ABSTRACT
The generation of two daughter cells with the same genetic information requires error-free chromosome segregation during mitosis. Chromosome transmission fidelity is dependent on spindle structure/function, which requires Asp1 in the fission yeast Schizosaccharomyces pombe. Asp1 belongs to the diphosphoinositol pentakisphosphate kinase (PPIP5K)/Vip1 family which generates high-energy inositol pyrophosphate (IPP) molecules. Here, we show that Asp1 is a bifunctional enzyme in vivo: Asp1 kinase generates specific IPPs which are the substrates of the Asp1 pyrophosphatase. Intracellular levels of these IPPs directly correlate with microtubule stability: pyrophosphatase loss-of-function mutants raised Asp1-made IPP levels 2-fold, thus increasing microtubule stability, while overexpression of the pyrophosphatase decreased microtubule stability. Absence of Asp1-generated IPPs resulted in an aberrant, increased spindle association of the S. pombe kinesin-5 family member Cut7, which led to spindle collapse. Thus, chromosome transmission is controlled via intracellular IPP levels. Intriguingly, identification of the mitochondrion-associated Met10 protein as the first pyrophosphatase inhibitor revealed that IPPs also regulate mitochondrial distribution.
KEYWORDS: PPIP5K family, Schizosaccharomyces pombe, chromosome segregation, inositol pyrophosphate, microtubule, mitosis, phosphatase, signaling molecules, yeast
INTRODUCTION
Inositol pyrophosphates (IPPs) are signaling molecules present in all eukaryotes and are synthesized by the two enzyme families inositol hexakisphosphate (IP6K)/Kcs1 and diphosphoinositol pentakisphosphate kinase (PPIP5K)/Vip1 (1–3). Numerous cellular processes are regulated by these high-energy molecules, including the activation of innate immune responses in mammals and plants, insulin signaling, telomere length maintenance, and cell death (4–8). IPP-generating enzymes control cell morphogenesis in fungi, including that of human fungal pathogens (9–11). In the fission yeast Schizosaccharomyces pombe the PPIP5K/Vip1 family member Asp1 is essential for the adaptation to nutrient limitation resulting in the dimorphic switch which allows yeast cells to grow in a substrate-invasive pseudohyphal manner (9). Alteration of the interphase microtubule (MT) cytoskeleton is an important contributor to efficient pseudohyphal growth in S. pombe (9), and Asp1 is needed for stability of interphase MTs (10). Assembly and function of the mitotic spindle also rely on Asp1: S. pombe cells expressing specific asp1 variants show aberrant bipolar spindle formation due to altered MT dynamics and spindle forces plus defects at the kinetochore-microtubule interface, leading to chromosome missegregation (12).
Two mechanisms have been described for modulation of biological processes by IPPs: pyrophosphorylation and the reversible binding to a protein (13, 14). IPP protein targets appear to be numerous as more than 150 Saccharomyces cerevisiae proteins were isolated in a screen using inositol polyphosphates/pyrophosphates as bait (15).
The best-studied IPPs are the two diphosphoinositol pentakisphosphate isoforms, 1-IP7 and 5-IP7, and bis-diphosphoinositoltetrakisphosphate, 1,5-IP8. They are synthesized from inositol hexakisphosphate (named IP6 in the text) by two classes of enzyme families: IP6Ks/Kcs1 and PPIP5Ks/Vip1. Synthesis of 5-IP7 is carried out by the 5-kinase activity of IP6Ks/Kcs1 (16, 17) while PPIP5Ks/Vip1 can add a diphosphate group to position 1 of IP6 and 5-IP7, thus generating 1-IP7 and 1,5-IP8 (named IP8 in the text), respectively (1, 2, 18, 19). The physiological in vivo substrate(s) of the kinase domain of the PPIP5K/Vip1 family has not been easy to define in a number of organisms analyzed to date (1–3). However, high-performance liquid chromatograph (HPLC) analysis of inositol phosphates in an S. cerevisiae VIP1 deletion strain suggested that Vip1 kinase activity might be responsible for the generation of IP8, as has been demonstrated for the PPIP5K/Vip1 family members in Cryptococcus neoformans and Arabidopsis thaliana (6, 11, 20). Also in mammalian cells PPIP5Ks are mainly responsible for IP8 synthesis since <2% of the IP7 pool is synthesized by PPIP5K (21).
Cellular IPP levels can be altered upon extrinsic signals. The jasmonate-mediated wound response of A. thaliana leads to an increase of IP8 (6). In Dictyostelium discoideum IPPs are greatly increased during the chemotactic response (22), while in mammalian cells IP8 levels are elevated upon hyperosmotic stress (3, 23). The mechanism(s) by which the relative abundance of IPPs is regulated is not understood. However, enzymes exist that can dephosphorylate IPPs in a nonspecific (24–26) or specific (27) manner. Thus, downregulation of such enzymes might contribute to increased cellular IPP pools.
In this context, the C-terminal domains of PPIP5K/Vip1 family members are of particular interest. PPIP5K/Vip1 proteins have an N-terminal kinase domain and a C-terminal domain with homology to histidine acid phosphatases (1). The signature motifs of histidine acid phosphatases, RHXXR and HD (28), are present in PPIP5K/Vip1 family members except for the aspartate next to the second histidine (1). Nevertheless, the C-terminal domain of the S. pombe PPIP5K/Vip1 member Asp1 has pyrophosphatase activity in vitro (10), which is inhibited by iron-sulfur clusters and is specific for the hydrolysis of the pyrophosphate at position 1 of the inositol ring (29).
In this work, we have dissected the function of the Asp1 kinase and pyrophosphatase domains in vivo and found that they control intracellular IP8 levels and thus the biological processes that require these specific IPPs.
RESULTS
The Asp1 kinase domain is responsible for the generation of IP8 in vivo.
To analyze the in vivo function of the Asp1 kinase domain, we measured inositol polyphosphates in a wild-type strain, a strain with a D-to-A change at position 333 encoded by the asp1 gene (asp1D333A), and an asp1Δ strain. The amino acid D333 is a key catalytic residue required for Asp1 kinase activity (2), while the entire asp1+ gene has been deleted in the asp1Δ strain (9).
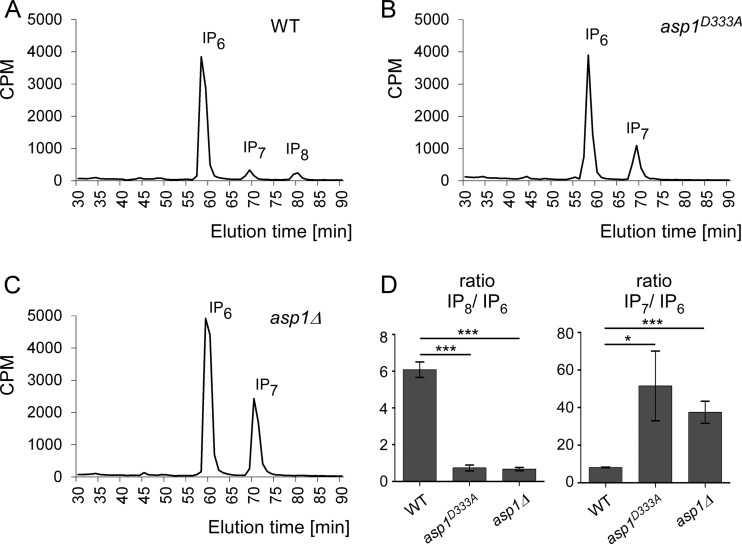
Inositol polyphosphates had not been assayed in S. pombe cells before, and thus we first defined the growth conditions needed. S. pombe is a natural inositol auxotroph and requires inositol in the medium (30; also our observations). Inositol at a minimum concentration of 10 μM was required for normal cell growth. Thus, cells of the three strains were radiolabeled with [3H]inositol in the presence of 10 μM cold inositol. Next, soluble inositol polyphosphates were extracted, and fractions were separated by HPLC and quantified by scintillation counting (31). The wild-type strain showed three prominent peaks; the most abundant was IP6, followed by IP7 and IP8 (Fig. 1A; for profiles of IPP standards, see Fig. S1 in the supplemental material). In the asp1D333A and asp1Δ strains, the IP8 peak was absent and the IP7 peak increased (Fig. 1B and C; quantification in panel D). Thus, Asp1 kinase has an enzymatic function in vivo, generating IP8 via the IP7 substrate. We have shown previously that strains without functional Asp1 kinase have defects in two biological processes: (i) chromosome segregation and (ii) the dimorphic switch (9, 10, 12). We can conclude now that these processes require IP8.
FIG 1.
Asp1 kinase generates IP8. (A to C) HPLC elution profiles of inositol polyphosphates of wild-type (WT), asp1D333A, and asp1Δ strains. S. pombe cells were radiolabeled with [3H]inositol, and cell lysates were separated using anion-exchange HPLC. CPM, counts per minute. (D) Diagrammatic representations of IP8 levels relative to those of IP6 and IP7 levels relative to those of IP6 (WT, n = 3; asp1D333A strain, n = 2; asp1Δ strain, n = 3; ***, P ≤ 0.001; *, P ≤ 0.05, t test). The fold change of IP8/IP6 is as follows (WT set at 1.00): 0.12 for the asp1D333A strain and 0.11 for the asp1Δ strain. Fold changes of IP7/IP6 are as follows: 6.26 for the asp1D333A strain and 4.56 for the asp1Δ strain. n, number of experiments done.
Asp1 pyrophosphatase activity leads to destabilized MTs and an inability to switch to pseudohyphal invasive growth.
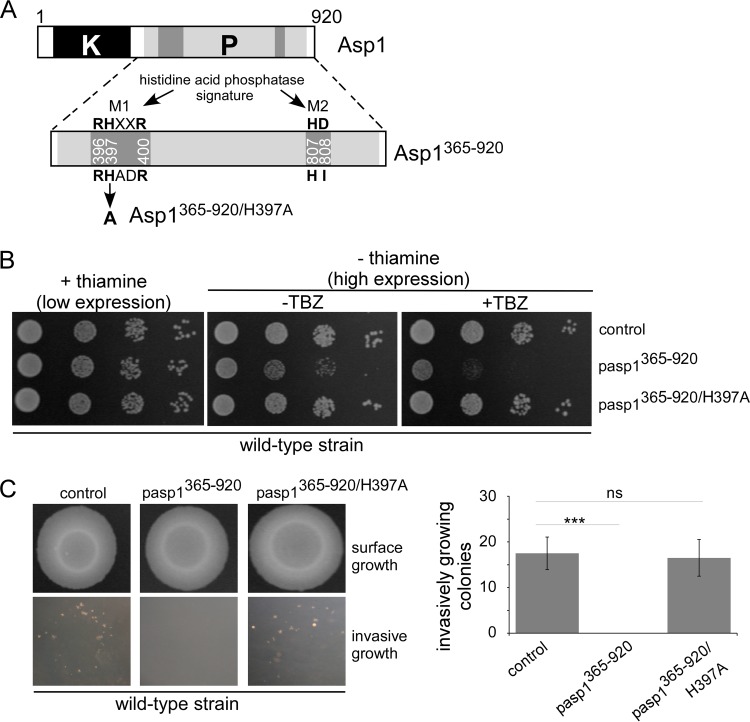
To understand the in vivo function of the Asp1 C-terminal domain, we assayed the consequences of overexpression of wild-type and mutant variants on MT stability and the dimorphic switch. The mutant Asp1 C-terminal proteins were generated by mutating conserved amino acids of the histidine acid phosphatase signature motifs (Fig. 2A, M1 and M2) (1).
FIG 2.
In vivo analysis of Asp1365–920 and Asp1365–920/H397A function. (A) Diagrammatic representation of the dual-domain structure of Asp1 with kinase (K) and pyrophosphatase (P, light gray box) regions. An enlargement of pyrophosphatase domain with the signature motifs M1 and M2 of histidine acid phosphatases is shown (1). In Asp1, the aspartate residue of M2 is replaced by isoleucine (HI instead of HD). (B) Serial dilution patch tests (104 to 101 cells) of a wild-type strain transformed with vector (control) or plasmids expressing asp1365–920 or asp1365–920/H397A from the thiamine-repressible promoter nmt1+. Transformants were grown under plasmid-selective conditions in the absence or presence of 7 μg/ml TBZ at 25°C for 7 days. (C) Invasive-growth assay. A total of 105 wild-type cells transformed with either a vector control or a plasmid expressing asp1365–920 or asp1365–920/H397A were spotted on plasmid-selective medium without thiamine and incubated for 21 days at 30°C (surface growth). Plates were washed, and all surface growth was rubbed off. Invasively growing colonies remained (bottom panels) and were counted. In the quantification shown on the right, three transformants were analyzed per plasmid in triplicate; ns, not significant; ***, P < 0.0005, t test. The numbers of agar-invading colonies of the asp1365–920/H397A transformants and the control transformants were 16.5 ± 4.0 and 17.5 ± 3.6, respectively.
A strain expressing the wild-type Asp1 C-terminal domain (amino acids 365 to 920, encoded by asp1365–920) (Fig. 2A) from the thiamine-repressible nmt1+ promoter was hypersensitive to the MT poison thiabendazole (TBZ), demonstrating that expression of the wild-type pyrophosphatase domain decreased MT stability (Fig. 2B, middle rows) (10). However, expression of a strain with an H-to-A change at position 397 encoded by an asp1365–920 mutant (asp1365–920/H397A) (Fig. 2A, M1 motif) did not lead to TBZ hypersensitivity (Fig. 2B, bottom rows), indicating that Asp1365–920/H397A was nonfunctional. Protein expression levels of Asp1365–920 and Asp1365–920/H397A were comparable (Fig. S2).
Similarly, the ability to grow in an invasive pseudohyphal manner was abolished in cells expressing asp1365–920. A wild-type strain expressing asp1365–920 on a plasmid via the nmt1+ promoter could not grow invasively (Fig. 2C, bottom middle panel). Growth per se was not affected in asp1365–920-expressing cells (Fig. 2C, surface growth). On the other hand, asp1365–920/H397A-expressing cells grew invasively in numbers comparable to those of the control (Fig. 2C, bottom right and left panels, respectively, and graph).
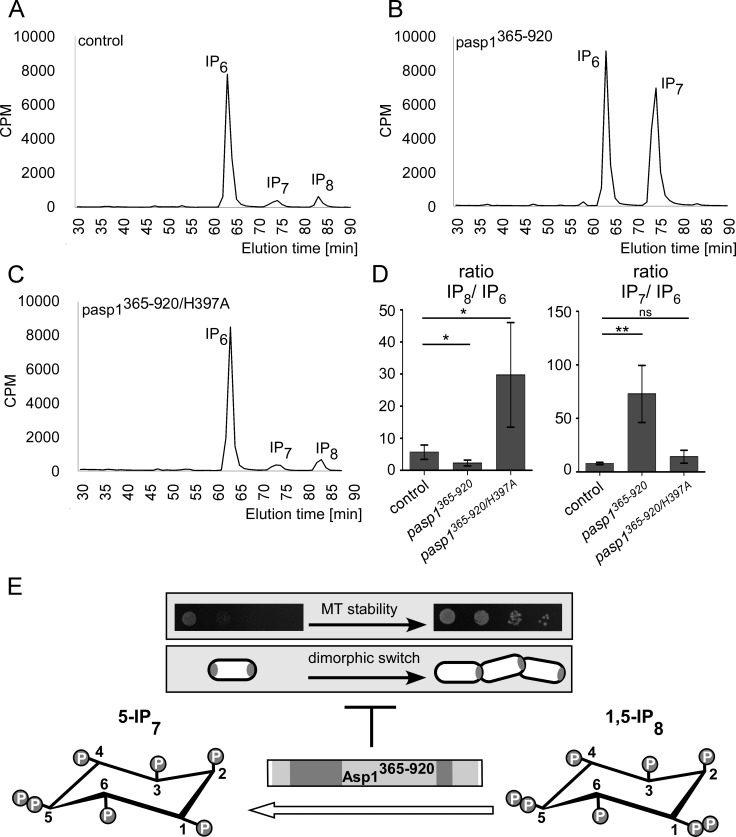
To understand the possible effect of asp1365–920 and asp1365–920/H397A expression on intracellular IPP levels, we measured inositol polyphosphates in strains expressing these variants. As expected, the wild-type strain transformed with the vector control showed the three peaks for IP6, IP7, and IP8 (Fig. 3A). Expression of asp1365–920 massively decreased IP8 levels and increased IP7 in comparison to the control levels (Fig. 3B; quantification in panel D). Thus, Asp1365–920 has in vivo pyrophosphatase activity and the substrate is IP8.
FIG 3.
Asp1365–920 has pyrophosphatase activity in vivo. (A to C) HPLC elution profiles of inositol polyphosphates of the wild-type strain transformed with a vector control (A) or asp1365–920- or asp1365–920/H397A-expressing plasmids (B and C, respectively). Cells were radiolabeled with [3H]inositol, and cell lysates were separated using anion-exchange HPLC. (D) Diagrammatic representation of IP8 levels relative to those of IP6 and IP7 levels relative to those of IP6 normalized to values for the vector control using data from panels A to C (control, n = 4; pasp1365–920 strain, n = 4; pasp1365–920/H397A strain, n = 4; **, P ≤ 0.01; *, P ≤ 0.05; ns, not significant, t test). The fold change of IP8/IP6 is as follows (control set at 1.00): 0.4 for the pasp1365–920 strain and 5.3 for the pasp1365–920/H397A strain. Fold changes of IP7/IP6 are as follows: 9.3 for the pasp1365–920 strain and 1.8 for the pasp1365–920/H397A strain. (E) MT stability and the dimorphic switch require intracellular IP8, which is downregulated by Asp1 pyrophosphatase activity.
The inositol polyphosphate profile of asp1365–920/H397A-expressing cells did not decrease IP8 levels as shown for wild-type pyrophosphatase expression (Fig. 3B and C; quantification in panel D), demonstrating that this Asp1 variant was enzymatically inactive in vivo. In fact, the HPLC profile of asp1365–920/H397A-expressing cells consistently showed higher IP8 peaks than the control strain (Fig. 3D). This result raises the interesting possibility that Asp1365–920/H397A acts as a dominant negative that might titrate away a protein/protein complex required for activation of the wild-type pyrophosphatase.
In summary, the Asp1 C-terminal domain has enzymatic activity in vivo using IP8 as the substrate. IP8 is required for MT stability and the ability to switch to pseudohyphal invasive growth. Thus, the Asp1 pyrophosphatase domain negatively regulates these two biological processes (Fig. 3E).
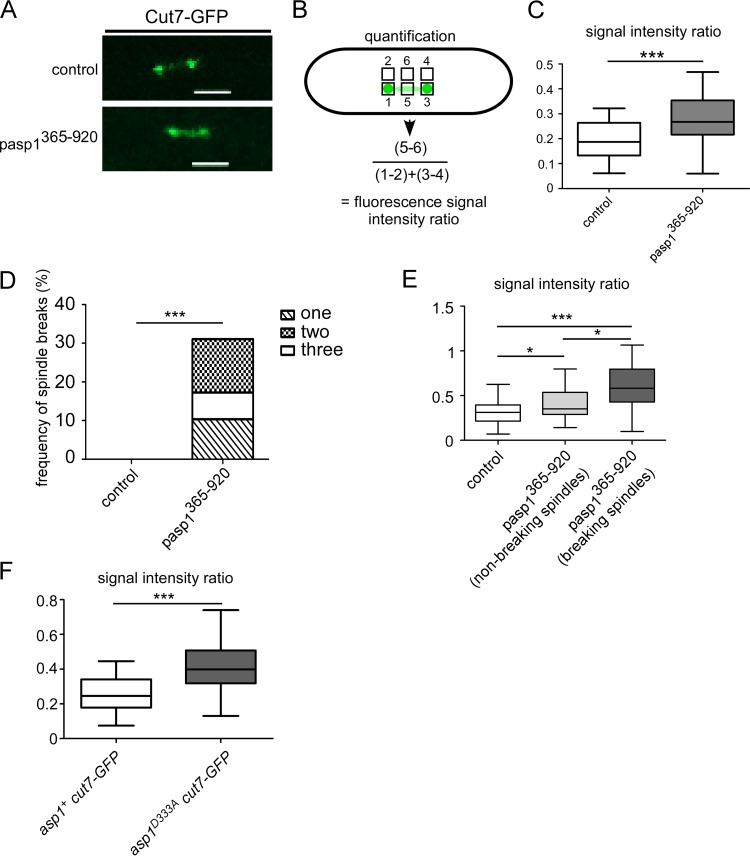
Correct spindle formation requires the concerted action of several motor proteins, and we have shown previously that ectopic expression of the Asp1 pyrophosphatase domain rescued the temperature-sensitive lethal phenotype of a cut7-446 strain (12). Cut7 belongs to the kinesin-5 Eg5 family of motor proteins, which localize to the spindle midzone and the spindle poles supporting bipolar spindle assembly (32). We assayed the consequences of asp1365–920 expression on mitotic Cut7 localization in a strain endogenously expressing cut7+-GFP (where GFP is green fluorescent protein) (33). Live-cell imaging of short spindles (2 to 3.5 μm) of a cut7+-GFP strain transformed with a control plasmid revealed fluorescence mainly at the two spindle poles and the spindle midzone (Fig. 4A). Cells expressing asp1365–920 had a significantly increased Cut7-GFP spindle midzone signal compared to that of control cells; quantification of the Cut7-GFP signal at the spindle middle in relation to the spindle pole signals (Fig. 4B) revealed that asp1365–920 expression led to an abnormal increase of Cut7-GFP fluorescence on the spindle (Fig. 4C).
FIG 4.
IP8 controls Cut7-GFP spindle association. (A) Photomicrographs of cut7+-GFP cells transformed with a vector control or an asp1365–920-expressing plasmid. Scale bars, 2 μm. (B) Quantification of the fluorescence signal of Cut7-GFP on short spindles. For comparison of the signal intensity at the spindle midzone to that at the spindle ends, the fluorescence signal at the midzone was normalized against the background (square 5 − square 6) and divided by the fluorescence intensity at spindle ends (square 1 − square 2 plus square 3 − square 4). (C) Diagrammatic representation of the ratio of the spindle midzone/spindle ends (control, n = 29; pasp1365–920 strain, n = 24; ***, P ≤ 0.001, t test; significant outliers were removed using Grubbs' test). (D) Diagrammatic representation of the frequency of spindle breaks in the indicated transformants (control, n = 30; pasp1365–920 strain, n = 29; ***, P ≤ 0.001, χ2 test). (E) Diagrammatic representation of the ratios of the spindle midzone/spindle ends (control, n = 30; pasp1365–920 strain [nonbreaking spindles], n = 23; pasp1365–920 strain [breaking spindles], n = 17 [9 cells]; *, P ≤ 0.05; ***, P ≤ 0.001, t test). (F) Diagrammatic representation of the ratios spindle midzone/spindle ends (asp1+ cut7-GFP strain, n = 29; asp1D333A cut7-GFP strain, n = 24; ***, P ≤ 0.001, t test; significant outliers were removed using Grubbs' test). Analysis was carried out at 33°C.
We had shown previously that asp1D333A-expressing mitotic cells showed spindle breakage of short spindles prior to sister chromatid separation (12). Spindle collapse was also observed in cut7+-GFP cells expressing plasmid-carried asp1365–920 (Fig. 4D). Thirty percent of such analyzed cells showed short spindles (<4.5 μm) that collapsed between one to three times during our analysis (Fig. 4D and Movie S1). Interestingly, asp1365–920-expressing cells with breaking spindles showed significantly higher Cut7-GFP spindle midzone fluorescence than cells with nonbreaking spindles (Fig. 4E), suggesting that spindle collapse might be mediated by abnormally large amounts of Cut7 on the spindle.
Cut7-GFP signal intensity was also assayed in an asp1D333A strain background. Again, we found that in the absence of Asp1-generated IP8, Cut7-GFP spindle fluorescence was increased significantly (Fig. 4F).
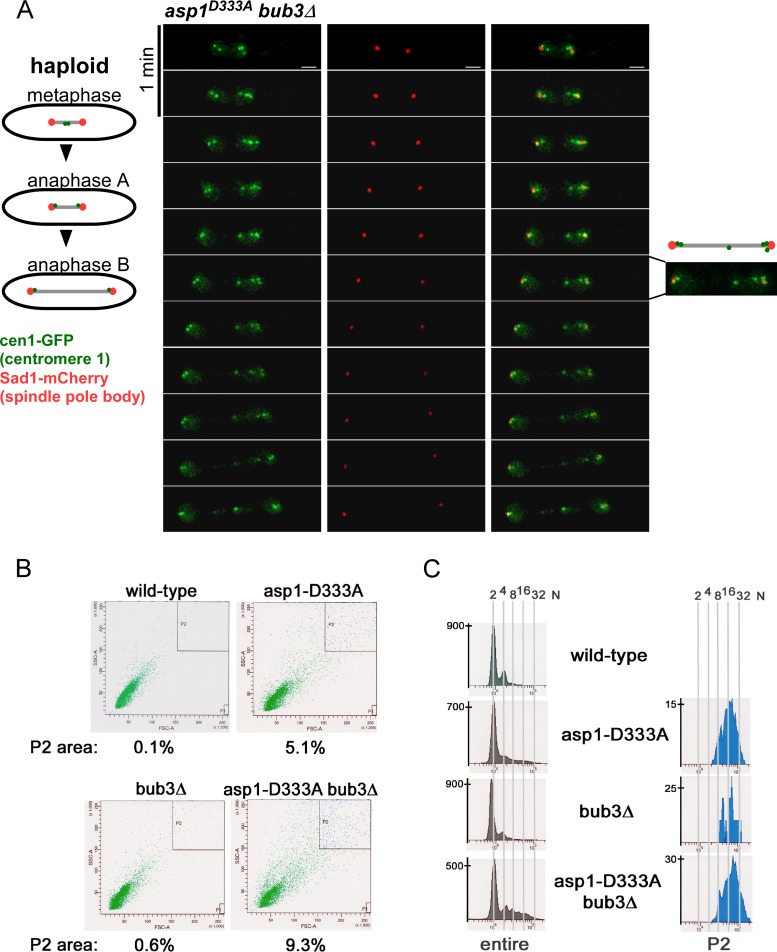
The human Eg5 kinesin-5 member is upregulated in many types of cancer, a feature that correlates with poor prognosis (34). S. pombe cells without a functional Asp1 kinase have defects in bipolar spindle formation and increased chromosome missegregation (12). As aberrant expression of human Eg5 results in polyploid cells in a mouse system (35), we reexamined chromosome segregation in IP8-less asp1D333A yeast strains. Time-lapse images of asp1D333A cells expressing cen1-GFP (marks chromosome I) and sad1+-mCherry (marks the spindle pole bodies) (36, 37) revealed several mitotic cells that had an aberrant number of cen1-GFP signals (Fig. 5A). S. pombe is a haploid organism; thus, during mitosis two segregating cen1-GFP signals representing the two chromosome I sisters are observed (36). In the photomicrographs in Fig. 5A, up to six cen1-GFP signals were observed, suggesting that these cells were polyploid. We therefore analyzed the ploidy state of wild-type, asp1D333A, bub3Δ, and asp1D333A bub3Δ strains via fluorescence-activated cell sorter (FACS) analysis (Fig. 5B). As shown in Fig. 5C, asp1D333A cell populations contain cells with an abnormally high DNA content (P2 population; P2 area is shown in Fig. 5B). This phenotype is increased in a bub3Δ strain background (Fig. 5C). P2 cells were longer and wider (on average 15%) than others in the entire cell population.
FIG 5.
asp1D333A cell population contains polyploid cells. (A) Photomicrographs of a mitotic asp1D333A bub3Δ cell expressing sad1+-mCherry and cen1-GFP. Time between images, 1 min; scale bar, 2 μm. Two of 11 analyzed asp1D333A bub3Δ double mutant cells showed this phenotype. (B) FACS analysis of the indicated strains. Cells were gated for size, revealing that cell populations with an asp1D333A strain background were much more heterogenous than asp1+ populations. The P2 area contains the largest cells. (C) Measurement of DNA content (2N to 32N) of the entire cell population and the P2 population. DNA content of peaks was defined by using the cdc11-123 strain as a standard (see Fig. S5 in the supplemental material) (65).
Intracellular IP8 levels are increased in strains without functional Asp1 pyrophosphatase.
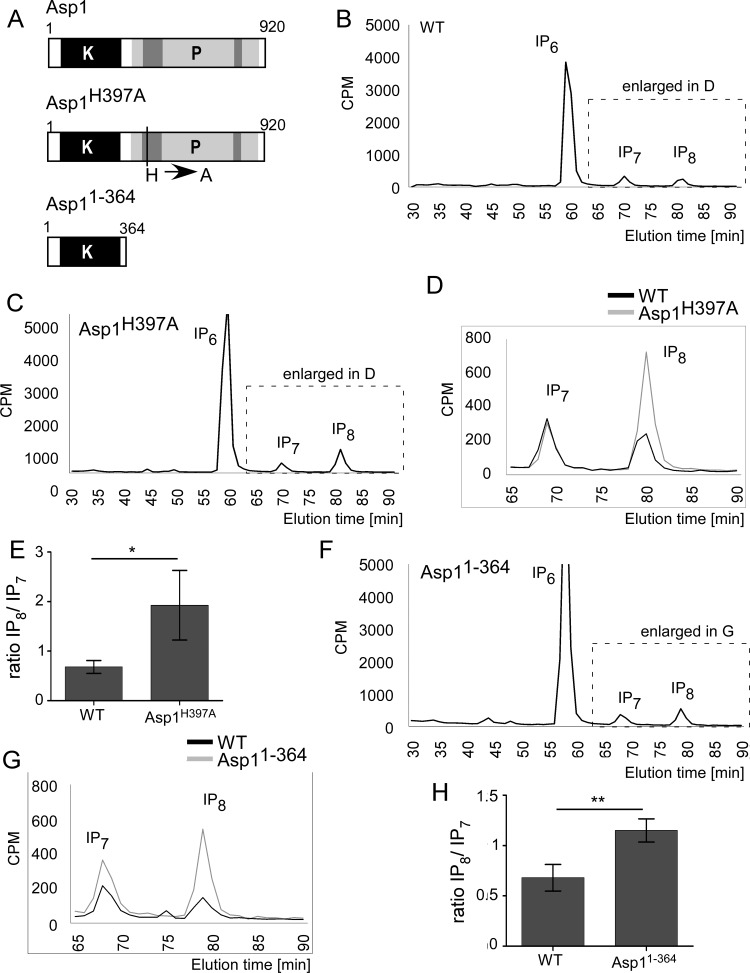
Expression of plasmid-carried asp1365–920 negatively affected intracellular IP8 levels while asp1365–920/H397A expression had no effect. Consequently, one would expect that a strain with an endogenous full-length asp1 variant with a mutation at position 397, i.e., the asp1H397A strain (Fig. 6A), would generate more IP8 than a wild-type strain. The HPLC profile of the asp1H397A strain (endogenous asp1+ open reading frame [ORF] replaced by asp1H397A [9]) showed a considerable increase of the IP8 peak compared with that of the wild-type asp1+ strain (Fig. 6B to D; quantification in panel E).
FIG 6.
Loss of a functional Asp1 pyrophosphatase domain results in increased IP8 levels. (A) Diagrammatic representation of Asp1 variants analyzed. All variants were expressed from the endogenous asp1+ locus. (B and C) HPLC elution profiles of inositol polyphosphates of the wild-type type (WT) and asp1H397A strains. (D) Comparison of part of the inositol pyrophosphate profiles of the wild-type and asp1H397A strains. (E) Diagrammatic representation of IP8 levels relative to those of IP7 (WT, n = 4; asp1H397A strain, n = 3; *, P ≤ 0.05, t test). The fold change of IP8/IP7 is 2.81 for the asp1H397A strain relative to the level of the wild-type strain. (F) HPLC elution profile of inositol polyphosphates of the asp11–364 strain. (G) Comparison of inositol pyrophosphate profiles of the wild-type and asp11–364 strains (data used for this wild-type strain were obtained from a strain grown in parallel to the asp11–364 strain). (H) Diagrammatic representation of IP8 levels relative to those of IP7 and normalized to the wild-type level (WT, n = 4; asp11–364 strain, n = 3; **, P ≤ 0.01, t test). The fold change of IP8/IP7 is 1.67 for the asp11–364 strain relative to the level of the wild-type strain.
Next, we investigated the consequences of the loss of the entire Asp1 pyrophosphatase domain on IP8 pools. For this we analyzed a strain which expressed an endogenous asp1 deletion variant consisting of only the Asp1 kinase domain Asp11–364 (asp11–364 strain) (Fig. 6A). The inositol polyphosphate profile of the asp11–364 strain also showed significantly higher IP8 levels (Fig. 6F and G; quantification in panel H). The similarity of the inositol polyphosphate profiles of the asp1H397A and asp11–364 mutant strains demonstrates that the change in IP8 levels observed for the asp1H397A strain is solely due to the missing pyrophosphatase activity.
All conserved residues of the M1 phosphatase motif are essential for enzymatic function.
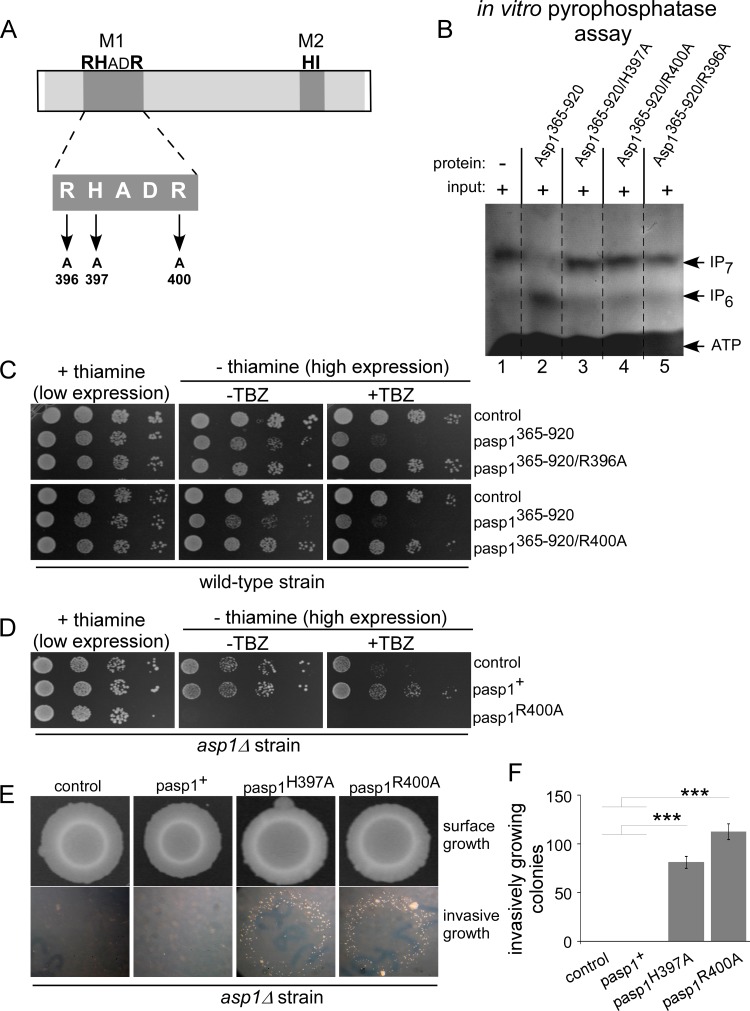
We have shown previously that bacterially produced, recombinant Asp1 protein generated IP7 in vitro using IP6 as a substrate (10). The addition of Asp1365–920 to the mixture for such a kinase assay reduced the IP7 amount in a dose-dependent manner. However, addition of Asp1365–920/H397A had no effect, demonstrating that this Asp1 pyrophosphatase variant had no enzymatic activity (10). To determine the role of the two other conserved amino acids of the M1 motif, we changed the arginine residues R396 and R400 to alanines individually (Fig. 7A) and tested the ability of these mutants to dephosphorylate Asp1 kinase-generated IP7 in vitro. It had been reported that recombinant bacterially expressed Asp1 is capable of incorporating an iron-sulfur cluster and that the presence of these iron-sulfur clusters inhibits the pyrophosphatase activity (29). Thus, we assessed the content of iron-sulfur clusters for all bacterially produced, recombinant Asp1 variants and found that our protein samples contained no iron-sulfur clusters (Fig. S3A).
FIG 7.
The conserved amino acids of the M1 motif are essential for pyrophosphatase activity. (A) Diagrammatic representation of Asp1 pyrophosphatase M1 motif mutants. (B) In vitro pyrophosphatase assay using Asp1365–920, Asp1365–920/H397A, Asp1365–920/R400A, or Asp1365–920/R396A. Eight micrograms of the indicated proteins was added to Asp1 kinase-generated IP7 (input is shown in lane 1) and incubated for 16 h, and the resulting inositol polyphosphates were resolved on a 35.5% PAGE gel and stained with toluidine blue (−, without added component; +, with added component). All pyrophosphatase variants were tested at least twice in the in vitro assay. (C) Serial dilution patch tests (104 to 101 cells) of a wild-type strain transformed with vector (control) or plasmids expressing the indicated asp1 variants via the nmt1+ promoter. Transformants were grown under plasmid-selective conditions with or without thiamine and with or without 7 μg/ml TBZ at 25°C for 7 days. (D) Serial dilution patch tests (104 to 101 cells) of an asp1Δ strain transformed with vector (control) or plasmids expressing asp1+ or asp1R400A from the nmt1+ promoter. Transformants were grown as described for panel C. (E) Invasive-growth assay. A total of 105 asp1Δ cells transformed with a vector control or plasmid expressing asp1+, asp1H397A, or asp1R400A were grown on plasmid-selective thiamine-supplemented medium for 21 days at 30°C (surface growth). Removal of surface growth by washing revealed invasively growing colonies (bottom panels). (F) Quantification of invasively growing colonies. Per plasmid, three transformants were analyzed in triplicate. ***, P < 0.0005, t test. Numbers of invasive colonies: 81 ± 6 for the asp1H397A strain and 113 ± 8 for the asp1R400A strain.
Recombinant glutathione S-transferase (GST)-Asp1365–920/R396A, GST-Asp1365–920/R400A, GST-Asp1365–920, and GST-Asp1365–920/H397A proteins were generated in bacteria, and the activity of these four Asp1 variants was tested in an in vitro pyrophosphatase assay. First, the IP7 substrate for the assay was synthesized using the Asp1 kinase domain (Asp11–364), which was heat inactivated after the reaction. Second, Asp1 pyrophosphatase variants were added to the mixture and incubated. The inositol polyphosphates present were then analyzed by PAGE (38). As shown previously (10) wild-type Asp1365–920 massively reduced the amount of IP7 (Fig. 7B, lane 2 versus input in lane 1) while the presence of Asp1364–920/H397A did not (Fig. 7B, lane 3). Similarly, GST-Asp1365–920/R396A and GST-Asp1365–920/R400A were unable to reduce the amount of IP7 in our assay (Fig. 7B, lanes 5 and 4, respectively), demonstrating that all conserved residues of the M1 motif were essential for in vitro enzymatic activity.
To investigate the in vivo function of Asp1365–920/R396A and Asp1365–920/R400A, the TBZ sensitivity of a wild-type strain expressing asp1365–920/R396A or asp1365–920/R400A on a plasmid via the nmt1+ promoter was examined. Western blot analysis showed that expression levels of these Asp1365–920 variants were similar (Fig. S2). In contrast to Asp1365–920, neither Asp1365–920/R396A nor Asp1365–920/R400A increased TBZ sensitivity of the strain (Fig. 7C).
Previously, we had shown that an asp1Δ strain was hypersensitive to TBZ and that this phenotype was rescued by high-level expression of plasmid-borne wild-type asp1+ or asp1R396A (10). To determine if Asp1R400A could rescue the TBZ hypersensitivity of the asp1Δ strain, we expressed this asp1+ version in the asp1Δ strain. However, high-level expression of asp1R400A led to cell death by lysis (Fig. 7D; also data not shown). The molecular basis for the lethality is unclear; however, we along with others had shown previously that high-level expression of plasmid-borne asp1H397A was also lethal due to cell lysis (2, 10).
Low-level expression of asp1R400A did not affect cell growth (Fig. 7D). Thus, we determined if low-level expression of asp1R400A could rescue the inability of the asp1Δ strain to switch to pseudohyphal invasive growth (9). This phenotype cannot be rescued by plasmid-carried wild-type asp1+ under low-level expression conditions (Fig. 7E). However, low-level expression of either asp1H397A or asp1R400A gave rise to invasively growing colonies (Fig. 7E; quantification in panel F). We conclude that Asp1R400A is able to generate more IP8 than the wild-type Asp1 protein.
Thus, all three conserved amino acids of the histidine acid phosphatase M1 motif RHADR (in bold) are essential for Asp1 pyrophosphatase activity.
Isoleucine 808 is critical for Asp1 pyrophosphatase function.
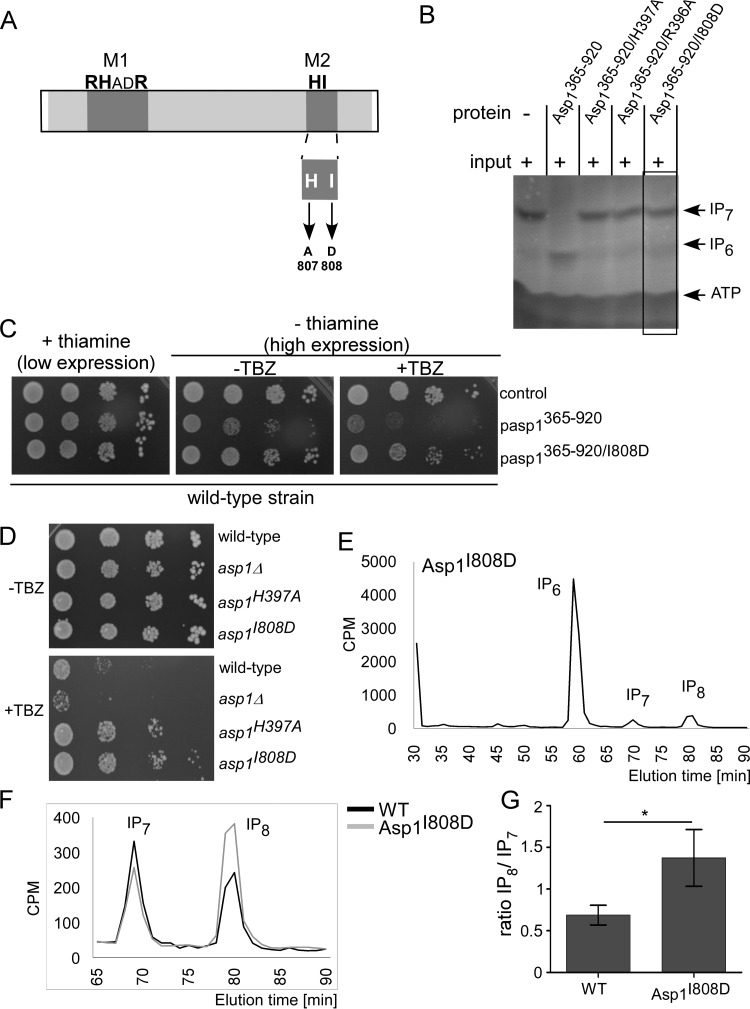
Histidine acid phosphatases require the presence of an aspartate in the M2 motif HD as a proton donor during the enzymatic reaction (28). No aspartate is found at this position in any Vip1 family member; in Asp1 an isoleucine residue is present at this position. To determine if a change to aspartate at this position influences pyrophosphatase activity, we assayed if Asp1365–920/I808D (Fig. 8A) could dephosphorylate IP7 in our in vitro assay. This was not the case (Fig. 8B).
FIG 8.
The I808D mutation abolishes Asp1 pyrophosphatase activity. (A) Diagrammatic representation of M2 motif mutants. (B) In vitro pyrophosphatase assay using 8 μg of protein of the indicated Asp1 variants. The assay was performed as described in the legend to Fig. 7B. The pyrophosphatase variants were tested at least twice in the in vitro assay. (C) Serial dilution patch tests (104 to 101 cells) of a wild-type strain transformed with a vector (control) or plasmid expressing asp1365–920 or asp1365–920/I808D via nmt1+. Transformants were grown under plasmid-selective conditions with or without thiamine and with or without 7 μg/ml TBZ at 25°C for 7 days. (D) Serial dilution patch tests (104 to 101 cells) of the wild-type, asp1Δ, asp1H397A, and asp1I808D strains grown on YE5S full medium at 25°C for 5 days with or without 12 μg/ml TBZ. (E) HPLC elution profile of inositol polyphosphates of the asp1I808D strain. (F) Comparison of inositol pyrophosphate profiles of wild-type and asp1I808D strains. (G) IP8 levels relative to those of IP7 and normalized to the wild-type level from data shown in panel F (WT, n = 4; asp1I808D strain, n = 3; *, P ≤ 0.05, t test). The fold change of IP8/IP7 is 2 for the asp1I808D strain compared to the level of the wild-type strain.
Furthermore, expression of plasmid-borne asp1365–920/I808D did not cause TBZ hypersensitivity (Fig. 8C). To test if intracellular IP8 levels were affected by the mutation at position 808 of Asp1, we constructed a strain in which the endogenous asp1+ gene was replaced by asp1I808D. Interestingly, the asp1I808D strain, similar to the asp1H397A strain, was more resistant to TBZ than a wild-type strain(Fig. 8D), showing that the Asp1I808D pyrophosphatase domain was nonfunctional.
Using HPLC-based analysis of inositol polyphosphates, we determined cellular IPP levels of the asp1I808D strain. IP8 was increased approximately 2-fold in the asp1I808D strain compared to the level in a wild-type strain (Fig. 8E to G). Therefore, alteration of amino acid 808 of Asp1 to aspartate, which is the proton donor in classical histidine acid phosphatases, abolished pyrophosphatase function.
Finally, we analyzed the function of the conserved histidine of the M2 motif (position H807) (Fig. 8A). A previous publication had described this residue to be essential for pyrophosphatase function in vitro (29). However, expression of the asp1365–920/H807A strain gave rise to TBZ hypersensitivity, indicating that this variant was functional (Fig. S4A). We thus tested this variant in our in vitro pyrophosphatase assay. The addition of 8 μg of either Asp1365–920 or Asp1365–920/H807A to the mixture for the assay reduced IP7, while the presence of Asp1365–920/R400A had no effect on IP7 levels (Fig. S4B, lanes 2 to 4). This result shows that Asp1365–920/H807A still retains pyrophosphatase activity. To understand the discrepancy between our data and those of Wang et al. (29), we repeated the assay with 4 and 2 μg of the relevant proteins. Four micrograms of protein led to a partial degradation of the IP7 input (Fig. S4B, lanes 5 to 7) while no pyrophosphatase activity was detected when 2 μg of the proteins was used (Fig. S4B, lanes 9 to 11). Thus, Asp1365–920/H807A retains residual pyrophosphatase activity.
Identification of the S. pombe Met10 protein, which inhibits Asp1 pyrophosphatase activity in vitro.
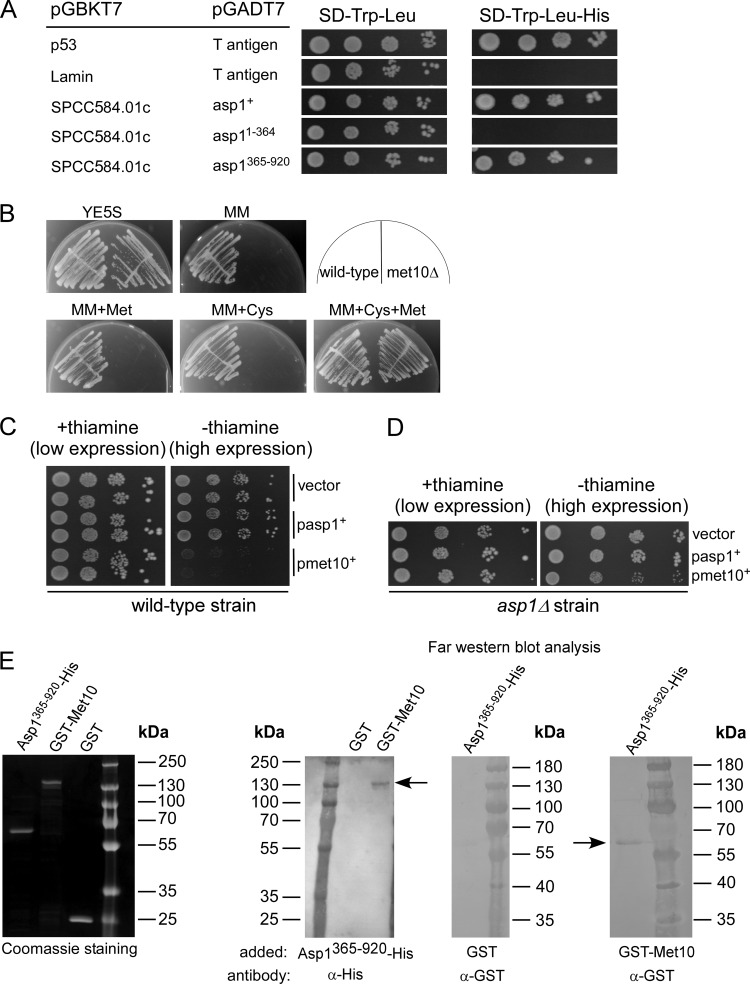
Our data show that the Asp1 protein harbors two enzymatic activities of opposing function and that MT stability and the dimorphic switch directly correlate with intracellular IP8 levels. To find Asp1-interacting proteins that influence the function of the two domains, we conducted an extensive yeast two-hybrid screen using pGBKT7-asp1+ as bait and an S. pombe cDNA library constructed in the pGAD GH vector (TaKaRa). Out of 2 × 107 transformants (4-fold coverage of the library), 150 plasmids with putative interacting candidates were isolated and retested. One of the Asp1-interacting proteins was encoded by the uncharacterized ORF SPCC584.01c, which interacted specifically with the Asp1 pyrophosphatase domain (Fig. 9A).
FIG 9.
Characterization of the Asp1 interaction partner Met10. (A) Yeast two-hybrid analysis of the interaction between Asp1 and Met10 (SPCC584.01c). S. cerevisiae strain AH109 was cotransformed with a plasmid expressing asp1+ fused to the GAL4 binding domain (pGBKT7) and a plasmid expressing an met10 variant (amino acids 544 to 1006) fused to the GAL4 activation domain (pGADT7). Cells were spotted on plasmid-selective SD medium with or without histidine and incubated for 5 days at 30°C. (B) Growth of wild-type and met10Δ strains on the indicated medium: full medium (YE5S), minimal medium (MM), MM plus 330 μM cysteine (MM+Cys), MM plus 140 μM methionine (MM+Met), or MM plus cysteine and methionine (MM+Cys+Met). (C) Serial dilution patch tests (104 to 101 cells) of a wild-type strain transformed with a vector (control) or plasmid expressing asp1+ or met10+ from the nmt1+ promoter. Transformants were grown at 25°C for 8 days. (D) Serial dilution patch tests (104 to 101 cells) of transformed asp1Δ cells with a vector (control) or plasmid expressing asp1+ or met10+ via nmt1+. Incubation was performed at 25°C for 11 days. (E) Far-Western analysis. A Coomassie-stained gel of 1 μg of the indicated purified proteins on the far left. Blots, from left to right, show the following: protein-protein interaction of GST-Met10 (blotted protein; 138 kDa, arrow) and Asp1365–920-His (probe protein) using His antibody for detection of GST-Met10; interaction of Asp1365–920-His (blotted protein) and GST (probe protein) with a GST antibody (control); protein-protein interaction of Asp1365–920-His (blotted protein; 65 kDa, arrow) and GST-Met10 (probe protein) using GST antibody for detection of Asp1365–920-His. One microgram of protein was loaded on the gel in all cases. Concentration of probe proteins, 10 μg/ml.
SPCC584.01c encodes a protein with a predicted size of 111.3 kDa which has 36% overall sequence identity and 52% similarity to Saccharomyces cerevisiae Met10, the alpha subunit of assimilatory sulfite reductase involved in methionine and cysteine synthesis (39). Due to this similarity, the ORF SPCC584.01c was named met10+ in the S. pombe database PomBase, and thus we refer to the protein as Met10.
To determine if the S. pombe Met10 protein had a function similar to that described for S. cerevisiae Met10, we analyzed the growth behavior of an S. pombe met10Δ (deletion of met10+ ORF) strain. The met10Δ strain required cysteine and methionine in the medium for growth (Fig. 9B), which is also the phenotype of the S. cerevisiae MET10 deletion strain (40). Overexpression of plasmid-borne met10+ was lethal in the wild-type strain (Fig. 9C). However, overexpression of met10+ was not lethal in the asp1Δ strain (Fig. 9D), indicating that the lethal phenotype requires the presence of Asp1. Thus, asp1+ and met10+ interact genetically. We tried to coimmunoprecipitate Asp1 and Met10 proteins in a strain in which the met10+ ORF had been fused with gfp using a GFP antibody followed by Western blot analysis with a polyclonal Asp1 antibody (41). However, coimmunoprecipitation using exponentially growing cells was not successful. Thus, we used far-Western blot analysis to determine whether Met10 and Asp1365–920 interact. Recombinantly produced and purified GST-Met10 interacted with His-Asp1365–920, demonstrating that the proteins can bind to each other (Fig. 9E).
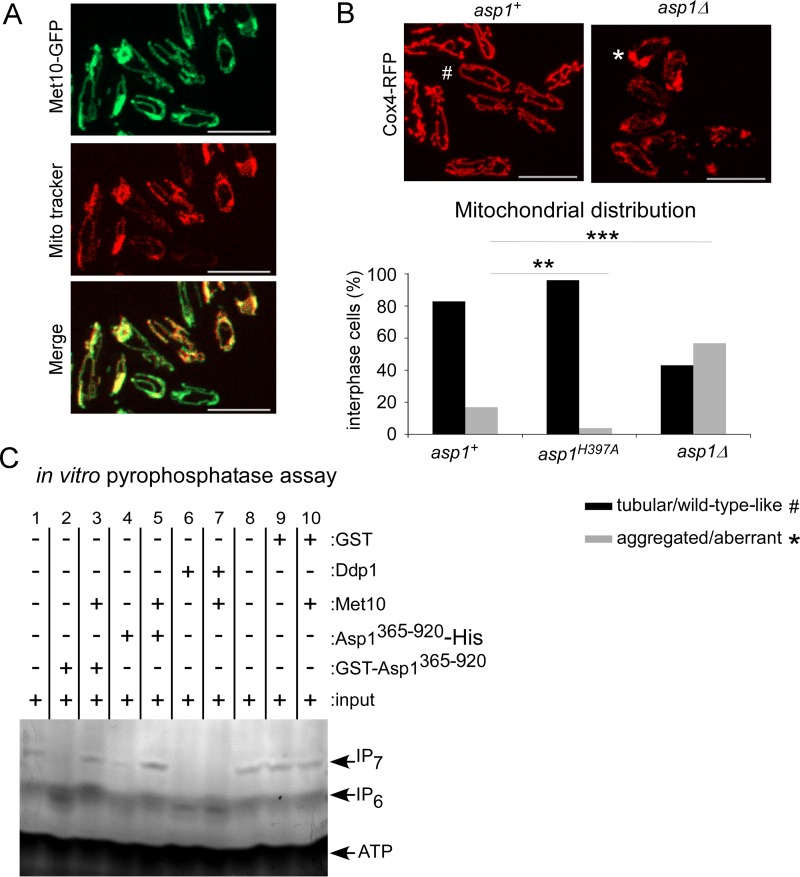
We next analyzed the subcellular localization of the Met10-GFP protein. Photomicroscopic analysis showed that Met10-GFP was associated with tubular-like structures, as has been observed for mitochondria (42). Staining of Met10-GFP cells with the mitochondrion-specific dye MitoTracker revealed colocalization (Fig. 10A). Thus, Asp1 can associate with a protein that colocalizes with mitochondria. Interestingly, in a screen for genes needed for survival under oxidative stress conditions, numerous genes related to mitochondrial function were identified, and the asp1+ ORF was one of the candidates (43). Indeed, we found that mitochondrion distribution depended on functional Asp1 kinase. In S. pombe the mitochondrion network is comprised of interconnected tubular-like structures that are MT associated (42), which guarantees proper mitochondrion positioning and inheritance (44). We found that in an asp1Δ strain, mitochondrial distribution was abnormal. Visualization of mitochondria via the mitochondrion inner membrane protein Cox4-red fluorescent protein (RFP) (44) showed that in the asp1+ and asp1H397A strain backgrounds, 83% and 96% of cells, respectively, showed the normal tubular-like mitochondrial structures (Fig. 10B). However, this number was reduced to 43% in asp1Δ cells. Instead, these cells had aberrant mitochondrial structures, the most prominent being aggregated mitochondria at the cell end(s) (Fig. 10B). This phenotype has been described previously for mutant mmb1 cells (44). The Mmb1 protein attaches the tubular mitochondria to the MT cytoskeleton (44). Intriguingly, when we expressed asp1 variants with a functional pyrophosphatase domain in an mmb1Δ strain (deletion of mmb1+ ORF) on a plasmid, the strains were unable to survive (data not shown). Thus, Asp1-generated IP8 has a role in mitochondrial function/organization.
FIG 10.
The mitochondrion-associated Met10 protein inhibits Asp1 pyrophosphatase activity in vitro. (A) Live-cell imaging of Met10-GFP cells stained with MitoTracker. Shown are maximum-intensity projection images of interphase cells grown at 25°C. Bars, 10 μm. (B) Live-cell imaging of the mitochondrial protein Cox4-RFP in asp1+ or asp1Δ cells was performed (top). Shown are maximum-intensity projection images of interphase cells grown at 25°C (#, normal mitochondrial distribution; *, abnormal mitochondrial distribution). Bars, 10 μm. Mitochondrial distribution is quantified in the graph: asp1+ strain, n = 143; asp1H397A strain, n = 77; asp1Δ strain, n = 44; **, P < 0.01; ***, P < 0.001, χ2 test). (C) In vitro pyrophosphatase assay. Lanes 1 and 8, input controls; lane 2, 4 μg of GST-Asp1365–920; lane 3, 4 μg of GST-Asp1365–920 plus 6 μg of Met10; lane 4, 4 μg of Asp365–920-His; lane 5, 4 μg of Asp365–920-His plus 8 μg of Met10; lane 6, 2 μg of Ddp1-GST; lane 7, 2 μg of Ddp1-GST plus 6 μg of Met10; lane 9, 2 μg of GST; lane 10, 6 μg of GST-Met10 and 2 μg of GST. In vitro pyrophosphatase assays involving Met10 protein were repeated four times. All assay mixtures were incubated for 16 h, and the resulting inositol polyphosphates were resolved by 35.5% PAGE and stained with toluidine blue (−, without added component; +, with added component). Sizes of proteins used for the experiment shown in panel C are as follows: GST-Asp1365–920, ∼91 kDa; GST-Met10, ∼138 kDa; Asp1365–920-His, ∼66 kDa; GST-Ddp1, ∼48 kDa.
To determine if Met10 affects Asp1 pyrophosphatase function, bacterially produced, recombinant GST-Met10 was added to an Asp1365–920-containing mixture for an in vitro pyrophosphatase assay. As the S. cerevisiae Met10 protein interacts with the cytoplasmic iron-sulfur assembly (CIA) component Mms19 (alias Met18) that is required for Fe-S protein maturation and is also a target of this complex, we first determined if the recombinant GST-Met10 protein contained an iron-sulfur cluster (45). This was not the case (Fig. S3B).
As shown previously, the presence of GST-Asp1365–920 in the pyrophosphatase assay mixture resulted in dephosphorylation of IP7 (Fig. 10C, lane 2). However, in the presence of equimolar amounts of GST-Met10 and GST-Asp1365–920 in the assay mixture, IP7 was not dephosphorylated (Fig. 10C, lane 3). Thus, Met10 inhibits the function of the Asp1 pyrophosphatase domain. As both proteins were GST tagged and as GST-GST interactions can occur, we repeated the assay using Asp1365–920-His and GST-Met10. Again, Asp1365–920-His dephosphorylated IP7 but not in the presence of GST-Met10 (Fig. 10C, lanes 4 and 5). Thus, in vitro the Met10 protein is an inhibitor of Asp1 pyrophosphatase activity. To determine whether the inhibitory effect of Met10 was specific for Asp1 pyrophosphatase, we tested if Met10 could inhibit another protein with pyrophosphatase activity. For this purpose recombinant GST-Ddp1 was generated and used in our in vitro assay. The S. cerevisiae Ddp1 protein has inositol pyrophosphatase activity (25). Ddp1 enzymatic activity dephosphorylated IP7 (Fig. 10C, lanes 6 and 7), and this ability was not altered in the presence of equimolar amounts of GST-Met10 (Fig. 10C, lane 7). Thus, in vitro Met10 inhibits specifically Asp1365–920 pyrophosphatase activity. However, as the inositol polyphosphate profiles of wild-type and met10Δ strains were similar, loss of Met10 was not sufficient to significantly downregulate Asp1 pyrophosphatase activity in vivo (data not shown).
DISCUSSION
In this work, we have established that Asp1 is a bifunctional enzyme in vivo responsible for the synthesis and hydrolysis of one specific inositol pyrophosphate, IP8. Functional dissection of the Asp1 pyrophosphatase by mutational analysis combined with our previous analysis of Asp1 function demonstrated that morphogenesis and chromosome transmission are regulated by IP8 in a dose-dependent manner (9, 10, 12). In fact, a direct correlation exists for the optimization of a cellular process and IP8 levels; for example, higher-than-wild-type IP8 levels resulted in higher-than-wild-type chromosome transmission fidelity. On the other hand, strains with less than wild-type IP8 levels or no IP8 showed decreased chromosome transmission fidelity (12). The output of the Asp1 kinase is altered by the Asp1 pyrophosphatase; thus, up- or downregulation of pyrophosphatase activity controls intracellular IP8 levels.
Identification of conserved amino acids essential for pyrophosphatase function.
We were the first to show in an in vitro assay that a member of the PPIP5K/Vip1 family proteins has pyrophosphatase activity: IP7 produced by the Asp1 kinase was reduced by Asp1365–920, demonstrating that the C-terminal Asp1 domain was enzymatically active (10). Pyrophosphatase activity depended on the two conserved signature motifs of histidine acid phosphatases M1 and M2. The conserved amino acids of M1 were essential for enzymatic function of the Asp1 pyrophosphatase in vitro and in vivo. Similarly, our in vivo readout assays for strains expressing asp1 variants with the mutation R396A or R400A imply that these are also pyrophosphatase negative (10). In metazoans, a mutation in either PPIP5K protein complementary to the Asp1R396 mutation had a similar effect (46).
Of particular interest was the second amino acid of the M2 motif HD as this amino acid is not conserved in PPIP5K/Vip1 family members (1). For Asp1 the M2 motif is HI. The catalytic mechanism of histidine acid phosphatases requires a proton donor, which is typically a glutamate or aspartate residue proximal to the active site (28). Replacement of the glutamate/aspartate residue resulted in a dramatic decrease in enzymatic activity (47, 48). Thus, it was of great interest to determine the enzymatic activity of a mutant Asp1 variant where the wild-type isoleucine had been replaced by aspartate, resulting in the “perfect” M2 signature motifs of histidine acid phosphatases. Asp1I808D variants had no in vitro or in vivo pyrophosphatase activity. Furthermore, replacement of isoleucine 808 by valine, which is found at this position in metazoan PPIP5K/Vip1 family members, also led to inactivation of pyrophosphatase function (data not shown) (1, 3).
Finally, the histidine in the M2 motif is conserved in histidine acid phosphatases and all PPIP5K/Vip1 family members (1). A previous publication showed that mutation of this residue generating Asp1397–920/H807A led to a loss of about 95% activity in vitro (29). However, we found that Asp1365–920/H807A retained residual pyrophosphatase activity. The difference in the results obtained might be due to a different experimental setup. Interestingly, it has been shown for the rat fructose 2,6-bisphosphatase that the replacement of the equivalent histidine did not significantly change the enzymatic activity (49).
Cellular levels of IP8 are regulated by Asp1 pyrophosphatase activity.
Ectopic expression of asp1365–920 massively reduced cellular IP8 amounts while endogenous pyrophosphatase-dead variants increased cellular IP8 levels. Thus, intracellular IP8 levels can be up- or downregulated by the enzymatic activity of the Asp1 pyrophosphatase domain. These high-energy molecules are generated solely by the Asp1 kinase domain as asp1Δ and asp1D333A strains had no detectable IP8 (2, 9, 10). Similarly, S. cerevisiae and C. neoformans strains with a deletion of the gene which encodes the PPIP5K/Vip1 protein have no or massively reduced IP8 levels but elevated lP7 levels, implying that in these organisms PPIP5K/Vip1 proteins generate IP8 (6, 11, 20). The in vivo function of the pyrophosphatase domain of PPIP5K/Vip1 proteins in other organisms remains to be studied.
The Asp1-interacting protein Met10 inhibits pyrophosphatase activity in vitro.
We identified the mitochondrion-associated Met10 protein that specifically interacted with the Asp1 pyrophosphatase domain and inhibited its function in vitro. Met10 belongs to a conserved protein family involved in the methionine biosynthesis pathway. Interestingly, the S. cerevisiae Met10 member interacts physically with the highly conserved Mms19 (alias Met18) protein (45). Mms19, which was identified previously to be required also for methionine biosynthesis, has since been shown to be a member of the Fe-S protein assembly (CIA) machinery (45, 50, 51). Incorporation of iron-sulfur clusters into proteins is mediated by a two-step mechanism occurring in the mitochondria and the cytosol (reviewed in reference 52). Mms19 serves as part of a CIA targeting complex responsible for iron-sulfur cluster insertion into proteins involved in specific cellular processes including methionine biosynthesis (45). Mms19 is needed for the sulfite reductase activity of the S. cerevisiae Met5-Met10 complex where Met10 represents a Fe-S-containing protein (45). As the Asp1 pyrophosphatase activity is inhibited by the incorporation of an iron-sulfur cluster in vitro (29), it is possible that such an iron-sulfur cluster transfer could occur via S. pombe Met10 in vivo. However, the consequences of such a transfer in vivo remain unclear. Inositol polyphosphate profiles of wild-type and met10Δ strains were comparable, and expression of an asp1 variant where one of the cysteine residues required for binding the iron-sulfur cluster was mutated (29) had no phenotypic consequences for yeast cell growth under various conditions (data not shown).
IP8 and its impact on the microtubule cytoskeleton.
The human MMS19 protein is part of the five-component MMXD complex required for chromosome transmission fidelity. MMS19 localizes to the mitotic spindle, and a knockdown of MMS19 gave rise to highly abnormal spindles (53). Thus, MMS19 is required for spindle formation/function. We have previously shown that S. pombe Asp1 kinase function controls bipolar spindle formation by modulating in- and outward pulling forces at the spindle (12). Our results raise the intriguing possibility that IP8-modulated MT regulation involves the Met10-Mms19 pathway. Although the impact of the Mms19 protein on the MT cytoskeleton has not been tested in S. pombe, it has been found that S. pombe cells with a deletion of the mms19+ gene have an abnormal cell shape, with branched and curved cells (54). Such cell shapes are indicative of a defective interphase MT cytoskeleton (reviewed in reference 55). Furthermore, an S. cerevisiae met10Δ bim1Δ double mutant strain is nonviable (56). Bim1 is a part of the EB1 family, which represents a central element of polymerizing MT plus ends (57). Thus, it is feasible that the Met10 and Mms19 proteins play a role in MT modulation.
Central elements in bipolar spindle assembly/function and segregation of spindle poles are kinesin-5 family members (58). The human kinesin-5 Eg5 protein has been the focus of research due to its important role in tumorigenesis. This motor protein is upregulated in many types of cancer, such as pancreatic cancer, is associated with poor prognosis, and can trigger genome instability in the mouse system (34, 35, 59). It is thus of great interest that intracellular IP8 levels control spindle association of the S. pombe kinesin-5 Cut7. This finding raises the exciting possibility that IP8 levels could be used as a tool to control Eg5 upregulation.
MATERIALS AND METHODS
Strains, plasmids, and media.
All strains used are listed in Table 1. Generation of asp1 mutant strains was performed as described previously (9). Gene deletions and ORF fusions to gfp were done by PCR-based gene targeting (60) using the kanamycin resistance (Kanr) cassette. Plasmids harboring asp1+, asp11–364, and asp1365–920 are derivatives of pJR2-3XL (9, 12, 61). For the plasmids containing asp1365–920/H397A, asp1365–920/H807A, asp1365–920/R396A, asp1365–920/R400A, and asp1365–920/I808D, PCR fragments were generated by directed mutagenesis using a QuikChange II site-directed mutagenesis kit (Agilent Technologies) and cloned into pJR2-3XL (61) via homologous recombination in S. cerevisiae (62).
TABLE 1.
Strains used in this study
| Strain | Genotypea | Source |
|---|---|---|
| S. pombe strains | ||
| UFY605 | his3-D1 ade6-M210 leu1-32 ura4-D18 h− | K. Gould |
| UFY1156 | asp1Δ::Kanr his3-D1 ade6-M216 leu1-32 ura4-D18 h− | U. Fleig |
| UFY1511 | asp1D333A::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h+ | U. Fleig |
| UFY1565 | cdc11-123 leu− h− | Yeast Genetic Research Center Osaka, Japan (FY8347)b |
| UFY1579 | asp1H397A::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h+ | U. Fleig |
| UFY1687 | cut7-GFP::Kanr cut12-CFP::Natr leu1 ura4 h− | Yeast Genetic Research Center Osaka, Japan (FY17673) |
| UFY2257 | bub3Δ::Kanr leu1− h− | Yeast Genetic Research Center Osaka, Japan (FY18583) |
| UFY2290 | bub3Δ::Kanr asp1D333A::Kanr | U. Fleig |
| UFY2294 | asp11−364::Kanr ura4-D18 leu1-32 his3-D1 ade6-M21x h+ | This study |
| UFY2386 | bub3Δ::Kanr asp1D333A::Kanr sad1-mCherry::Kanr LacI-GFP::his7+ LacO-repeat::lys1+ lys1-131 his7-366 h− | U. Fleig |
| UFY2553 | asp1I808D::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h− | This study |
| UFY2758 | met10Δ::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h− | This study |
| UFY2795 | met10-GFP::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h− | This study |
| UFY2805 | met10Δ::Kanr asp1D333A::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h− | This study |
| UFY2807 | met10Δ::Kanr asp1H397A::Kanr his3-D1 ade6-M210 leu1-32 ura4-D18 h− | This study |
| UFY2860 | met10Δ::Kanr mal3Δ::his3 ade6-M210 leu1-32 ura4-D18 his3-D h+ | This study |
| UFY2940 | asp1D333A-GFP::ura4+ cox4-RFP::LEU2 ade6-M210 leu1-32 ura4-D18 his3-D1 h− | This study |
| UFY2941 | asp1-pk-GFP::ura4+ cox4-RFP::LEU2 ade6-M21x leu1-32 ura4-D18 his3-D1 h+ | This study |
| UFY2951 | asp1Δ::Kanr cox4-RFP::LEU2 ura4-D18 leu1-32 his3-D1 ade6-M21x h− | This study |
| UFY3035 | cut7-GFP::Kanr leu1 ura4 his3-D1 | This study |
| UFY3039 | asp1D333A::Kanr cut7-GFP::Kanr his3-D1 ade6− leu1 ura4 | This study |
| S. cerevisiae strains | ||
| AH109 | MATα ura3-52 trp1-901 leu2-3112 his3-200 gal4Δ gal80Δ LYS2::GAL1UAS-GAL1TATA-HIS3 GAL2UAS-GAL2TATA-ADE2 URA3::MEL1UAS-MEL1TATA-lacZ | Clontech |
| Y187 | MATα ura3-52 trp1-901 leu2-3112 his3-200 gal4Δ met− gal80Δ URA3:: GAL1UAS-GAL1TATA-lacZ | Clontech |
| E. coli Rosetta(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) pRARE (Camr) | Novagen |
CFP, cyan fluorescent protein; UAS, upstream activation sequence.
The strain identification number is shown in parentheses.
S. pombe strains were grown in rich medium (YE5S) or minimal medium (MM) with supplements (63). To control the nmt1+ promoter, cells were grown in MM with or without 5 μg/ml thiamine. Experiments were carried out at 25°C, except for the invasive-growth experiments and the labeling with [3H]inositol, which were performed at 30°C. Microscopy was performed at temperatures stated in the respective figure legends.
Western blot analysis.
Transformants expressing plasmid-borne asp1 variants were grown under plasmid-selective conditions without thiamine for 24 h at 25°C before protein extraction. Protein extraction was carried out as described previously (9) using an anti-GFP antibody when asp1 variants were fused to gfp (monoclonal mouse; Roche) or using an anti-Asp1 antibody (41) and an anti-γ-tubulin antibody (monoclonal mouse; Sigma).
In vitro enzymatic activity of Asp1 variants.
Recombinant proteins Asp11–364 and Asp1365–920 were previously described (10). PCR 1,751-bp fragments containing asp1365–920/R396A, asp1365–920/H397A, asp1365–920/R400A, asp1365–920/H807A, and asp1365–920/I808D, a 3,101-bp fragment containing the entire met10+ ORF, and a 649-bp fragment containing the entire ScDDP1 ORF were cloned into the E. coli expression vector pKM36 to generate GST-tagged proteins or into the E. coli expression vector pFT25 to generate His-tagged proteins. Proteins were expressed and purified from the E. coli Rosetta(DE3) strain according to protocol (Sigma-Aldrich). Enzymatic reactions were performed as described previously (10, 38). For the kinase reaction, 4 μg of purified Asp11–364 protein was incubated for 16 h at 37°C with 300 μM IP6 (Sigma-Aldrich), followed by Asp11–364 inactivation (65°C for 20 min). Inactivation was verified by performing a kinase assay with the treated Asp11–364 protein. Thirty microliters of the generated IP7 was incubated with 8 μg of Asp1365–920 variants for 16 h at 37°C, followed by PAGE analysis. For the experiment shown in Fig. 10C, 8 μg of GST-Met10, 2 μg of GST-Ddp1, and 4 μg of Asp1365–920 were used in the assay.
[3H]inositol labeling and HPLC analysis.
[3H]inositol labeling of S. pombe cultures was performed as described previously (31). Cells were grown overnight at 30°C in MM with 55 μM inositol, then diluted to an optical density at 600 nm (OD600) of 0.05 in 5 ml of MM with 10 μM inositol supplemented with 6 μCi/ml of [3H]inositol, and incubated until the OD600 reached 0.8 to 1.6 (30 to 48 h). Extraction of inositol polyphosphates was performed as described previously (31) and resolved by anion-exchange HPLC (using a PartiSphere SAX 4.6- by 125-mm column; Whatman). Collected fractions were analyzed by scintillation counting. Soluble inositol polyphosphate levels were normalized against total lipid inositol content. Statistics for the ratios of IP8/IP6, IP8/IP7, and IP7/IP6 were performed using GraphPad Prism, version 5.
Electronic absorption spectroscopy.
Electronic absorption spectroscopy was used to determine the iron-sulfur cluster content of Asp1365–920. Electronic absorption spectra were recorded using a double-beam Jasco V-650 spectrophotometer at room temperature (RT). Spectra were obtained using a 1-cm-path-length cuvette for samples with a protein concentration of ∼1 μg/ml.
Flow cytometry.
Yeast flow cytometry was carried out as described previously using Sytox green (64) and a FACSAria instrument (BD Biosciences). Ten thousand cells were counted per sample, and all strains were counted at least twice and were grown at different temperatures before fixation (20 to 36°C). The data shown in Fig. 5B and C were obtained from cells incubated at 30°C but are representative of all other temperatures. DNA content of cells was defined using the temperature-sensitive cdc11-123 strain as a standard (65).
Invasive-growth assay.
Transformants were grown overnight in plasmid-selective medium with or without thiamine. Cells were diluted to an end concentration of 2 × 106 cells/ml, and 5 μl of cells was patched on plasmid-selective agar plates at equal distances from each other. Incubation was done at 30°C for 21 days (66). To analyze invasive growth, surface-grown cells were removed by washing, and plates were dried and then photographed using a binocular microscope and Sony digital single-lens reflex (DSLR) camera. Quantification of invasive growth was done by determining the number of invasive colonies per mutant in three different transformants in at least three different experiments.
Yeast two-hybrid screen.
A yeast two-hybrid screen was performed using the AH109 strain transformed with pGBKT7-asp1+ as bait and mated with Y187 transformed with an S. pombe cDNA library constructed in the pGAD GH vector (Matchmaker cDNA library [XL4000AA; TaKaRa]). Mating was plated on synthetic dextrose (SD)-Leu-Trp-His medium and incubated for 8 days. Plasmids from positive candidates were cotransformed with pGBKT7-asp1+ into strain AH109 and further analyzed.
Microscopy.
Live-cell imaging was performed using a Zeiss spinning-disk confocal microscope equipped with a Rolera EM-C (QImaging) camera. Transformants expressing cut7+-GFP were pregrown for 20 h at 30°C in plasmid-selective medium. Videos were taken at 30°C. For asp1+ cut7+-GFP and asp1D333A cut7+-GFP strains, growth and imaging were done at 33°C. A maximum intensity projection (MIP) picture (25 z-slices for transformants or 35 z-slices for strains in 0.5-μm intervals) of the time point with the strongest fluorescence signal on a short spindle was generated and used for analysis. Analysis was performed using Zen2012 and AxioVision software. Image processing was done with Canvas 14 and Adobe Photoshop CS2. Intensity of GFP fluorescence signals was measured via ImageJ, version 1.44 (NIH). The asp1D333A bub3Δ strain was pregrown for 24 h at 30°C. Shown in Fig. 5A are MIP images of a single cell. For live-cell imaging of met10+-GFP-expressing cells stained with MitoTracker or cox4+-RFP cells expressing different asp1 variants, cells were recorded at 25°C with a z-stack of 25 z-slices with a distance of 0.5 μm, and an MIP image was generated. Statistics for fluorescence signal intensity ratios and spindle break frequencies were performed using GraphPad Prism, version 5.
Far-Western blot analysis.
GST-Met10 and Asp1365–920-His were purified from the E. coli Rosetta(DE3) strain. One microgram of GST-Met10 or Asp1365–920-His (prey proteins) was separated by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane. After denaturation with 6 M guanidine-HCl, the prey protein was gradually renatured on the membrane by incubation with decreasing concentrations of guanidine-HCl in a buffer containing 10% glycerin, 0.1 M NaCl, 20 mM Tris, pH 7.5, 1 mM EDTA, 0.1% Tween 20, 2% milk, and 1 mM dithiothreitol (DTT). After an overnight incubation at 4°C with the buffer containing no guanidine-HCl, 10 μg/ml of Asp1365–920-His, GST-Met10, or GST (bait proteins) was incubated for 5 h at RT with the regenerated membrane. After three washes with phosphate-buffered saline (PBS), protein interactions were detected using His (Roche) or GST (Thermo Fisher) antibodies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Boris Topolski for his help with Fig. 4B and Katja Mölleken and Tim Fechtner for plasmids (all Heinrich Heine University, Düsseldorf, Germany), Phong Tran (University of Pennsylvania, USA) for strains, Klaus Meyer (Heinrich Heine University, Düsseldorf, Germany) for help with the FACS analysis, and Anna Feoktistova and Kathleen Gould (Vanderbilt University, Nashville, TN) for the very generous gift of Asp1 antibody. We thank the Center for Advanced Imaging at the Heinrich Heine University.
This work was supported by the Deutsche Forschungsgemeinschaft (http://www.dfg.de/) project FOR1334 and the Manchot Graduate school MOI II (Juergen Manchot Stiftung) to U.F., Fonds der Chemischen Industrie to I.S., and Medical Research Council (MRC) core support to the MRC/UCL Laboratory for Molecular Cell Biology University Unit (MC_UU_1201814) to A.S.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00047-18.
REFERENCES
- 1.Fridy PC, Otto JC, Dollins DE, York JD. 2007. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J Biol Chem 282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- 2.Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. 2007. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Williams J, Cho J, Falck JR, Shears SB. 2007. Purification, sequencing, and molecular identification of a mammalian PP-InsP5 kinase that is activated when cells are exposed to hyperosmotic stress. J Biol Chem 282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. 2005. Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc Natl Acad Sci U S A 102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. 2005. Inositol diphosphate signaling regulates telomere length. J Biol Chem 280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- 6.Laha D, Johnen P, Azevedo C, Dynowski M, Weiss M, Capolicchio S, Mao H, Iven T, Steenbergen M, Freyer M, Gaugler P, de Campos MK, Zheng N, Feussner I, Jessen HJ, Van Wees SC, Saiardi A, Schaaf G. 2015. VIH2 Regulates the synthesis of inositol pyrophosphate InsP8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell 27:1082–1097. doi: 10.1105/tpc.114.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulloor NK, Nair S, Kostic AD, Bist P, Weaver JD, Riley AM, Tyagi R, Uchil PD, York JD, Snyder SH, Garcia-Sastre A, Potter BV, Lin R, Shears SB, Xavier RJ, Krishnan MN. 2014. Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in type-I interferon response. PLoS Pathog 10:e1003981. doi: 10.1371/journal.ppat.1003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, Snowman AM, Moran TH, Mezey E, Snyder SH. 2010. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pöhlmann J, Fleig U. 2010. Asp1, a conserved 1/3 inositol polyphosphate kinase, regulates the dimorphic switch in Schizosaccharomyces pombe. Mol Cell Biol 30:4535–4547. doi: 10.1128/MCB.00472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pöhlmann J, Risse C, Seidel C, Pohlmann T, Jakopec V, Walla E, Ramrath P, Takeshita N, Baumann S, Feldbrugge M, Fischer R, Fleig U. 2014. The Vip1 inositol polyphosphate kinase family regulates polarized growth and modulates the microtubule cytoskeleton in fungi. PLoS Genet 10:e1004586. doi: 10.1371/journal.pgen.1004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lev S, Li C, Desmarini D, Saiardi A, Fewings NL, Schibeci SD, Sharma R, Sorrell TC, Djordjevic JT. 2015. Fungal inositol pyrophosphate IP7 is crucial for metabolic adaptation to the host environment and pathogenicity. mBio 6:e00531-. doi: 10.1128/mBio.00531-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topolski B, Jakopec V, Kunzel NA, Fleig U. 2016. Inositol pyrophosphate kinase Asp1 modulates chromosome segregation fidelity and spindle function in Schizosaccharomyces pombe. Mol Cell Biol 36:3128–3140. doi: 10.1128/MCB.00330-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YS, Mulugu S, York JD, O'Shea EK. 2007. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. 2004. Phosphorylation of proteins by inositol pyrophosphates. Science 306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 15.Wu M, Chong LS, Perlman DH, Resnick AC, Fiedler D. 2016. Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms. Proc Natl Acad Sci U S A 113:E6757–E6765. doi: 10.1073/pnas.1606853113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draskovic P, Saiardi A, Bhandari R, Burton A, Ilc G, Kovacevic M, Snyder SH, Podobnik M. 2008. Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chem Biol 15:274–286. doi: 10.1016/j.chembiol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. 1999. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol 9:1323–1326. doi: 10.1016/S0960-9822(00)80055-X. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Falck JR, Hall TM, Shears SB. 2011. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nat Chem Biol 8:111–116. doi: 10.1038/nchembio.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, Falck JR, Shears SB, York JD, Mayr GW. 2009. Structural analysis and detection of biological inositol pyrophosphates reveal that the family of VIP/diphosphoinositol pentakisphosphate kinases are 1/3-kinases. J Biol Chem 284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onnebo SM, Saiardi A. 2009. Inositol pyrophosphates modulate hydrogen peroxide signalling. Biochem J 423:109–118. doi: 10.1042/BJ20090241. [DOI] [PubMed] [Google Scholar]
- 21.Gu C, Wilson MS, Jessen HJ, Saiardi A, Shears SB. 2016. Inositol pyrophosphate profiling of two HCT116 cell lines uncovers variation in InsP8 levels. PLoS One 11:e0165286. doi: 10.1371/journal.pone.0165286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. 2003. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 114:559–572. doi: 10.1016/S0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 23.Pesesse X, Choi K, Zhang T, Shears SB. 2004. Signaling by higher inositol polyphosphates. Synthesis of bisdiphosphoinositol tetrakisphosphate (“InsP8”) is selectively activated by hyperosmotic stress. J Biol Chem 279:43378–43381. [DOI] [PubMed] [Google Scholar]
- 24.Safrany ST, Caffrey JJ, Yang X, Bembenek ME, Moyer MB, Burkhart WA, Shears SB. 1998. A novel context for the “MutT” module, a guardian of cell integrity, in a diphosphoinositol polyphosphate phosphohydrolase. EMBO J 17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. 2011. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem 286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher DI, Safrany ST, Strike P, McLennan AG, Cartwright JL. 2002. Nudix hydrolases that degrade dinucleoside and diphosphoinositol polyphosphates also have 5-phosphoribosyl 1-pyrophosphate (PRPP) pyrophosphatase activity that generates the glycolytic activator ribose 1,5-bisphosphate. J Biol Chem 277:47313–47317. doi: 10.1074/jbc.M209795200. [DOI] [PubMed] [Google Scholar]
- 27.Steidle EA, Chong LS, Wu M, Crooke E, Fiedler D, Resnick AC, Rolfes RJ. 2016. A Novel Inositol Pyrophosphate Phosphatase in Saccharomyces cerevisiae: Siw14 protein selectively cleaves the beta-phosphate from 5-diphosphoinositol pentakisphosphate (5PP-IP5). J Biol Chem 291:6772–6783. doi: 10.1074/jbc.M116.714907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigden DJ. 2008. The histidine phosphatase superfamily: structure and function. Biochem J 409:333–348. doi: 10.1042/BJ20071097. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Nair VS, Holland AA, Capolicchio S, Jessen HJ, Johnson MK, Shears SB. 2015. Asp1 from Schizosaccharomyces pombe binds a [2Fe-2S]2+ cluster which inhibits inositol pyrophosphate 1-phosphatase activity. Biochemistry 54:6462–6474. doi: 10.1021/acs.biochem.5b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez S, Homann MJ, Henry SA, Carman GM. 1986. Metabolism of the phospholipid precursor inositol and its relationship to growth and viability in the natural auxotroph Schizosaccharomyces pombe. J Bacteriol 166:779–786. doi: 10.1128/jb.166.3.779-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azevedo C, Saiardi A. 2006. Extraction and analysis of soluble inositol polyphosphates from yeast. Nat Protoc 1:2416–2422. doi: 10.1038/nprot.2006.337. [DOI] [PubMed] [Google Scholar]
- 32.Hagan I, Yanagida M. 1990. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- 33.Toya M, Sato M, Haselmann U, Asakawa K, Brunner D, Antony C, Toda T. 2007. Gamma-tubulin complex-mediated anchoring of spindle microtubules to spindle-pole bodies requires Msd1 in fission yeast. Nat Cell Biol 9:646–653. doi: 10.1038/ncb1593. [DOI] [PubMed] [Google Scholar]
- 34.Jin Q, Huang F, Wang X, Zhu H, Xian Y, Li J, Zhang S, Ni Q. 2017. High Eg5 expression predicts poor prognosis in breast cancer. Oncotarget 8:62208–62216. doi: 10.18632/oncotarget.19215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Wang X, Yang Y, Li D, Ren H, Zhu Q, Chen Q, Han S, Hao J, Zhou J. 2010. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol 221:221–228. doi: 10.1002/path.2706. [DOI] [PubMed] [Google Scholar]
- 36.Nabeshima K, Nakagawa T, Straight AF, Murray A, Chikashige Y, Yamashita YM, Hiraoka Y, Yanagida M. 1998. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol Biol Cell 9:3211–3225. doi: 10.1091/mbc.9.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagan I, Yanagida M. 1995. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol 129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loss O, Azevedo C, Szijgyarto Z, Bosch D, Saiardi A. 2011. Preparation of quality inositol pyrophosphates. J Vis Exp 55:e3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen J, Cherest H, Kielland-Brandt MC. 1994. Two divergent MET10 genes, one from Saccharomyces cerevisiae and one from Saccharomyces carlsbergensis, encode the alpha subunit of sulfite reductase and specify potential binding sites for FAD and NADPH. J Bacteriol 176:6050–6058. doi: 10.1128/jb.176.19.6050-6058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas D, Surdin-Kerjan Y. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61:503–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feoktistova A, McCollum D, Ohi R, Gould KL. 1999. Identification and characterization of Schizosaccharomyces pombe asp1+, a gene that interacts with mutations in the Arp2/3 complex and actin. Genetics 152:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaffe MP, Harata D, Verde F, Eddison M, Toda T, Nurse P. 1996. Microtubules mediate mitochondrial distribution in fission yeast. Proc Natl Acad Sci U S A 93:11664–11668. doi: 10.1073/pnas.93.21.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuin A, Gabrielli N, Calvo IA, Garcia-Santamarina S, Hoe KL, Kim DU, Park HO, Hayles J, Ayte J, Hidalgo E. 2008. Mitochondrial dysfunction increases oxidative stress and decreases chronological life span in fission yeast. PLoS One 3:e2842. doi: 10.1371/journal.pone.0002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu C, Jain D, Costa J, Velve-Casquillas G, Tran PT. 2011. mmb1p binds mitochondria to dynamic microtubules. Curr Biol 21:1431–1439. doi: 10.1016/j.cub.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJ, Pierik AJ, Wohlschlegel JA, Lill R. 2012. MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337:195–199. doi: 10.1126/science.1219723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu C, Nguyen HN, Hofer A, Jessen HJ, Dai X, Wang H, Shears SB. 2017. The significance of the bifunctional kinase/phosphatase activities of diphosphoinositol pentakisphosphate kinases (PPIP5Ks) for coupling inositol pyrophosphate cell signaling to cellular phosphate homeostasis. J Biol Chem 292:4544–4555. doi: 10.1074/jbc.M116.765743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostanin K, Van Etten RL. 1993. Asp304 of Escherichia coli acid phosphatase is involved in leaving group protonation. J Biol Chem 268:20778–20784. [PubMed] [Google Scholar]
- 48.Lin K, Li L, Correia JJ, Pilkis SJ. 1992. Glu327 is part of a catalytic triad in rat liver fructose-2,6-bisphosphatase. J Biol Chem 267:6556–6562. [PubMed] [Google Scholar]
- 49.Mizuguchi H, Cook PF, Tai CH, Hasemann CA, Uyeda K. 1999. Reaction mechanism of fructose-2,6-bisphosphatase. A mutation of nucleophilic catalyst, histidine 256, induces an alteration in the reaction pathway. J Biol Chem 274:2166–2175. [DOI] [PubMed] [Google Scholar]
- 50.Masselot M, De Robichon-Szulmajster H. 1975. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol Gen Genet 139:121–132. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- 51.Gari K, Leon Ortiz AM, Borel V, Flynn H, Skehel JM, Boulton SJ. 2012. MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 337:243–245. doi: 10.1126/science.1219664. [DOI] [PubMed] [Google Scholar]
- 52.Netz DJ, Mascarenhas J, Stehling O, Pierik AJ, Lill R. 2014. Maturation of cytosolic and nuclear iron-sulfur proteins. Trends Cell Biol 24:303–312. doi: 10.1016/j.tcb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Ito S, Tan LJ, Andoh D, Narita T, Seki M, Hirano Y, Narita K, Kuraoka I, Hiraoka Y, Tanaka K. 2010. MMXD, a TFIIH-independent XPD-MMS19 protein complex involved in chromosome segregation. Mol Cell 39:632–640. doi: 10.1016/j.molcel.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 54.Hayles J, Wood V, Jeffery L, Hoe KL, Kim DU, Park HO, Salas-Pino S, Heichinger C, Nurse P. 2013. A genome-wide resource of cell cycle and cell shape genes of fission yeast. Open Biol 3:130053. doi: 10.1098/rsob.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huisman SM, Brunner D. 2011. Cell polarity in fission yeast: a matter of confining, positioning, and switching growth zones. Semin Cell Dev Biol 22:799–805. doi: 10.1016/j.semcdb.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Pan X, Yuan DS, Xiang D, Wang X, Sookhai-Mahadeo S, Bader JS, Hieter P, Spencer F, Boeke JD. 2004. A robust toolkit for functional profiling of the yeast genome. Mol Cell 16:487–496. doi: 10.1016/j.molcel.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Beinhauer JD, Hagan IM, Hegemann JH, Fleig U. 1997. Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J Cell Biol 139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wojcik EJ, Buckley RS, Richard J, Liu L, Huckaba TM, Kim S. 2013. Kinesin-5: cross-bridging mechanism to targeted clinical therapy. Gene 531:133–149. doi: 10.1016/j.gene.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castillo A, Morse HC III, Godfrey VL, Naeem R, Justice MJ. 2007. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res 67:10138–10147. doi: 10.1158/0008-5472.CAN-07-0326. [DOI] [PubMed] [Google Scholar]
- 60.Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943–951. [DOI] [PubMed] [Google Scholar]
- 61.Moreno MB, Duran A, Ribas JC. 2000. A family of multifunctional thiamine-repressible expression vectors for fission yeast. Yeast 16:861–872. doi:. [DOI] [PubMed] [Google Scholar]
- 62.Jakopec V, Walla E, Fleig U. 2011. Versatile use of Schizosaccharomyces pombe plasmids in Saccharomyces cerevisiae. FEMS Yeast Res 11:653–655. doi: 10.1111/j.1567-1364.2011.00752.x. [DOI] [PubMed] [Google Scholar]
- 63.Moreno S, Klar A, Nurse P. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194:795–823. doi: 10.1016/0076-6879(91)94059-L. [DOI] [PubMed] [Google Scholar]
- 64.Sabatinos SA, Forsburg SL. 2009. Measuring DNA content by flow cytometry in fission yeast. Methods Mol Biol 521:449–461. doi: 10.1007/978-1-60327-815-7_25. [DOI] [PubMed] [Google Scholar]
- 65.Creanor J, Mitchison JM. 1984. Protein synthesis and its relation to the DNA-division cycle in the fission yeast Schizosaccharomyces pombe. J Cell Sci 69:199–210. [DOI] [PubMed] [Google Scholar]
- 66.Prevorovsky M, Stanurova J, Puta F, Folk P. 2009. High environmental iron concentrations stimulate adhesion and invasive growth of Schizosaccharomyces pombe. FEMS Microbiol Lett 293:130–134. doi: 10.1111/j.1574-6968.2009.01515.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.