in several M3 populations of putative acquired thermotolerance mutants in Arabidopsis. Individual bars represent the level of chlorophyll (Chl) accumulation as percentage of RLD control values following a 4-h pre-incubation at 38°C and a 30-min challenge at 50°C. B, Temperature optimum for chlorophyll accumulation in cotyledons of RLD (●) and AtTS02 (○) Arabidopsis seedlings. C, Chlorophyll accumulation at 20°C (a) and 28°C (b) in RLD (●) and AtTS02 (○) seedlings. The rates of chlorophyll accumulation in μg cotyledon−1 h−1 are presented above the respective panels. D, Pre-incubation temperatures that induce acquired thermotolerance of chlorophyll accumulation in cotyledons of RLD (●) and AtTS02 (○) Arabidopsis seedlings to an otherwise lethal 48°C challenge.
Characterization of the Acquired Thermotolerance Deficient Mutant: AtTS02
Determination of the Temperature for Maximum Chlorophyll Accumulation
To validate that our screen did indeed identify mutants in acquired thermotolerance we undertook a more detailed evaluation of the temperature characteristics of one of the first lines identified, AtTS02.
A comparison of the temperature response curve for chlorophyll accumulation in AtTS02 and RLD is shown in Figure 4B. Chlorophyll levels in the RLD seedlings increased with temperature from 10°C to 25°C and then declined to minimal levels at 40°C. Chlorophyll levels accumulated to maximum levels at 20°C in AtTS02 and declined with increasing temperatures. The maximum chlorophyll level in AtTS02 was approximately 90% of the level seen for RLD at 20°C. AtTS02 only accumulated chlorophyll to 64% of the RLD level at 25°C and the differential in chlorophyll levels between RLD and AtTS02 increased with increasing temperature. The chlorophyll accumulation data presented in Figure 4B provides a static picture of the endpoint for chlorophyll accumulation. The question raised was whether the low chlorophyll level in AtTS02 at 25°C (compared to RLD) was the result of a difference in the rate of chlorophyll accumulation or was due to a similar rate of accumulation between the two lines with the AtTS02 chlorophyll level peaking before the RLD. To address this question, a study evaluating the time course of chlorophyll accumulation in AtTS02 and RLD was performed at 20°C and 28°C. The rate of chlorophyll accumulation in AtTS02 at 20°C (Fig. 4Ca, white circles) was similar to that of RLD (black circles). The RLD seedlings accumulated more chlorophyll than the AtTS02 during the first 4 h, but exhibited similar rates of chlorophyll accumulation thereafter. The rate of chlorophyll accumulation in RLD seedlings at 28°C (Fig. 4Cb) was approximately twice that of the AtTS02 seedlings across all time points. Comparison of chlorophyll accumulation rates between 20°C and 28°C revealed a slight reduction in accumulation within RLD seedlings at the warmer temperature. The comparison of AtTS02 seedlings across the same two temperatures showed a 2-fold reduction in chlorophyll accumulation at the warmer temperature. Having identified the reduced rate of chlorophyll accumulation at 28°C in AtTS02, subsequent light treatments were at or near 20°C to minimize the treatment differences in chlorophyll accumulation.
Pre-Incubation Temperature Determination
A comparison of the effectiveness of pre-incubation temperatures in protecting the RLD and AtTS02 seedlings from a subsequent 50°C challenge is shown in Figure 4D. The RLD seedlings showed increased chlorophyll accumulation (our measure of thermotolerance) during the 20°C light treatment when pre-incubated at 40°C prior to the 50°C challenge. A pre-incubation temperature of 38°C also showed significant chlorophyll accumulation with values reaching 80% of that observed for the 40°C pre-incubated seedlings. The level of chlorophyll accumulation was negligible in seedlings that had been pre-incubated at 34°C, 36°C, 42°C, 44°C, or 46°C, thereby suggesting insufficient induction of thermotolerance outside the 38°C to 40°C range to protect against the more severe challenge imposed by a 50°C, 30-min treatment. AtTS02 seedlings (white circles) also showed maximum chlorophyll accumulation when pre-incubated at 40°C, although the absolute level of chlorophyll accumulation was only 29% of that obtained in the RLD seedlings.
Genetic Analysis
Since chlorophyll accumulation was preserved in RLD seedlings incubated for 4 h at 38°C before they were challenged for 30 min at 48°C to 50°C and the mutant AtTS02 was more sensitive to the 48°C to 50°C challenge temperatures than RLD despite the pre-incubation for 4 h at 38°C, we decided to investigate the genetic basis for the observed temperature sensitivity. An M7 population of the AtTS02 line was evaluated for the characteristic phenotype of the mutant. Seedlings were pre-incubated at 38°C for 4 h and challenged at 46°C for 30 min. The parent RLD seedlings accumulated high chlorophyll levels (Fig. 3I), whereas the AtTS02 showed the characteristic inhibition of chlorophyll accumulation observed in the original selection (Fig. 3J). Because of the use of the non-lethal 46°C challenge temperature, the AtTS02 mutant was able to recover from this high temperature inhibition (Fig. 3L) and continued to grow and develop like the RLD seedlings (Fig. 3K).
The inheritance of the mutant phenotype observed in AtTS02 was evaluated by performing crosses between an M8 population of AtTS02 and wild-type RLD plants. The F1 progeny were evaluated for the acquired thermotolerance phenotype. The chlorophyll levels observed in F1 progeny were the same as the wild-type RLD seedlings. F1 seedlings were allowed to self-fertilize and F2 seedlings were evaluated for the mutant phenotype. When AtTS02 was used as the maternal parent, the phenotype segregated with 108 individuals having the RLD phenotype and 42 individuals having the AtTS02 phenotype. When RLD was the maternal parent, 79 individuals showed the RLD phenotype and 26 individuals showed the AtTS02 phenotype. The segregation pattern of the mutant phenotype suggested a single, recessive, nuclear-encoded Mendelian trait.
Two-Dimensional Gel Protein Analysis
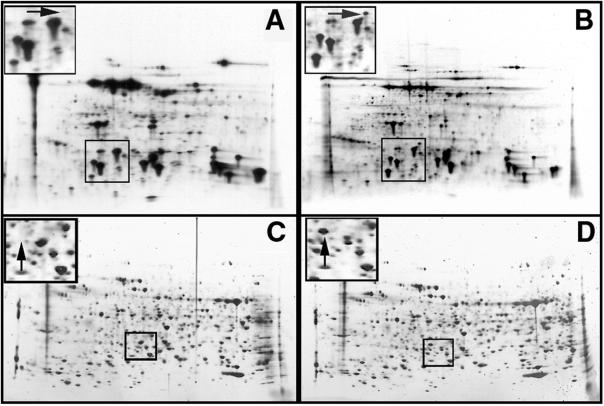
Once it had been established that the AtTS02 line was deficient in the ability to acquire thermotolerance compared to the wild-type RLD Arabidopsis, we were interested in determining if this phenotype could be linked to a concomitant change in the synthesis of HSPs. To accomplish this, RLD seedlings and AtTS02 seedlings were treated in the dark at 22°C and 38°C for 4 h in the presence of 35S-labeled amino acid mix. Labeled proteins were separated by two-dimensional gel electrophoresis and detected by fluorography. Protein patterns for RLD and AtTS02 treated at 22°C were qualitatively identical and only minor comparative quantitative differences could be determined (data not shown). The protein patterns from each line are also essentially identical following a 4-h, 38°C pre-incubation (heat shock) treatment, with one notable exception. A low-Mr putative HSP is consistently absent in the heat-shocked AtTS02 line, as evidenced by the absence of this protein in the AtTS02, 38°C fluorograph presented in Figure 5A (left panel) compared with RLD 38°C (Fig. 5B). This protein has an apparent molecular mass of 27 kD, and a pI value of approximately 5. This protein is considered a HSP as it is synthesized during the heat shock treatment, and is not detectable in the control treated at 22°C. The loss of the 27-kD protein is the only consistent and reproducible change in the pattern of proteins synthesized by AtTS02 in response to heat shock when compared to control patterns. To further study the relationship between the absence of the 27-kD protein and the mutant phenotype, homozygous dominant, heterozygous, and homozygous recessive individuals were identified from backcrossed material. Protein analysis of these individuals demonstrated that the absence of the 27-kD HSP was always associated only with the mutant phenotype (homozygous recessive seedlings). The results of this analysis are shown in Figure 5C (heat-shocked homozygous AtTS02) and Figure 5D (heat-shocked RLD).
Figure 5.
Autoradiographs (A and B) and silver stains (C and D) of 2D-PAGE electrophoresis separation of proteins from AtTS02 (A and C) and RLD (B and D) seedlings following pre-incubation at 38°C for 4 h to induce acquired thermotolerance. Insets represent magnified sections of the gels showing the absence of a 27-kD protein in the AtTS02 seedlings (A and C, arrow) compared with the RLD seedlings (B and D, arrow).
Characterization of Whole Plant Acquired Thermotolerance
The chlorophyll accumulation assay has proven to be useful, not only in identifying acquired thermotolerance (Burke, 1994, 1998), but also in allowing for the identification and recovery of acquired thermotolerance deficient mutants. Because the phenotype that this procedure uses is based upon chlorophyll accumulation, it is possible that the observed temperature characteristics are unique to that pathway and may not reflect the true temperature characteristics of the entire plant. To evaluate this possibility, we compared the temperature sensitivity of the RLD and AtTS02 lines determined by the chlorophyll accumulation assay with a different measure of thermotolerance. The measure of thermotolerance that we chose for comparison was the viability of light grown seedlings 2 d after a 4-h, 38°C pre-incubation and 30-min challenge at 46°C, 48°C, 50°C, or 52°C. The results of the viability test are shown in Figure 3M. RLD seedlings that had an activated acquired thermotolerance system maintained high chlorophyll levels and seedling turgor following the 46°C, 48°C, and 50°C challenges. The 52°C challenge was sufficient to overwhelm the protection system and result in RLD seedling death. AtTS02, on the other hand, maintained high chlorophyll levels and seedling viability following the 46°C treatment, but showed a loss of chlorophyll and viability at challenge temperatures of 48°C and above. Some seedling death was apparent at 48°C, with an increasing number of seedlings dying at 50°C, and all of the seedlings dying at 52°C. The 2°C to 4°C difference in protection between RLD and AtTS02 observed in the chlorophyll accumulation assay, clearly translates to a similar difference in survival of the whole plant.
DISCUSSION
In this study we have developed and utilized a protocol for the characterization of the thermal sensitivity of Arabidopsis seedlings to isolate and characterize mutants that are deficient in the process that underlies the trait of acquired thermotolerance. We have also demonstrated a link between the synthesis of HSPs and acquired thermotolerance in one mutant line AtTS02.
The protocol we describe is based on the ability of etiolated tissues to synthesize and accumulate chlorophyll in the light at optimal temperatures following exposure to an elevated temperature. This bioassay was first developed to investigate the temperature sensitivity and heat shock response in cucumbers (Burke, 1994), and later as an assay of acquired thermotolerance in soybean (Burke, 1998). We chose chlorophyll as a screening tool based upon previous experience with the chlorophyll a/b light harvesting pigment protein complex of photosystem II (Burke et al., 1978). Because this complex is encoded in the nucleus, translated in the cytoplasm, and transported to the chloroplast thylakoid, it is a candidate for high temperature injury. Previous work on temperature optima in plants established that accumulation of this complex tracked total chlorophyll accumulation in the plant and therefore chlorophyll levels could be used directly for monitoring temperature sensitive changes (Burke and Oliver, 1993).
To utilize this bioassay for the isolation of Arabidopsis mutants deficient in (or more proficient in) acquiring thermotolerance it was necessary to identify optimum temperature and time parameters for the wild type that yield maximum chlorophyll accumulation, injury, and acquisition of thermotolerance. The temperature optimum for chlorophyll accumulation in etiolated 2-d-old Arabidopsis seedlings exposed to continuous light at 115 μmol m−2 s−1 was 25°C, while exposure of the same seedlings to 48°C or 50°C for 30 min blocked chlorophyll accumulation upon subsequent exposure to light at 25°C and was eventually lethal. However, a milder treatment, 44°C for 30 min, simply delayed chlorophyll accumulation but was not lethal allowing recovery of putative mutants. Arabidopsis seedlings were protected against the effects of both the 44°C and 50°C 30-min challenge by a 4-h pre-incubation at 38°C. These thermal characteristics were identical for each of the Arabidopsis ecotypes tested: Columbia, C24, and RLD. The values obtained in this study for temperature sensitivity, challenge temperatures, and protective pretreatments using chlorophyll accumulation as a measure of cellular damage are consistent with other Arabidopsis studies (Hugly et al., 1989; Kunst et al., 1989).
The sensitivity of Arabidopsis to high temperature exposure was highlighted by Binelli and Mascarenhas (1990). They observed that Arabidopsis seedlings exposed to 37°C for 2 h and returned to 23°C showed no apparent short- or long-term damage, whereas seedlings exposed to a 42°C treatment for 2 h showed no apparent immediate damage; but 48 h after returning to 23°C severe damage symptoms were visible, and after 96 h all of the seedlings were dead. Using high temperature challenges of shorter duration we demonstrated that challenges of 48°C for 30 min prevented chlorophyll accumulation at 25°C resulting in seedling death. Seedlings challenged at temperatures below 48°C for 30-min periods did recover from the initial injury and developed normally thereafter. We also demonstrated that a 50°C challenge only required a 20-min incubation to provide the same level of injury showing that both challenge temperature and time of exposure determined the level of injury.
Throughout the literature, the appearance of HSPs is strongly correlated to the development of acquired thermotolerance. In soybean induction of the heat shock response is rapid with a change in protein synthesis patterns occurring within the first 30 min following a shift from 30°C to 41°C. The production of HSPs becomes a substantial portion of the total protein synthesis over the next 3 to 4 h (Key et al., 1985). Analysis of acquired thermotolerance in soybean cotyledons via the chlorophyll bioassay showed a time course of development similar to that of HSP synthesis (Burke, 1998). The heat shock response in Arabidopsis has been characterized by many laboratories (for reviews, see Vierling, 1991; Nover, 1997; Schöffl et al., 1999). In this study, evaluation of the pre-incubation temperatures contributing to the acquired thermotolerance of chlorophyll accumulation (Fig. 1C) revealed protection across the 35°C to 40°C temperature range with 36°C and 38°C providing the greatest protection. In Arabidopsis leaves incubated at 37°C HSP111, 90, 76 and 22 were shown to be induced within 30 min, and HSP 19 and 27 induced within 60 min (Wu et al., 1988). This is consistent with results presented here, which demonstrate that the induction of acquired thermotolerance correlated with increased levels of AtHSP101 after 30 min, and the appearance of AtHSP17.6 after 60 min at 38°C (Fig. 5).
Using the time and temperature parameters established for the wild type, we screened for mutants that exhibited reduced chlorophyll accumulation (deficient in acquired thermotolerance), despite a 4-h, 38°C pre-incubation. To date, more than 60% of the putative mutants identified during the initial screen maintain a reduced acquired thermotolerance level compared with control seedlings (Fig. 4A). The relative protection levels as measured by the level of chlorophyll accumulation following a pre-incubation and challenge temperature range from 10% (TS47) to 50% of control levels (TS14, TS15, TS25, TS29, TS45, TS46, TS50, TS101, and TS102). It is important to remember that these are values obtained from seeds following self-fertilization of the identified F2 seedling, and therefore represent a possible composite of a series of distinct mutations within an isolate. Therefore backcrosses against the wild-type parents are essential. We have characterized one of the first mutants identified (AtTS02), and demonstrate that the phenotype segregates in a manner representative of a single recessive trait.
The temperature sensitivity of chlorophyll accumulation in AtTS02 compared with the RLD parent ecotype (Fig. 4B) showed that similar levels of chlorophyll were detected at 20°C and below, whereas the mutant exhibited reduced chlorophyll accumulation above 20°C compared to the RLD. Differential temperature sensitivity of chlorophyll accumulation in Arabidopsis mutants is not unique to this study. Markwell and Osterman (1992), in a study of temperature-sensitive phenotypic plasticity in chlorophyll-deficient mutants of Arabidopsis, showed that several individuals exhibited greater chlorophyll accumulation at 20°C compared with 26°C, and that the degree of phenotypic plasticity in response to growth temperature was not strictly correlated with chlorophyll content at the growth temperature used for the initial screening. They also noted a marked temperature modulation of plant height in a number of dwarf mutants, and concluded that temperature sensitivity will prove to be common in mutants affected in pathways other than chlorophyll biosynthesis. The reduction in AtTS02 chlorophyll accumulation at 28°C compared with the RLD parent raises several questions. One question is whether this mutant is in fact an acquired thermotolerance deficient mutant or simply a temperature sensitive mutant not associated with the inducible high temperature protection system. To determine this, we evaluated RLD and AtTS02 plants grown at 20°C where similar growth habits were observed and similar chlorophyll accumulation rates were observed (Fig. 4Ca). Comparative experiments at 20°C demonstrated a loss in acquired thermotolerance levels compared with the RLD controls (Fig. 4D). These experiments clearly suggest that AtTS02 is an acquired thermotolerance mutant. If AtTS02 is an acquired thermotolerance mutant, then why is there a reduction in chlorophyll at 25°C, a temperature below that seen for induction of the heat shock response in Arabidopsis? One possibility is that the 27-kD protein missing in the mutant but present in the RLD parent following heat shock might also be synthesized at a low level under non-heat shock temperatures. Our analysis of two-dimensional gels of RLD at 22°C (data not shown) could not rule out this possibility as several proteins of similar molecular mass and pI were synthesized at this temperature and identifying a low level of this specific 27-kD protein unequivocally would require the use of monospecific antibodies. Another Arabidopsis mutant JB1 reported to have an increased sensitivity to high temperature was reported by Browse et al. (1986). This mutant was shown to be a fadD mutant and it exhibited altered lipid unsaturation. It is interesting to note that the JB1 mutant also exhibited a slight reduction in chlorophyll content (10%) at 23°C, but it's growth was very vigorous and not readily distinguished from the wild type by any criterion other then leaf lipid composition. We cannot rule out the possibility of a similar change in fatty acids in the AtTS02 as no measure of lipid saturation has been determined. Comparison of the molecular mass of fadD, however, with that of the 27-kD protein suggests that these are not the same proteins, and that the AtTS02 mutation is distinct from the JB1 mutant. Future genetic and physiological analyses of the AtTS02 mutant will help to identify the underlying reason for the reduced chlorophyll accumulation at 25°C.
The AtTS02 mutant does not have a fully functional high temperature protection system, thereby making it more susceptible to injury at elevated temperatures. However, pre-incubation at 38°C is not lethal to the mutant since it survives a subsequent challenge at 46°C (Fig. 3, C–E, J, and L). In fact we show that pre-incubation at 38°C and 40°C actually induced acquired thermotolerance in AtTS02, albeit to a lesser extent than the wild type (Fig. 4D). Additional analysis of other acquired thermotolerance deficient mutants is required to determine if the enhanced temperature sensitivity of chlorophyll accumulation at 25°C is a common feature of this class of mutants.
Protein analysis of the AtTS02 mutant revealed a consistent reduction in the level of a 27-kD protein following heat shock (Fig. 5) that tracked the phenotype throughout the backcross analysis. Wu et al. (1988) also reported the presence of a 27-kD protein whose synthesis was enhanced by exposure to temperatures 15°C above their normal growth temperatures. They reported that the HSP27 appeared within 60 min of the initial exposure to elevated temperature. In the present study, we incubated the mutant at heat shock induction temperatures for 4 h and still observed the reduction of the 27-kD protein. We are at present actively pursuing the identity of the 27-kD protein that is lacking or reduced in the AtTSO2 mutant. If we can manipulate the expression of this protein in transgenic experiments or identify TDNA tagged lines that map to the same locus as AtTSO2, we may be able to directly demonstrate the link between the heat shock response and acquired thermotolerance.
Finally, to validate our screening procedure, we analyzed the whole plant response of AtTS02 to elevated temperatures (Fig. 3M). This clearly showed it had a reduced protection, thus substantiating the usefulness of the chlorophyll accumulation bioassay for identifying acquired thermotolerance mutants.
In summary, we have described the characteristics of acquired thermotolerance in Arabidopsis, described a bioassay for identifying acquired thermotolerance deficient mutants, and characterized one of the selected mutants to show that the deficiencies can be linked to a reduction in a particular HSP and that the observed reduction in chlorophyll accumulation correlate with deficiencies observed at the whole plant level.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis ecotypes C24, Columbia, and RLD seedlings were sown on 1% (w/v) agarose in 35-mm-diameter × 10-mm-deep Petri dishes. The Petri dishes were sealed with Parafilm2 (American National Can, Greenwich, CT) and placed in a refrigerator at 4°C for 6 to 7 d. Following cold incubation, the Petri dishes were unsealed and placed in the dark at 23°C to 25°C for 48 h prior to use.
Determination of the Temperature for Maximum Chlorophyll Accumulation
Chlorophyll accumulation was evaluated across a range of temperatures from 10°C to 40°C. Temperatures were obtained by placing the Petri dishes containing the Arabidopsis seedlings on moistened filter paper (3MM, Bio-Rad Laboratories, Richmond, CA) on the temperature blocks of an electronically controlled eight position thermal plate system named the CELTEC (Burke and Mahan, 1993). The temperature blocks containing the moistened filter paper and Petri dishes were covered with plastic wrap to prevent the filter paper from drying while allowing gas exchange to occur. In this way the temperature of the cotyledons could be rapidly altered (within 30 s) and accurately maintained to within 0.5°C. Chlorophyll accumulation in cotyledons of Arabidopsis was determined by measuring the chlorophyll content of whole tissue acetone extracts according to the procedure described by Arnon (1949) following exposure of the seedlings to continuous light at 115 μmol m−2 s−1 (two F40/AGRO AGRO-LITE fluorescent bulbs [Philips, Dallas] and two 75-W incandescent bulbs) for 16 h. Forty to 45 seedlings were combined for each determination. Six measurements at 10°C, 15°C, 20°C, 25°C, 30°C, 35°C, and 40°C were used in the identification of the optimum temperature for chlorophyll accumulation.
Challenge Temperature Determination
Etiolated Arabidopsis seedlings were incubated in the dark for 30 min at 25°C (control), 42°C, 44°C, 46°C, 48°C, 50°C, 52°C, or 54°C. Temperatures were obtained by placing the Petri dishes on moistened 3MM filter paper on the temperature blocks of the CELTEC. The temperature was returned to 25°C following the high temperature challenge and the seedlings exposed to continuous light at 115 μmol m−2 s−1 (two Philips F40/AGRO-LITE fluorescent bulbs and two 75-W incandescent bulbs) for 24, 48, and 72 h. The chlorophyll content of 40 to 45 seedlings per sample was determined according to the procedure described by Arnon (1949). The challenge temperature selected for subsequent experiments was the temperature that prevented chlorophyll accumulation over a 72-h period.
Pre-Incubation Temperature Determination
Etiolated Arabidopsis seedlings were placed on moistened 3MM filter paper on the temperature blocks of the CELTEC and pre-incubated for 4 h at 28°C, 34°C, 36°C, 38°C, 40°C, 42°C, or 44°C in the dark. Following the pre-incubation period, the temperature blocks were set to 48°C, taking approximately 30 s to come to temperature, and the seedlings were incubated at this challenge temperature in the dark for 30 min. The temperature blocks were then adjusted to 25°C, again coming to temperature within 30 s, and the seedlings incubated for 16 h under continuous light. The optimal pre-incubation temperature was identified as the temperature providing the maximum chlorophyll accumulation.
Duration of Acquired Thermotolerance
Etiolated Arabidopsis seedlings were pre-incubated for 4 h at 38°C to induce acquired thermotolerance. The seedlings were challenged at 48°C at 0, 30, 60, 90, 120, 150, 180, 240 and 1,200 min in the dark and then transferred to continuous light at 25°C for 24 h. Chlorophyll accumulation was compared with 25°C control seedlings that had not been pre-incubated or challenged.
Time Course for the Induction of Acquired Thermotolerance
Etiolated Arabidopsis seedlings were pre-incubated at 38°C for 0, 15, 30, 60, 120, 180, and 240 min in the dark prior to exposure to a temperature challenge of 48°C for 30 min. Seedlings were placed in continuous light at 25°C and chlorophyll content was determined after 24 and 48 h.
Screening for Thermotolerance Mutants
M2 RLD Arabidopsis seeds from plants derived from ethyl methane sulfonate treated seeds were purchased from Lehle Seeds (Round Rock, TX) and treated as described in “Plant Growth Conditions.”
The mutant screening procedure involved pre-incubating the seedlings at 38°C for 4 h in the dark and challenging the seedlings at 44°C for 30 min. The seedlings were then transferred to continuous light at 25°C at 115 μmol m−2 s−1 (two Philips F40/AGRO-LITE fluorescent bulbs and two 75-W incandescent bulbs) for 16 h. Putative mutants were identified by their light green or yellow cotyledons, similar to control seedlings challenged at 44°C without a 38°C pre-incubation. The putative acquired thermotolerance mutants were allowed to grow for 2 d in the light after which those seedlings that accumulated chlorophyll to control levels were transferred to arabaskets (Lehle Seeds) containing Sunshine 3-Mix soil (Sun Gro Horticulture Canada, Bellevue, WA). This was done to separate putative thermotolerance mutants from mutants that were deficient in chlorophyll biosynthesis or deposition. The arabaskets were in turn embedded in rock wool pads (Hummert International, Earth City, MO) that had been saturated with a complete nutrient solution. The seedlings received additional nutrients every 2 d by applying nutrient solution to the pads through an automated drip system. The plants were grown to maturity in controlled environment rooms with a 16-h/8-h day/night cycle at a constant 25°C. The light intensity at plant height was 150 μmol m−2 s−1. Seeds isolated from the putative mutants were evaluated further to determine the level of acquired thermotolerance exhibited compared to control seedlings.
In Vivo Labeling and Protein Isolation
Leaf proteins were labeled in vivo by immersing the cut surface of the stems of derooted seedlings for 4 h in water containing 0.5 mCi/mL 35S-trans label (ICN, Costa Mesa, CA) at control (22°C) or at a temperature providing a high level of acquired thermotolerance (38°C). This labeling procedure enabled the incorporation of label into proteins at a rate independent of uptake rates (data not presented). Following the labeling period, seedlings were washed in distilled water to remove excess radioactivity, and homogenized in Tris/Gly extraction buffer (12.2 g/L Tris base, pH 8.4, and 7.6 g/L Gly) using a plastic pestle sized to fit a 1.5-mL microfuge tube mortar. Cell debris was removed by centrifugation at 14,000g for 10 min. Proteins were extracted from the supernatant by an equal volume of water saturated phenol. The phenol phase was re-extracted with 0.5 volume of extraction buffer and proteins were precipitated overnight at −20°C by addition of 2.5 volumes of 0.1 m ammonium acetate in methanol. After recovery by centrifugation the protein pellet was washed once in 0.1 m ammonium acetate in methanol, air dried, and resuspended in IEF buffer (0.54 g/mL urea, 10 mg/mL DTT, 0.02 mL/mL 3-10 Pharmalyte, 0.005 mL/mL Triton X-100, and 0.001% [w/v] bromphenol blue). Following resuspension in IEF buffer, insoluble material was removed by centrifugation at 14,000g for 2 min, the supernatant moved to a new tube, and stored at −20°C. The quantity of labeled protein in each sample was determined by liquid scintillation analysis using a Tri-Carb 1500 liquid scintillation counter (Packard Instruments, Meriden, CT).
Protein Isolation and Western Analysis
Protein was isolated from shoot tissue by pulverizing the tissue in extraction buffer (0.5 m Tris-Cl, pH 8.65, 50 mm EDTA, 0.1 m KCl, and 2% [v/v] β-mercaptoethanol). Pulverized tissue was cleared of cell debris by centrifugation in 1.5-mL microcentrifuge tubes in a refrigerated benchtop microcentrifuge at 10,000g for 5 min. The clear supernatant was removed to fresh microcentrifuge tubes and protein concentrations were estimated by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA) using BSA as standard. Proteins were fractionated by 12% (w/v) SDS-PAGE using the Bio-Rad minigel system and transferred to PVDF membrane (Pierce, Rockford, IL) using the Bio-Rad TransBlot in transfer buffer (5.8 g/L Tris base, 2.9 g/L Gly, and 200 mL/L 100% [v/v] methanol) for 1 h at 0.2 amps.
Membranes were blocked for 1 h in TBS containing 0.1% (v/v) Tween 20 (TTBS) and 5% (w/v) non-fat dried milk. Primary antibody (1:5,000 for both HSP 101 and HSP 17.6) was incubated with the membrane in TTBS for 1 h at room temperature. Following three 5-min washes in TTBS, goat anti rabbit horseradish peroxidase-conjugated secondary antibody (Pierce) was incubated with the membrane at a dilution of 1:10,000 in TTBS for 1 h. Following three 5-min washes in TTBS, signal detection was achieved using the SuperSignal Substrate system (Pierce).
Two-Dimensional Gel Electrophoresis
Two-dimensional separation of radiolabeled proteins was achieved using the Immobiline DryStrip Kit and ExcelGel SDS on the Multiphor II electrophoresis system (Pharmacia Biotech, Piscataway, NJ). Procedures followed the manufacturer's instructions with some modifications. Acetic acid was used instead of Pharmalyte 3-10 in the rehydration solution for IEF dry strips to improve the quality of the second dimension and to remove contaminating ampholytes. Approximately 200,000 cpm of each sample were loaded on each first-dimension Immobiline strip. The SDS-PAGE gel, following the final protein separation step, was treated with fixer (10% [v/v] acetic acid and 30% [v/v] methanol) for 30 min and fluor (55% [v/v] acetic acid, 15% [v/v] ethanol, 30% [v/v] xylene, and 0.8% [w/v] 2, 5-diphenyl oxazole) for 1 h. The gel was washed twice in distilled water for 2 min, covered with wet cellulose acetate sheet and dried for 2 h at 45°C. Labeled proteins were detected by fluorography by exposure to x-ray film (BIOMAX-MR, Eastman-Kodak, Rochester, NY) in the presence of a single enhancer screen at −80°C.
Characterization of Whole Plant Acquired Thermotolerance
Arabidopsis ecotype RLD and AtTS02 mutant seedlings were surface sterilized and sown on 1% (w/v) agarose containing MS minimal media (4.5 g/L) as described in “Plant Growth Conditions.” The germinating seedlings were moved to a growth chamber (model E-30B, Percival, Boone, IA) at 25°C with continuous light for 5 d. After the 5-d light treatment the Petri dishes containing the seedlings were placed on moistened 3MM filter paper on the temperature blocks of the CELTEC and covered with plastic wrap to prevent the filter paper from drying. The plates containing seedlings were to the temperature blocks of the CELTEC as described in “Determination of the Temperature for Maximum Chlorophyll Accumulation” and exposed to a 38°C pre-incubation temperature for 4 h, followed by a 30-min challenge at 46°C, 48°C, 50°C, or 52°C. The plates were then returned to the 25°C growth chamber for 48 h under light conditions prior to analysis. Plant viability was determined visually.
Genetic Analysis
RLD and AtTS02 plants were crossed and F1 seeds produced. Some F1 seeds were analyzed for the level of acquired thermotolerance relative to the parental lines. Other F1 seeds were planted in arabaskets and grown as described earlier under light at a constant 25°C. These F1 plants were allowed to self-fertilize, and the resulting F2 seeds were collected and tested in segregation studies of the mutant phenotype. Eighty F2 seeds were planted in arabaskets and grown to maturity. These F2 plants were allowed to self-fertilize and the F3 seed from each individual F2 plant was characterized for the acquired thermotolerance phenotype. Individual F3 seed lots were separated based upon their homozygous dominant, homozygous recessive, or their heterozygous phenotypes. Representatives from these seed lots were analyzed by two-dimensional gel electrophoresis to determine if the absence of any specific protein tracked the mutant phenotype.
ACKNOWLEDGMENTS
The authors wish to express gratitude to Norma Zuñiga, Jacob Sanchez, and Thomas Mahan for their technical assistance throughout the course of this research.
Footnotes
This work was supported in part by the National Research Initiative Competitive Grants Program/U.S. Department of Agriculture (grant no. 96–35100–3168).
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture, and does not imply its approval to the exclusion of other products that may also be suitable.
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beator J, Potter E, Kloppstech K. The effect of heat shock on morphogenesis in barley: coordinated circadian regulation of mRNA levels for light-regulated genes and of the capacity for accumulation of chlorophyll protein complexes. Plant Physiol. 1992;100:1780–1786. doi: 10.1104/pp.100.4.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli G, Mascarenhas JP. Arabidopsis: sensitivity of growth to high temperature. Dev Genet. 1990;11:294–298. [Google Scholar]
- Brodl MR, Ho T-HD. Heat shock causes selective destabilization of secretory protein mRNAs in barley aleurone cells. Plant Physiol. 1991;96:1048–1052. doi: 10.1104/pp.96.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodl MR, Ho T-HD. Heat shock in mechanically wounded carrot root disks causes destabilization of stable secretory protein mRNA and dissociation of endoplasmic reticulum lamellae. Physiol Plant. 1992;86:253–262. [Google Scholar]
- Browse J, McCourt P, Somerville C. A mutant of Arabidopsis deficient in C18:3 and C16:3 leaf lipids. Plant Physiol. 1986;81:859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JJ. Integration of acquired thermotolerance within the developmental program of seed reserve mobilization. In: Cherry JH, editor. Biochemical and Cellular Mechanisms of Stress Tolerance in Plants. Berlin: Springer-Verlag; 1994. pp. 191–200. [Google Scholar]
- Burke JJ. Characterization of acquired thermotolerance in soybean seedlings. Plant Physiol Biochem. 1998;36:601–607. [Google Scholar]
- Burke JJ, Ditto CL, Arntzen CJ. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. J Arch Biochem Biophys. 1978;187:252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Burke JJ, Mahan TC. An electronically controlled eight position thermal plate system. Appl Eng Agric. 1993;9:483–486. [Google Scholar]
- Burke JJ, Oliver MJ. Optimal thermal environments for plant metabolic processes (Cucumis sativus L.): light-harvesting chlorophyll a/b pigment-protein complex of photosystem II and seedling establishment in cucumber. Plant Physiol. 1993;102:295–302. doi: 10.1104/pp.102.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Caldwell C, Pitto L. Heat shock disrupts cap and poly(A) tail function during translation and increases mRNA stability of introduced reporter mRNA. Plant Physiol. 1995;108:1703–1713. doi: 10.1104/pp.108.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S, Kunst L, Browse J, Somerville C. Enhanced thermal tolerance of photosynthesis and altered chloroplast ultrastructure in a mutant of Arabidopsis deficient in lipid saturation. Plant Physiol. 1989;90:1134–1142. doi: 10.1104/pp.90.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key JL, Kimpel J, Vierling E, Lin C-Y, Nagao RT, Czarnecka E, Schoffl F. Physiological and molecular analyses of the heat shock response in plants. In: Atkinson BG, Walden DB, editors. Changes in Eukaryotic Gene Expression in Response to Environmental Stress. New York: Academic Press; 1985. pp. 327–348. [Google Scholar]
- Kunst L, Browse J, Somerville C. Enhanced thermal tolerance in a mutant of Arabidopsis deficient in palmitic acid unsaturation. Plant Physiol. 1989;91:401–408. doi: 10.1104/pp.91.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hübel A, Schöffl F. De-repression of the activity of genetically engineered heat shock factor causes constitutive synthesis of heat shock proteins and increased thermotolerance in transgenic Arabidopsis. Plant J. 1995;8:603–609. doi: 10.1046/j.1365-313x.1995.8040603.x. [DOI] [PubMed] [Google Scholar]
- Lee Y-RJ, Nagao RT, Key JL. A soybean 101-kD heat shock protein complements a yeast HSP104 deletion mutation in acquiring thermotolerance. Plant Cell. 1994;6:1889–1897. doi: 10.1105/tpc.6.12.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell J, Osterman JC. Occurrence if temperature-sensitive phenotypic plasticity inchlorophyll-deficient mutants of Arabidopsis thaliana. Plant Physiol. 1992;98:392–394. doi: 10.1104/pp.98.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L. Heat stress factors and transcription factors. Cell Mol Life Sci. 1997;53:80–103. doi: 10.1007/PL00000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B, Ohad I, Kloppstech K. Temperature treatments of dark-grown pea seedlings cause an accelerated greening in the light at different levels of gene expression. Plant Mol Biol. 1992;18:887–896. doi: 10.1007/BF00019203. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Taulien J, Lindquist S. The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci. 1993;339:279–286. doi: 10.1098/rstb.1993.0026. [DOI] [PubMed] [Google Scholar]
- Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F. HSF3, a new heat shock factor from Arabidopsis thaliana, de-represses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet. 1998;258:269–278. doi: 10.1007/s004380050731. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Lindquist SL. HSP104 required for induced thermotolerance. Science. 1990;248:1112–1114. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Lindquist S, Vierling E. An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell. 1994;6:1899–1909. doi: 10.1105/tpc.6.12.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Molecular responses to heat stress. In: Shinozaki K, Yamaguchi-Shinozaki K, editors. Molecular Responses to Cold, Drought, Heat, and Salt Stresses in Higher Plants. R.G. Austin, TX: Landes; 1999. pp. 81–98. [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Wu CH, Caspar T, Browse J, Lindquist S, Somerville C. Characterization of an HSP70 cognate gene family in Arabidopsis. Plant Physiol. 1988;88:731–740. doi: 10.1104/pp.88.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-T, Wallner SJ. Heat stress responses in cultured plant cells development and comparison of viability tests. Plant Physiol. 1983;72:817–820. doi: 10.1104/pp.72.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Chang PFL, Yeh KW, Lin WC, Chen YM, Lin CY. Expression of a gene encoding a 16.9-kDa heat-shock protein, Oshsp16.9, in Escherichia coli enhances thermotolerance. Proc Natl Acad Sci USA. 1997;94:10967–10972. doi: 10.1073/pnas.94.20.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]