Abstract
Trichomoniasis, caused by Trichomonas vaginalis infection, is the most prevalent sexually transmitted disease in female and male globally. However, the mechanisms by innate immunity against T. vaginalis infection have not been fully elucidated. Toll-like receptor2 (TLR2) has been shown to be involved in pathogen recognition, innate immunity activation, and inflammatory response to the pathogens. Nonetheless, the function of TLR2 against T. vaginalis remains unclear. In the present study, we investigated the role of TLR2 in mouse macrophages against T. vaginalis. RT-qPCR analysis revealed that T. vaginalis stimulation increased the gene expression of TLR2 in wild-type (WT) mouse macrophages. T. vaginalis also induced the secretion of IL-6, TNF-α, and IFN-γ in WT mouse macrophages, and the expression of these cytokines significantly decreased in TLR2-/- mouse macrophages and in WT mouse macrophages pretreated with MAPK inhibitors SB203580 (p38) and PD98059 (ERK). Western blot analysis demonstrated that T. vaginalis stimulation induced the activation of p38, ERK, and p65 NF-κB signal pathways in WT mouse macrophages, and the phosphorylation of p38, ERK, and p65 NF-κB significantly decreased in TLR2-/- mouse macrophages. Taken together, our data suggested that T. vaginalis may regulates proinflammatory cytokines production by activation of p38, ERK, and NF-κB p65 signal pathways via TLR2 in mouse macrophages. TLR2 might be involved in the defense and elimination of T. vaginalis infection.
Keywords: Trichomonas vaginalis, TLR2, TLR2-/-, MAPK, NF-κB, cytokines
Introduction
Trichomoniasis is caused by Trichomonas vaginalis infection. As the most prevalent sexually transmitted disease worldwide, about 280 million people are infected with T. vaginalis every year (World Health Organization, 2012). In addition to causing serious discomfort, trichomoniasis has also been linked to vaginitis, preterm delivery, low birth weight, infertility, and cervical cancer (Grodstein et al., 1993; Cotch et al., 1997; Viikki et al., 2000). In infected men, T. vaginalis can be parasitic in prostate, epididymis or foreskin capsule and cause male urinary tract disease (Seña et al., 2007; Johnston and Mabey, 2008; Ryan et al., 2011). Although at least 80% of T. vaginalis infections are asymptomatic, epidemiological studies have also found that trichomoniasis is a risk factor of human immunodeficiency virus transmission (Rottingen et al., 2001). It is obvious that T. vaginalis infection has important medical, social, and economical implications. However, the mechanisms by innate immunity against T. vaginalis infection have not been fully elucidated.
The innate immunity plays a crucial role on the elimination of pathogen infections and defense against invading microorganisms. TLRs are a well-known group of pattern recognition receptors that recognize conserved pathogen-associated molecular patterns. Different TLR family members are expressed by a variety of cells in many animal species which are critical in generating innate immune responses to multiple stimuli (Aderem and Ulevitch, 2000; Anderson, 2000; Akira et al., 2001). Inflammatory responses mediated by TLRs can be triggered by a variety of pathogens, including parasite, bacteria, fungi, and virus (Kawai and Akira, 2005; Oliveira-Nascimento et al., 2012). TLR activation not only leads to inflammatory responses but is also involved in the development of adaptive immunity for specific antigens. Stimulation of adaptive immunity can promote a series of host immune defense mechanisms, such as the activation of mitogen-activated protein kinases (MAPKs) and the secretion of proinflammatory cytokines which participate in the elimination of pathogens (Takeda et al., 2003). Activation of TLR in turn activates downstream MAPK signal pathways especially the extracellular signal-regulated kinase p38 and ERK which regulates a variety of cellular responses, such as inflammatory, differentiation, and apoptosis. Previous studies have demonstrated that NF-κB participates in the modulation of inflammatory responses during early infection stage, and parasites like Leishmania and Toxoplasma gondii could interfere with the activation of the NF-κB signal pathways (Shapira et al., 2005; Reinhard et al., 2012).
TLR2 recognizes PAMPs and informs immune cells of invading pathogens. TLR2 activation leads to the proinflammatory cytokine production through the activation of MAPK, AKT, and NF-κB signal pathways (Soilleux et al., 2002; Vasselon et al., 2002; Asehnoune et al., 2005). TLR2 can be activated by glycosylphosphatidylinositols (GPIs) presented on some protozoa and participates in the host defense against parasite infection (Oliveira-Nascimento et al., 2012), including Toxoplasma gondii and Trypanosoma cruzi (Campos et al., 2001; Debierre-Grockiego et al., 2007) The expression of TLR2 has been confirmed in various cells, such as endothelial cells, epithelial cells, and macrophages (Flo et al., 2001; Yadav and Schorey, 2006; Brzezińska-Błaszczyk and Wierzbicki, 2010). Macrophages are important for innate immune system during infection and are involved in a series of inflammatory reactions, such as the secretion of IL-6, TNF-α, and IFN-γ (Gessani and Belardelli, 1998; Butcher and Denkers, 2002; Goral et al., 2004). Previous studies have shown that T vaginalis infection in women caused high level production of proinflammatory cytokines including IL-6, IL-1β, and TNF-α in cervicovaginal mucosa (Riezzo et al., 2007; Han et al., 2009). However, the role of TLR2 in the host defense against T. vaginalis remains unclear.
In this study, we examined the expression of TLR2; the activation of p38, ERK, and NF-κB signal pathways; the secretion of IL-6, TNF-α, and IFN-γ in WT and TLR2-/- mouse macrophages by RT-qPCR, western-blot, and ELISA, respectively.
Materials and Methods
T. vaginalis and Mouse Peritoneal Macrophages (PMϕ)
The T. vaginalis isolate used in this study was isolated from the vaginal secretions of trichomonas vaginitis in the gynecological clinic of the First Clinical Hospital of Jilin University (Zhao et al., 2007). T. vaginalis were cultivated in Diamond’s Typticase-yeast extract-maltose medium with 10% FBS at 37°C, only the late-logarithmic-phase trophozoites were used for the assays. Wild-type (WT) female C57BL/6 mice and TLR2-/- mice were euthanized with over dose of ether (Rutkowski et al., 2007; Li et al., 2017) and soaked in 75% ethanol for 15 min, peritoneal cavity were flushed twice with 10 ml PBS (pH 7.4), then cell suspension were centrifuged at 1,000 g for 10 min, and washed twice with PBS.The primary culture cell viability of macrophages obtained by the lavage fluid was determined by trypan blue method (>99%). 3 × 106 cells were plated in a well of 6-well tissue culture plates (JET BIOFIL, China) in 1 ml RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin and incubated overnight at 37°C with 5% CO2. Cells were washed twice with PBS to remove the non-adherent cells before stimulation.
Real-Time PCR Measurement for TLR2 Gene Expression
3 × 106 WT peritoneal macrophages (PMϕ) were co-incubated for 2 h with 1 × 106 T. vaginalis or Pam3Cys-Ser-(Lys) 4 (Pam3CSK4) (TLR2/TLR1 Agonist, InvivoGen, United States, 10 μg/ml) (Summers et al., 2014) in 1 ml RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C with 5% CO2. After treatments, cell culture supernatants were removed and the cells were washed twice with RPMI 1640. Total RNA was isolated from the cells using the TRIzol Reagent (Invitrogen, United States). First-strand cDNA was synthesized by reverse transcription using the total RNA Transcript II reverse transcriptase (TransGen Biotech Company, Beijing, China). RNA expression levels of the analyzed genes were normalized to the amount of β-actin. The PCR program ran for 30 cycles with three steps: 95°C for 3 min; 95°C for 45 s, 60°C for 45 s, and 72°C for 45 s; 72°C for 10 min. Melting curves were acquired after PCR to ensure the homogeneity of the PCR products. All primers were synthesized by Sangon (Shanghai, China), and their sequences were as follows:
β-actin, sense: 5′-TGCTGTCCCTGTATGCCTCT-3′, antisense: 5′-GGTCTTTACGGATGTCAACG-3′; TLR2, sense: 5′-CCCACTTCAGGCTCTTTGAC-3′, antisense: 5′-GCCACTCCAGGTAGGTCTTG-3′.
Detection of Cytokines by ELISA
3 × 106 WT or TLR2-/- PMϕ were co-incubated with 1 × 106 T. vaginalis or Pam3CSK4 (10 ug/ml) for 18 h in RPMI 1640 containing 2% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C with 5% CO2. In the inhibition experiment, WT PMϕ were pretreated with specific inhibitor of p38 (SB203580; 30 μM), ERK (PD98059; 40 μM) (Sigma-Aldrich, United States) for 1 h at 37°C with 5% CO2 and the viability of the macrophages was determined by trypan blue method (>95%). Then the PMϕ were co-incubated with T. vaginalis in cell culture medium containing 2% FBS. Culture supernatants were collected and IL-6, TNF-α, and IFN-γ levels were analyzed by ELISA Ready-SET-Go kits (eBioscience, San Diego, CA, United States) according to the manufacturer.
Western Blot Analysis
3 × 106 WT or TLR2-/- PMϕ in monolayer were co-incubated with 1 × 106 T. vaginalis for 0.5, 1, 2, and 4 h. In parallel experiments, WT PMϕ were pretreated with specific inhibitor of p38 (SB203580; 30 μM), ERK (PD98059; 40 μM). Pam3CSK4 (10 ug/ml) were used to stimulate macrophages for the positive control (TLR2, NF-κB p65). Non-treated PMϕs were used as negative control. After the incubation, cells were harvested with 25 cm cell scrapers (SRASTEDT, Germany) and centrifuged at 12,000 g for 30 min at 4°C. The pellets were washed twice with sterile PBS, then treated with RIPA lysis buffer (1% NP-40, 0.5% Sodium deoxycholate, 0.1% SDS) (BOSTER, China) containing proteinase inhibitor (1/100) and phosphatase inhibitor (1/100) (Sangon Biotech, Shanghai, China). Protein concentrations were measured using the Bradford protein-quantification assay. 30 μg of sample protein/lane was separated with 10% SDS-PAGE electrophoresis, then transferred to polyvinyldifluoride membranes (Millipore, Bedford, MA, United States) and blocked in 5% skim milk in TBST for 2 h at room temperature. The membranes were incubated overnight at 4°C with primary rabbit antibodies anti-TLR2 (1/1,000), anti-ERK (1/1,000), anti-p38 (1/1,000), anti-IκBα (1/1,000), anti-p65 (1/1,000), anti-phospho-ERK (1/1,000), anti-phospho-p38 (1/1,000), anti-phospho-IκBα (1/1,000) and anti-phospho-p65 (1/1,000) (Cell Signaling Technology, United States) and mouse anti-β-actin (1/2,000) (Bioss, United States). After 1 h of washing with TBST, membranes were incubated with secondary HRP conjugated goat anti-rabbit IgG or anti-mouse IgG (Cell Signaling Technology, dilution 1/5,000) for 1 h at room temperature and washed three times with TBST. Bands were detected using enhanced chemiluminescence (Vigorous, Beijing, China). The Relative Gray Value (target protein/internal reference) of the western blot results were analyzed by Image J.
Subcellular Localization of NF-κB p65
The localization of NF-κB p65 were determined after 7.5 × 105 PMϕ cultivated on glass coverslips in a well of 24-well culture plates co-incubated with 1 × 106 T. vaginalis or Pam3CSK4 (10 ug/ml) at 37°C. After stimulation for 0 or 1 h, cells were washed twice with PBS, then fixed with 4% paraformaldehyde (diluted in PBS) for 15 min at room temperature and washed three times with PBS. The macrophages were permeabilized with 0.25% Triton X-100 for 10 min, then incubated with mouse anti-phosphor NF-κB p65 (Santa Cruz, CA, United States) at a 1:100 dilution at 4°C overnight. Cells were then washed and incubated with FITC-conjugated goat anti-mouse IgG (Proteintech, United States, dilution 1/5,000) secondary antibody for 1 h at room temperature. The cells were washed, and the coverslips were stained with DAPI at room temperature for 5 min. NF-κB p65 localization was observed using a Zeiss LSM 710 confocal microscope equipped with a 63X, 1.4-NA, oil-immersion objective (Carl Zeiss).
Statistical Analysis
All data were presented as mean ± SD of triplicate experiments. GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, United States) was utilized to analyze the data detected by ELISA. Image J was used to analyze the results of western blot and confocal microscope. SPSS version 19.0 (SPSS, Inc., Chicago, IL, United States) was used for the statistical analysis using ANOVA followed by Tukey test. p-Values < 0.05 were considered statistically significant.
Results
TLR2 Activation and Proinflammatory Cytokine Production in PMϕ Induced by T. vaginalis
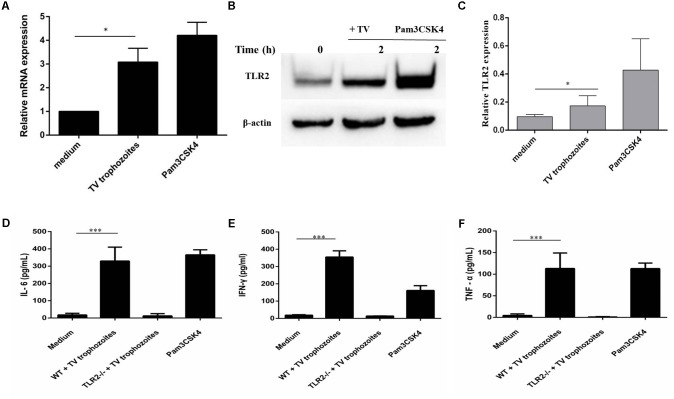
RT-qPCR and western blot results showed that TLR2 gene expression increased in WT PMϕ co-incubated with T. vaginalis for 2 h compared with PMϕ without T. vaginalis (Figures 1A,B). The most significant TLR2 expression was detected in Pam3CSK4 (10 ug/ml) stimulated macrophages. Relative Gray analysis further confirmed this result (Figure 1C). Secretion of IL-6 (Figure 1D), IFN-γ (Figure 1E), and TNF-α (Figure 1F) increased in WT PMϕ with T. vaginalis co-incubation compared to TLR2-/- PMϕ with T. vaginalis or WT PMϕ without T. vaginalis.
FIGURE 1.
Trichomonas vaginalis induces cytokines secretion in a TLR2-dependent way in mouse peritoneal macrophages. RT-qPCR analysis for TLR2 in total RNA isolated from mouse peritoneal macrophages incubated in medium alone, with T. vaginalis or Pam3CSK4 (10 μg/ml), respectively. RT-qPCR demonstrated that T. vaginalis stimulation significantly enhances TLR2 gene expression in WT macrophages (A). WT macrophages were co-incubated with T. vaginalis or Pam3CSK4 for 2 h (B). Relative Gray analysis of western blot (C). WT and TLR2-/- mouse peritoneal macrophages were co-incubated with T. vaginalis. The levels of IL-6, TNF-α, and IFN-γ in cell culture supernatant were detected by ELISA. Compared with TLR2-/- mouse peritoneal macrophages, the production of IL-6, TNF-α, and IFN-γ in WT macrophages were significantly increased (D–F). Data are expressed as the mean ± SD from three separate experiments (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
T. vaginalis Activated p38 and ERK Signal Pathways in WT PMϕ
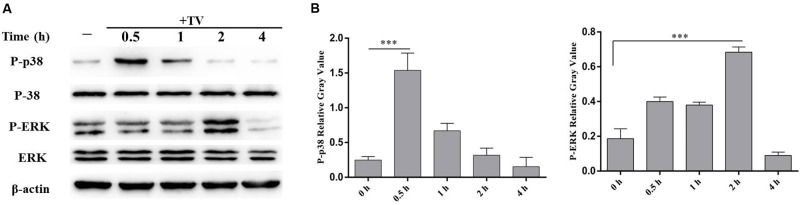
The phosphorylation of p38 and ERK were detected by western blot after co-incubation of WT PMϕ with T. vaginalis for 0.5 and 2 h. The phosphorylation of p38 peaked at 0.5 h and returned to baseline at 2 h, while phosphorylated ERK peaked at 2 h in WT PMϕ with T. vaginalis stimulation compared to control PMϕ without T. vaginalis stimulation (Figure 2).
FIGURE 2.
Trichomonas vaginalis activates p38 and ERK signal pathways in WT mouse peritoneal macrophages. WT macrophages were co-incubated with T. vaginalis for different times (0, 0.5, 1, 2, and 4 h), phosphorylation of p38 and ERK were detected by western blot (A). Relative Gray analysis of western blot (B). The phosphorylated of p38 (at 0.5 h) and ERK (at 2 h) were observed obviously. Data are expressed as the mean ± SD from three separate experiments (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
T. vaginalis Induced Cytokines Secretion in PMϕ Through Phosphorylation of p38 and ERK via TLR2
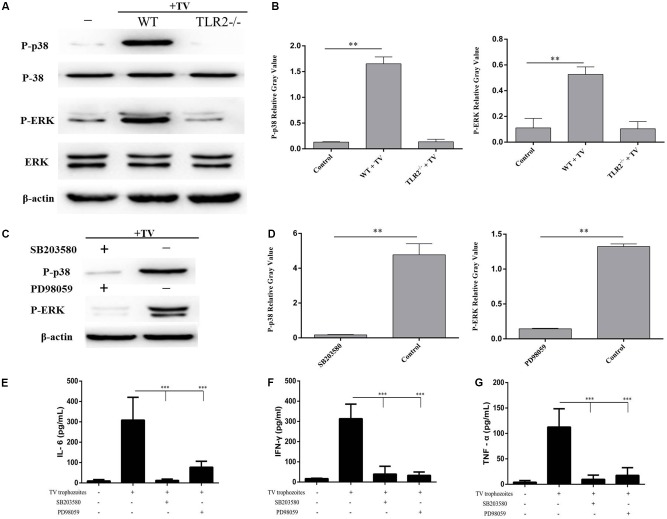
To investigate whether the phosphorylation of p38 and ERK induced by T. vaginalis was mediated through TLR2, TLR2-/- PMϕ were incubated with T. vaginalis for 0.5 or 2 h. The phosphorylation of p38 and ERK in TLR2-/- PMϕ was significantly reduced in TLR2-/- PMϕ with T. vaginalis co-incubation compared to WT PMϕ with T. vaginalis or WT PMϕ without T. vaginalis. These data demonstrated that T. vaginalis induced phosphorylation of p38 and ERK via TLR2 (Figure 3A).
FIGURE 3.
Trichomonas vaginalis induces cytokines production regulated by p38 and ERK via TLR2. Phosphorylationof p38 and ERK in TLR2-/- mouse peritoneal macrophages were significantly reduced after co-incubated with T. vaginalis for 0.5 h (p38) or 2 h (ERK) compared to in WT mouse peritoneal macrophages (A). Inhibitors of p38 (SB203580; 30 μM) or ERK (PD98059; 40 μM) were used to pretreated WT mouse peritoneal macrophages for 1 h before co-incubated by T. vaginalis. Phosphorylation of p38 and ERK in SB203580 and PD98059 pre-treated WT macrophages (C). Relative Gray analysis of western blot (B,D). The production of IL-6, TNF-α, and IFN-γ induced by T. vaginalis were significantly inhibited by the inhibitors compared to in WT mouse peritoneal macrophages (E–G). Data are expressed as the mean ± SD from three separate experiments (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
To estimate the role of p38 and ERK signaling pathways in the regulation of IL-6, TNF-α, and IFN-γ productions, two MAPK inhibitors SB203580 (p38) and PD98059 (ERK) were used to pretreat WT PMϕ for 60 min at 37°C. Phosphorylation of p38 and ERK in inhibitor pretreated PMϕ (Figure 3C). Relative Gray analysis of western blot (Figures 3B,D). Macrophages with or without inhibitor treatments were incubated with T. vaginalis for 18 h, then cytokines in different samples were measured by ELISA. The results indicated that the production of IL-6, TNF-α, and IFN-γ induced by T. vaginalis were significantly reduced in WT PMϕ treated with the two inhibitors compared to PMϕ without inhibitors (Figures 3E–G). These results indicated that p38 and ERK signaling were activated via TLR2 which in turn upregulated the expression of IL-6, TNF-α, and IFN-γ in PMϕ in response to T. vaginalis.
T. vaginalis Induced Translocation of NF-κB p65 to Nucleus via TLR2 in WT PMϕ
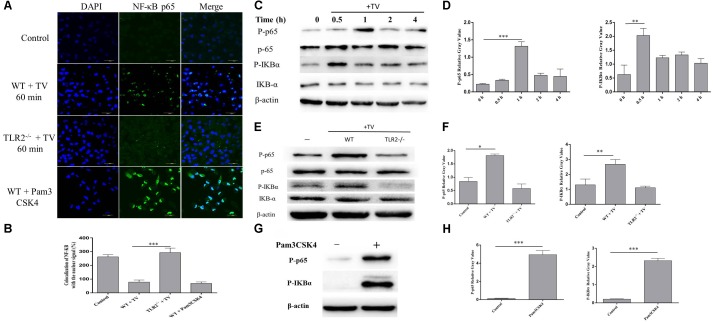
The activation of NF-κB by T. vaginalis was detected by immunofluorescence staining and western blot. Phosphorylated NF-κB p65 was observed in the WT PMϕ nucleus after co-incubation with T. vaginalis (Figure 4A) and its phosphorylation increased, as well as of IKB-α (Figure 4C). The percentage of colocalization of p65 with nuclei signal (Figure 4B). To investigate whether the activation of NF-κB p65 was via TLR2, TLR2-/- PMϕ was co-incubated with T. vaginalis and NF-κB p65 localization was observed. NF-κB p65 was located primarily in the cytoplasm of TLR2-/- PMϕ after co-incubation with T. vaginalis (Figure 4A). In addition, western blot analysis also showed that phosphorylated NF-κB p65 and IκB-α in TLR2-/- PMϕ with T. vaginalis were significantly reduced compared to WT PMϕ (Figure 4E). Pam3CSK4 (10 ug/ml) stimulated macrophages showed the most significant translocation and phosphorylation of NF-κB p65 (Figure 4G). Relative Gray analysis of western blot (Figures 4D,F,H). These data demonstrated that T. vaginalis may induce the phosphorylation of NF-κB p65 via TLR2 in WT PMϕ.
FIGURE 4.
Trichomonas vaginalis induces changes of subcellular localization of NF-κB p65 via TLR2. Confocal microscopy analysis revealed the effects of T. vaginalis or Pam3CSK4 on the translocation of NF-κB p65 from the cytoplasm to the nucleus in WT macrophages after the incubation with T. vaginalis for 1 h (A). The percentage of colocalization of NF-κB with the nuclear signal under the different treatments (B). WT mouse macrophages were incubated with T. vaginalis for different times (0–4 h), cell lysates were used for western blot analysis (C). WT and TLR2-/- mouse macrophages were incubated with T. vaginalis for 1 h, phosphorylation of NF-κB p65 and IκBα was detected by western blot (E). Phosphorylation of NF-κB p65 in Pam3CSK4 treated macrophages (G). Relative Gray analysis of western blot (D,F,H).
Discussion
Toll-like receptors are important molecules for the recognition of different pathogens, including parasites, bacteria, viruses, and fungi. Components of microorganisms were recognized by different TLRs, which result in the activation of intracellular signals and cytokine production by leukocytes and other cells that are necessary for pathogenic microorganisms elimination (Medzhitov, 2001; Kawai and Akira, 2007). TLR2 mediates cell activation by the recognition of microorganisms and microbial products, and also is involved in the defense and elimination of parasites infection, including Plasmodium falciparum (Zhu et al., 2011), Toxoplasma gondii (Mun et al., 2003), and Trypanosoma cruzi (Campos et al., 2001). HeLa cells treated with T. vaginalis showed significant up-regulation of TLR2, TLR4, and TLR9 (Chang et al., 2006). In this study, we analyzed TLR2, TLR4, and TLR9 gene expression in WT macrophages and the results from RT-qPCR and western blot demonstrated that T. vaginalis stimulation significantly enhanced TLR2 gene expression in WT macrophages.
Previous studies also implicated that T. vaginalis infection induced inflammatory responses in epithelial (Yang et al., 2015), neutrophils (Ryu et al., 2004), and macrophages (Han et al., 2009). Proinflammatory cytokines such as IL-6 from human prostate epithelial cells and TNF-α from rat peritoneal mast cells have been demonstrated to be necessary for the T. vaginalis control and the initiation of subsequent adaptive immune response in the process of T. vaginalis infection (Im et al., 2011; Han et al., 2016). Furthermore, human monocyte-derived macrophages (HMDMs) co-cultured with T. vaginalis increased the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (Han et al., 2009). In our study, we found that the production of proinflammatory cytokines IL-6, TNF-α, and IFN-γ in WT macrophages were significantly higher than that in TLR2-/- macrophages, which indicated that T. vaginalis induced proinflammatory cytokines production in macrophages was partially dependent on TLR2.
Innate immune system recognizes microorganism and activates a variety of signal transduction pathways which initiate the subsequent immune response (Beutler, 2009). The GPI from parasite could lead the activation of MAPK and NF-κB signal pathways via TLR2 (Gowda, 2007). Human macrophages and SiHa Cervical Mousosal Epithelial cells infected with T. vaginalis increased the activity of phospho NF-κB p65 and p38, ERK, and JNK, respectively (Han et al., 2009; Yang et al., 2015). HeLa cells treated with T. vaginalis showed significant up-regulation of TLR2, TLR4, TLR9, which was a p38 MAPK signaling pathway dependent process (Medzhitov, 2001). Our results showed that the phosphorylation of p38, ERK in WT macrophages significantly increased compared to in TLR2-/- macrophages. Furthermore pretreatment with inhibitors of p38 and ERK obviously inhibited the production of IL-6, TNF-α, and IFN-γ. Taken together, our data suggested that T. vaginalis induced proinflammatory cytokines production in macrophages through the activation of MAPK via TLR2.
Nuclear transcription factor NF-κB, formed by five different Rel family proteins, is known to be crucial in the regulation of inflammatory gene transcription for both innate and adaptive immunity responses (Caamaño et al., 1999). Recent evidence demonstrated that a shift in NF-κB subunits from p50 to p65 heterodimer to p50 homodimers is associated with inflammation (Lawrence et al., 2001; Ashall et al., 2009), in addition, degradation of IkBα is a classical way for NF-κB p65 phosphorylation and translocation to the nucleus (Hayden and Ghosh, 2008). Studies on ultraviolet light (UV) and recombinant MPT83 derived from Mycobacterium tuberculosis showed that the activation of TLR2 was involved in the phosphorylation of NF-κB (Wang et al., 2011; Chen et al., 2012). The NF-κB activation also participated in the regulation of NO production and some NF-κB dependent proinflammatory gene expression can be up-regulated by CD40 (Song et al., 2011; Adesso et al., 2013). Western blot and immune fluorescence assays from the present study indicated that T. vaginalis induced the phosphorylation and accumulation in nucleus of NF-κB p65 in WT macrophages but not in TLR2-/- macrophage. The results demonstrated that T. vaginalis could activate NF-κB signal pathway via TLR2 in mouse macrophage.
In summary, T. vaginalis could regulate pro-inflammation cytokines production by activation of p38, ERK, and NF-κB p65 signal pathways via TLR2 in mouse peritoneal macrophages. These data could enrichour understanding on host defense against T. vaginalis infection through innate immune system.
Ethics Statement
WT C57BL/6 mice (Huafukang Experimental Animal Center, Beijing, China) and TLR2-/- mice (Model Animal Research Center of Nanjing University, Nanjing, China) were housed in filter-top cages in an air-conditioned animal facility in the National Experimental Teaching Demonstration Centre of Jilin University (Changchun, China). Water and normal mouse food were given ad libitum. All animal experimental procedures were performed in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved through the State Council of People’s Republic of China (1988.11.1) and with approval of the Animal Welfare and Research Ethics Committee at Jilin University.
Author Contributions
LL and PG drafted the main manuscript and performed the data analysis. LL and XL planned and performed the experiments. LL, ZY, JY, and JL were responsible for experimental design. XZ and JL responsible for guiding and supporting the experiments and manuscript revisions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was funded by National Natural Science Foundation of China (Grant Nos. 31772732 and 31672288). The experiments conducted in this study comply with the current laws of China.
References
- Aderem A., Ulevitch R. J. (2000). Toll-like receptors in the induction of the innate immune response. Nature 406 782–787. 10.1038/35021228 [DOI] [PubMed] [Google Scholar]
- Adesso S., Popolo A., Bianco G., Sorrentino R., Pinto A., Autore G., et al. (2013). The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS One 8:e76778. 10.1371/journal.pone.0076778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Takeda K., Kaisho T. (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2 675–680. 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- Anderson K. V. (2000). Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12 13–19. 10.1016/S0952-7915(99)00045-X [DOI] [PubMed] [Google Scholar]
- Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. (2005). Involvement of PKCα/β in TLR4 and TLR2 dependent activation of NF-κB. Cell. Signal. 17 385–394. 10.1016/j.cellsig.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Ashall L., Horton C. A., Nelson D. E., Paszek P., Harper C. V., Sillitoe K., et al. (2009). Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 324 242–246. 10.1126/science.1164860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. A. (2009). TLRs and innate immunity. Blood 113 1399–1407. 10.1182/blood-2008-07-019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezińska-Błaszczyk E., Wierzbicki M. (2010). [Mast cell Toll-like receptors (TLRs)]. Postepy Hig. Med. Dosw. 64 11–21. [PubMed] [Google Scholar]
- Butcher B. A., Denkers E. Y. (2002). Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect. Immun. 70 5216–5224. 10.1128/IAI.70.9.5216-5224.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamaño J., Alexander J., Craig L., Bravo R., Hunter C. A. (1999). The NF-kappa B family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J. Immunol. 163 4453–4461. [PubMed] [Google Scholar]
- Campos M. A., Almeida I. C., Takeuchi O., Akira S., Valente E. P., Procopio D. O., et al. (2001). Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167 416–423. 10.4049/jimmunol.167.1.416 [DOI] [PubMed] [Google Scholar]
- Chang J. H., Park J. Y., Kim S. K. (2006). Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells. Immunology 118 164–170. 10.1111/j.1365-2567.2006.02347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Li J. Y., Zhang Y., Gao X., Cai H. (2012). Recombinant MPT83 derived from Mycobacterium tuberculosis induces cytokine production and upregulates the function of mouse macrophages through TLR2. J. Immunol. 188 668–677. 10.4049/jimmunol.1102177 [DOI] [PubMed] [Google Scholar]
- Cotch M. F., Pastorek J. G., Nugent R. P., Hillier S. L., Gibbs R. S., Martin D. H., et al. (1997). Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex. Transm. Dis. 24 353–360. 10.1097/00007435-199707000-00008 [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego F., Campos M. A., Azzouz N., Schmidt J., Bieker U., Resende M. G., et al. (2007). Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179 1129–1137. 10.4049/jimmunol.179.2.1129 [DOI] [PubMed] [Google Scholar]
- Flo T. H.,, Halaas Ø.,, Torp S.,, Ryan L.,, Lien E.,, Dybdahl B.,, et al. (2001). Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69 474–481. [PubMed] [Google Scholar]
- Gessani S., Belardelli F. (1998). IFN-γ Expression in Macrophages and Its Possible Biological Significance. Cytokine Growth Factor Rev. 9 117–123. 10.1016/S1359-6101(98)00007-0 [DOI] [PubMed] [Google Scholar]
- Goral J., Choudhry M. A., Kovacs E. J. (2004). Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J. Leukoc. Biol. 75 553–559. 10.1189/jlb.0703350 [DOI] [PubMed] [Google Scholar]
- Gowda D. C. (2007). TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 23 596–604. 10.1016/j.pt.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Grodstein F., Goldman M. B., Cramer D. W. (1993). Relation of tubal infertility to history of sexually transmitted diseases. Am. J. Epidemiol. 137 577–584. 10.1093/oxfordjournals.aje.a116711 [DOI] [PubMed] [Google Scholar]
- Han I. H., Goo S. Y., Park S. J., Hwang S. J., Kim Y. S., Yang M. S., et al. (2009). Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J. Parasitol. 47 205–212. 10.3347/kjp.2009.47.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I. H., Kim J. H., Kim S. S., Ahn M. H., Ryu J. S. (2016). Signalling pathways associated with IL-6 production and epithelial-mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunol. 38 678–687. 10.1111/pim.12357 [DOI] [PubMed] [Google Scholar]
- Hayden M. S., Ghosh S. (2008). Shared principles in NF-kappaB signaling. Cell 132 344–362. 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Im S. J., Ahn M. H., Han I. H., Song H. O., Kim Y. S., Kim H. M., et al. (2011). Histamine and TNF-alpha release by rat peritoneal mast cells stimulated with Trichomonas vaginalis. Parasite 18 49–55. 10.1051/parasite/2011181049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston V. J., Mabey D. C. (2008). Global epidemiology and control of Trichomonas vaginalis. Curr. Opin. Infect. Dis. 21 56–64. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2005). Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17 338–344. 10.1016/j.coi.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2007). TLR signaling. Semin. Immunol. 19 24–32. 10.1016/j.smim.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Lawrence T., Gilroy D. W., Colvillenash P. R., Willoughby D. A. (2001). Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 7 1291–1297. 10.1038/nm1201-1291 [DOI] [PubMed] [Google Scholar]
- Li X., Zhang X., Gong P., Xia F., Li L., Yang Z., et al. (2017). TLR2(-/-) mice display decreased severity of giardiasis via enhanced proinflammatory cytokines production dependent on AKT signal pathway. Front. Immunol. 8:1186. 10.3389/fimmu.2017.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. (2001). Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1 135–145. 10.1038/35100529 [DOI] [PubMed] [Google Scholar]
- Mun H. S., Aosai F., Norose K., Mei C., Piao L. X., Takeuchi O., et al. (2003). TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 15 1081–1087. 10.1093/intimm/dxg108 [DOI] [PubMed] [Google Scholar]
- Oliveira-Nascimento L., Massari P., Wetzler L. M. (2012). The role of TLR2 in infection and immunity. Front. Immunol. 3:79. 10.3389/fimmu.2012.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard K., Huber M., Lohoff M., Visekruna A. (2012). The role of NF-kappaB activation during protection against Leishmania infection. Int. J. Med. Microbiol. 302 230–235. 10.1016/j.ijmm.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Riezzo I., Neri M., De S. F., Fulcheri E., Ventura F., Pomara C., et al. (2007). Kinetics of serum and local cytokine profile in experimental intravaginal trichomoniasis induced with Trichomonas vaginalis isolates from symptomatic and asymptomatic women. Parasite Immunol. 29 101–105. [DOI] [PubMed] [Google Scholar]
- Rottingen J. A., Cameron G. P., Garnett D. W. (2001). A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex. Transm. Dis. 28 579–597. 10.1097/00007435-200110000-00005 [DOI] [PubMed] [Google Scholar]
- Rutkowski M., Mcnamee L., Harmsen A. (2007). Neutrophils and inducible nitric-oxide synthase are critical for early resistance to the establishment of Tritrichomonas foetus infection. J. Parasitol. 93 562–574. 10.1645/GE-976R.1 [DOI] [PubMed] [Google Scholar]
- Ryan C. M., De M. N., Johnson P. J. (2011). Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. 51 161–175. 10.1042/bse0510161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. S., Kang J. H., Jung S. Y., Shin M. H., Kim J. M., Park H., et al. (2004). Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect. Immun. 72 1326–1332. 10.1128/IAI.72.3.1326-1332.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seña A. C., Miller W. C., Hobbs M. M., Schwebke J. R., Leone P. A., Swygard H., et al. (2007). Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin. Infect. Dis. 44 13–22. 10.1086/511144 [DOI] [PubMed] [Google Scholar]
- Shapira S., Harb O. S., Margarit J., Matrajt M., Han J., Hoffmann A., et al. (2005). Initiation and termination of NF-kappaB signaling by the intracellular protozoan parasite Toxoplasma gondii. J. Cell Sci. 118 3501–3508. 10.1242/jcs.02428 [DOI] [PubMed] [Google Scholar]
- Soilleux E. J., Morris L. S., Leslie G., Chehimi J., Luo Q., Levroney E., et al. (2002). Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71 445–457. [PubMed] [Google Scholar]
- Song Z., Jin R., Yu S., Rivet J. J., Smyth S. S., Nanda A., et al. (2011). CD40 Is essential in the upregulation of TRAF proteins and NF-KappaB-dependent proinflammatory gene expression after arterial injury. PLoS One 6:e23239. 10.1371/journal.pone.0023239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers S. A., Odobasic D., Khouri M. B., Steinmetz O. M., Yang Y., Holdsworth S. R., et al. (2014). Endogenous interleukin (IL)-17A promotes pristane-induced systemic autoimmunity and lupus nephritis induced by pristane. Clin. Exp. Immunol. 176 341–350. 10.1111/cei.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Kaisho T., Akira S. (2003). TOLL-LIKE RECEPTORS. Annu. Rev. Immunol. 21 335–376. 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- Vasselon T., Hanlon W. A., Wright S. D., Detmers P. A. (2002). Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. J. Leukoc. Biol. 71 503–510. [PubMed] [Google Scholar]
- Viikki M., Pukkala E., Nieminen P., Hakama M. (2000). Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 39 71–75. 10.1080/028418600431003 [DOI] [PubMed] [Google Scholar]
- Wang X., Bi Z., Wang Y., Wang Y. (2011). Increased MAPK and NF-kappaB expression of Langerhans cells is dependent on TLR2 and TLR4, and increased IRF-3 expression is partially dependent on TLR4 following UV exposure. Mol. Med. Rep. 4 541–546. 10.3892/mmr.2011.450 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2012). Global incidence and prevalence of selected curable sexually transmitted infections: 2008. Reprod. Health Matters 20 207–209. 10.1016/S0968-8080(12)40660-7 [DOI] [Google Scholar]
- Yadav M., Schorey J. S. (2006). The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108 3168–3175. 10.1182/blood-2006-05-024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. B., Quan J. H., Kim Y. E., Rhee Y. E., Kang B. H., Choi I. W., et al. (2015). Involvement of PI3K/AKT and MAPK pathways for TNF-α production in SiHa Cervical Mucosal Epithelial Cells Infected with Trichomonas vaginalis. Korean J. Parasitol. 53 371–377. 10.3347/kjp.2015.53.4.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wu S., Zhang X. (2007). In vitro culture of Trichomonas vaginalis carrying virus strains. J. Hebei North Univ. 23 34–37. [Google Scholar]
- Zhu J., Krishnegowda G., Li G., Gowda D. C. (2011). Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp. Parasitol. 128 205–211. 10.1016/j.exppara.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]