Abstract
Objective
The phenotype of the cells present in the ductal region of salivary glands have been well characterized. However, it is imperative to identify novel biomarkers that can identify different cell types present in other glandular components for the development of therapeutic strategies and diagnostics of salivary gland disorders and malignancies. Our study aimed at the characterization of the expression and distribution of various cell surface markers, especially with a focus on CD29 in human fetal as well as adult glands.
Materials and methods
Paired human midgestation fetal and adult parotid, sublingual and submandibular glands were collected. Phenotypic expression of various lineage-specific cell surface markers including CD29 was investigated in freshly collected glands. The findings were further corroborated by immunohistochemistry assay.
Results
Enriched expression of CD29 was found on acinar and ductal epithelial, mesenchymal stromal and myoepithelial cells; CD29+ cells co-expressed epithelial (CD324, CD326, NKCC1 and CD44), mesenchymal (CD73, CD90, vimentin and CD34) and myoepithelial (α-SMA) cell-specific progenitor markers in both fetal as well as adult salivary glands.
Conclusion
CD29 is widely expressed in human salivary glands and, it could serve as a potential biomarker for devising novel cellular therapeutic and diagnostic strategies for salivary gland disorders and malignancies.
Keywords: Adult, CD29, fetal, progenitor, salivary gland, stem cells
Introduction
Salivary glands (SGs) belong to a group of branched organs, in which specialized secretory units consisting of acini and ducts are developed through a key process known as branching morphogenesis (Davies, 2002). Branching morphogenesis is regulated by the differential cellular adhesion properties determined by surface receptor expressions as well as distribution and composition of extracellular matrix (ECM) and basement proteins. Interactions between cell receptors and ECM proteins lead to a cascade of downstream intracellular signaling pathways that guide the primitive epithelial progenitors to undergo proliferation and branching right from the primitive bud stage to their differentiation into acinar lobules and ducts (Hoffman et al., 2002; Nakanishi, Nogawa, Hashimoto, Kishi & Hayakawa, 1988; Sakai, Larsen & Yamada, 2003; Spooner & Faubion, 1980; Spooner, Thompson-Bletscher, Stokes & Bassett, 1986). Among the various cell adhesion molecules, integrins are mainly involved in cell to ECM interactions, and are involved in the regulation of several signaling pathways that influence cell proliferation, polarization and differentiation.
Integrins are heterodimeric proteins containing α and β subunits, and based on the type of β subunit, are further divided into sub-families. The CD29 or integrin β1, subfamily of integrin proteins is involved in the interaction of cells with the ECM proteins such as collagen, laminin and fibronectin (Hynes, 1987; Hynes, 1992). Integrins are expressed on various cell lineages, including mesenchymal and epithelial cells which are the main cellular constituents of SGs that play critical roles in the event of branching morphogenesis and embryonic SG development (Goessler et al., 2008; Lee & Streuli, 2014).
CD29 has been reported to be widely expressed on different cell types including stem cells, in tissues like blood, skin and especially in glandular organs like mammary glands (Laird et al., 2008; Shackleton et al., 2006; Stingl et al., 2006; Taddei et al., 2008). Moreover, studies on SG derived cultured monolayer cells and salispheres have identified CD29 as a stem cell marker of SGs (David, Shai, Klauser & Denny, 2008; Nanduri et al., 2014; Neumann et al., 2012; Palmon et al., 2012). Several studies suggest that CD29 in combination with other markers such as CD24, CD49f, CD90 or CD117 could be used as markers to isolate SG stem cells (Banh et al., 2011; Feng, van der Zwaag, Stokman, van OS & Coppes, 2009; Jeong et al., 2013; Maria, Maria, Cai & Tran, 2012; Palmon et al., 2012; Sato et al., 2007). Some of the published studies focused on the demonstration of the in situ localization of CD29 in human fetal and adult SGs have reported its presence on the acinar, ductal and myoepithelial cells (Ianez et al., 2010; Lam, Zhang, Bewick & Lafrenie, 2005; Lourenco & Kapas, 2005; Lourenco, Lima, Uyekita, Schultz & de Brito, 2007). However, these studies differ among each other with respect to a definite CD29 expression pattern among various cell lineages of SGs. Furthermore, most of the studies that have demonstrated expression of CD29 on SGs, have investigated salispheres, monolayer cultures or cell lines derived from the SG cells, and none of the studies have clearly demonstrated its in situ expression pattern in freshly isolated human SGs (Baek et al., 2014; David et al., 2008; Matsumoto et al., 2007; Maimets et al., 2016; Nanduri et al., 2014; Sato et al., 2007). Phenotypic expression profile of cell surface markers might differ between in vivo and in vitro conditions. Even though cell culture serves as a powerful tool for the study of cells, it can induce phenotypic alterations in cell signatures (Boquest et al., 2005). Therefore, it is important to understand the innate phenotypic expression profile of various cell types in freshly isolated SG cells, to precisely explore their downstream functional implications.
Owing to the functional significance of CD29 in cell adhesion, organ development, tissue repair and homeostasis, and taking into consideration the conflicting reports and wide range of distribution of CD29 on different cell types, our study aimed at the extensive characterization of expression of CD29 in human prenatal as well as postnatal parotid (PAGs), sublingual (SLGs) and submandibular (SMGs) glands. The goal of this study is to determine the expression of CD29 on cell types belonging to various specialized structures such as acini, ducts and connective tissues of SGs, which might in turn aid in the progress of diagnostics and cell based therapeutics.
Material and methods
Human fetal and adult SG tissue collection
The study was performed in accordance with the Declaration of Helsinki. Research with human fetal tissues was approved by the University of California at San Francisco Institutional Review Board (UCSF IRB; approval number: 10-00768), and all methods were performed in accordance with the relevant guidelines and regulations. Paired whole PAGs, SLGs and SMGs (n=5 each) were obtained from 16 to 24 weeks' midgestation fetuses of consented women undergoing elective abortions at the San Francisco General Hospital, CA, USA. Collection of fetal SGs was performed after obtaining written consents from the women undergoing elective abortions.
A small portion (approximately 1 cm in size) of the adult PAGs (n=1), SLGs (n=3) and SMGs (n=3) were collected from anonymous non-irradiated donors (age 31-88 years; 5 males and 2 females) undergoing head and neck resection at the Department of Otolaryngology and Head-Neck surgery division of University of California San Francisco, CA, USA. The collected SG tissues were normal in structure because, the donor patients did not receive any irradiation or had SG tumors or associated diseases at the time of surgery. According to UCSF IRB's policies and federal guidelines, UCSF IRB review is not required for the research involving unidentifiable biological specimens. The tissues were not collected specifically for the purposes of this study, but were annonymous residual clinical specimens which were made available for research use.
Isolation of cells from SGs
Human fetal PAGs, SLGs and SMGs were digested with collagenase IV (1mg/ml) (Life Technologies, Grand Island, NY, USA) and dispase II (2.5mg/ml) (Roche, San Francisco, CA, USA) at 37°C for 1 hour. Single cell suspensions of fetal SG cells were prepared by filtering the digested tissue lysate through a 40μM filter (Millipore, Billerica, Massachusetts, USA). These cells were used for the flow cytometry analysis.
Flow cytometry
Freshly isolated human fetal SG single cells were stained with a panel of monoclonal antibodies listed in Table S1, and cells were analyzed on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). Propidium iodide was used to differentiate between live and dead cells. The data was further analyzed using FlowJo software (FlowJo, Ashland, OR, USA).
Immunohistolocalization assay and quantification of expression
For immunohistolocalization assays, freshly isolated fetal and adult PAG, SLG and SMG tissues were fixed and embedded as described previously (Zhu et al., 2014). 10μm cryostat sections were subsequently stained with primary and secondary antibodies (Table S2) and were counterstained with ProLong® Gold antifade reagent with 4′6-diamidino-2-phenylindole (DAPI) (Life Technologies, Grand Island, NY, USA). Images were captured on Leica CTR6500 (Leica Microsystems, Buffalo Grove, IL, USA). Images were further analyzed and quantification of expression was performed using ImageJ software (NIH, Bethedsa, Maryland, USA) as described previously (Rezvani et al., 2016). Quantification of the images was done on 6 to 10 random images at 63× magnification from ≥3 fetal and adult SG sections using NIH-ImageJ software.
Statistics
Data are represented as mean ± SD. The significance of differences was tested by two-tailed nonparametric Mann-Whitney t-test and two-way ANOVA followed by Tukey's multiple comparison test using GraphPad Prism version 6.00 (GraphPad software, La Jolla, CA, USA). p≤0.05 is considered significant.
Results
CD29 is highly expressed among the mesenchymal stromal cells (MSCs) and epithelial cells of SGs
Phenotypic expression analysis of a panel of stem/progenitor markers consisting of CD29, CD44, CD73, CD90, CD133, CD271, CD324 (E-cadherin) and CD326 (EpCAM) showed that CD29 was highly expressed on freshly collected fetal PAG, SLG and SMG derived cells (Figure 1A). We further focused on the detailed characterization of the lineage specificity of the CD29+ SG cells. Expression of CD29 with respect to mesenchymal markers such as CD73 and CD90 showed two distinctive subpopulations: (a) CD29+CD73- and CD29+CD90- and (b) CD29+CD73+ and CD29+CD90+ cells. Further analysis of CD29+CD73- and CD29+ CD90- cells showed that >65% of these cells expressed pan-epithelial markers such as CD326 and CD324, in SLGs and SMGs (Figure 1B). Even though expression of CD326 was high in the CD29+CD73- cell fraction, low expression of CD324 was observed in the CD29+CD90- cells (Figure 1B).
Figure 1.
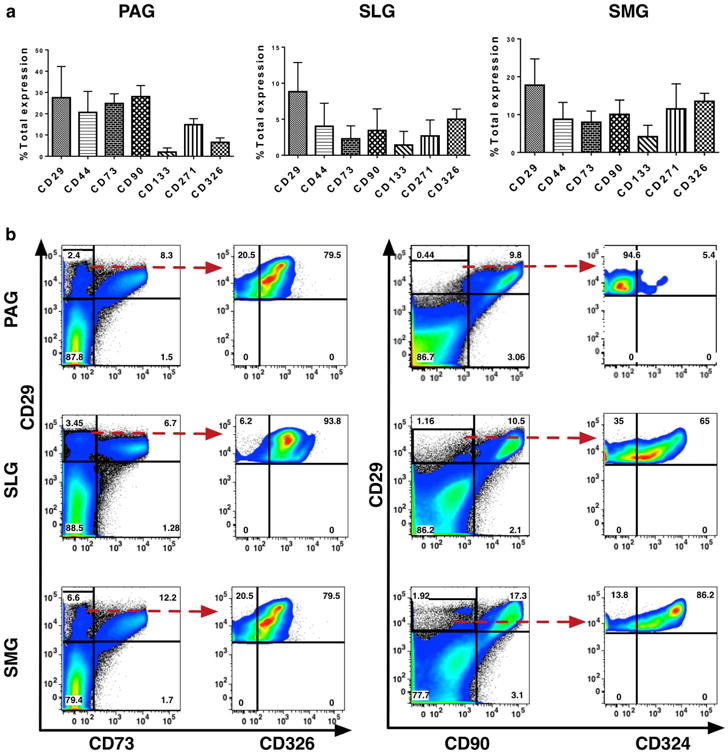
(a) Phenotypic expression analysis of CD29, CD44, CD73, CD90, CD133, CD271, CD324 (E-cadherin) and CD326 (EpCAM) in human fetal parotid, sublingual and submandibular glands (n=5, 16-24 weeks'). Error bars represent mean±SD. (b) Dot plot representation of expression of CD29 with respect mesenchymal stromal cell-specific marker CD73, CD90 and epithelial-specific markers CD326 and CD324.
CD29 is widely expressed by the MSCs, ductal and acinar epithelial and myoepithelial cells of human fetal and adult SGs
Analysis of the in situ distribution pattern of CD29 in both fetal and adult PAGs, SLGs and SMGs demonstrated that it was expressed on the acinar and ductal epithelial cells (Figure 2). Interestingly, CD29 was expressed by both serous and mucous type of acinar cells (Figure 2A, B, white and magenta arrows, respectively). In SMGs, serous cells present in the serous demilunes encircling mucous acinar cells showed rich expression of CD29 (Figure 2C, gray arrow). Its expression could also be seen on striated, intercalated (Figure 2D, green arrow) and excretory ductal cells of SGs (Figure 2E, blue arrow). A representative example of the pattern of CD29 distribution on developing branching ducts is shown in Figure 2F. Furthermore, CD29 was also expressed on the encircling myoepithelial cells (Figure 2A, F, yellow arrows) and mesenchymal stromal connective tissue cells lining the acinar and ductal cells (Figure 2C, E, red arrows) of SGs.
Figure 2.
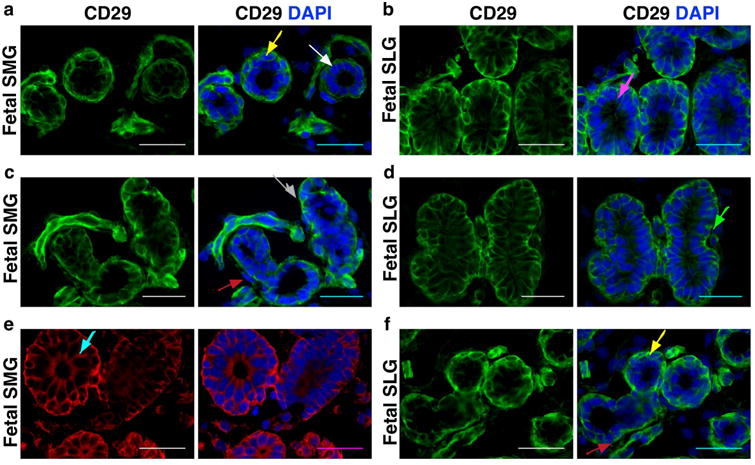
(a-f) In situ co-localization of CD29 in human fetal sublingual and submandibular glands. Different colored arrows indicate as follows: White=serous acini; Magenta=mucous; Gray=serous demilunes; Green=intercalated ducts; Blue=excretory ducts; Yellow=myoepithelial cells; Red=mesenchymal cells. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Epithelial and myoepithelial cell-specific expression of CD29 on different structural components of SGs was further confirmed by the co-localization of CD29+ cells with respect to some of the known pan-acinar, -ductal and -myoepithelial progenitor markers. NKCC1 has been shown to be expressed on the basolateral membrane of acinar and ductal cells where it plays an important role in the secretion and transport of fluids and electrolytes in SGs (Maria et al., 2012). It was previously shown by Maria and colleagues that CD44 could serve as a potential biomarker to identify specifically serous acinar progenitors in SGs (Maria et al., 2012). In our study, CD29+ cells from the acinar and ductal epithelial cells were found to strongly co-express pan-epithelial markers CD326, NKCC1 and CD44 (Figures 3, 4, 5). In contrast to the study by Maria and colleagues, we found expression of CD44 on both serous and mucous type of acinar as well as on the ductal cells of SGs (Figure 5) (Maria et al., 2012). Additionally, CD44 was also found to be expressed on the MSCs of SGs (Figure 5). Further, co-expression of CD29+ cells with α-smooth muscle actin (α-SMA) expressing cells confirmed their expression on myoepithelial cells of SGs (Figure S1).
Figure 3.
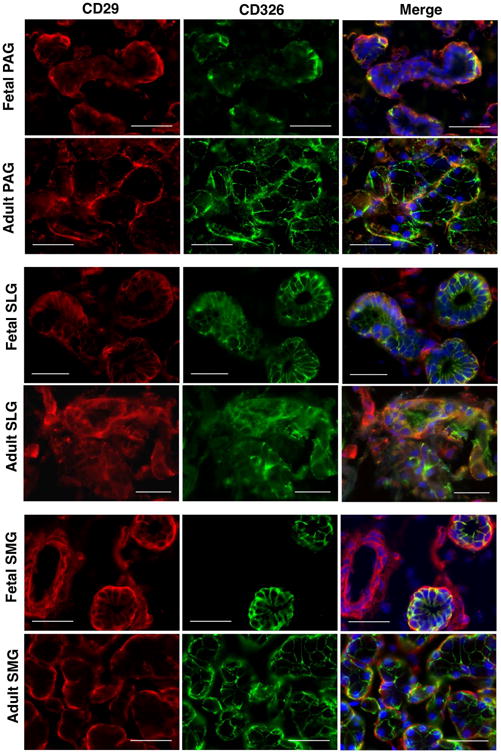
In situ localization of CD29 (red) and CD326 (green) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Figure 4.
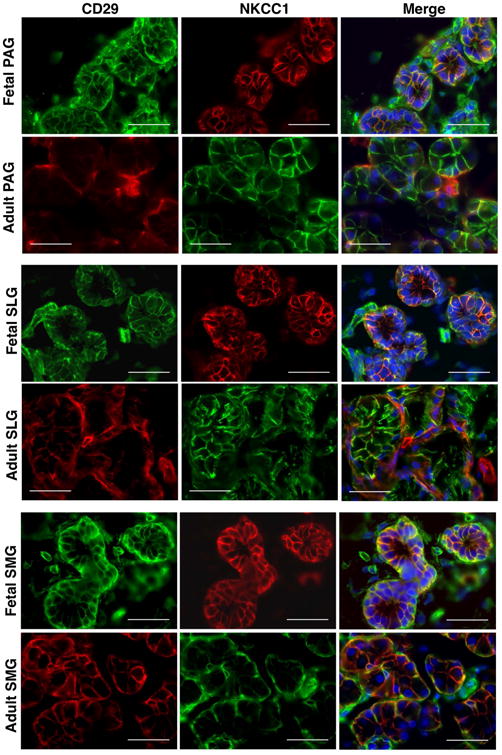
In situ localization of CD29 and NKCC1 in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Figure 5.
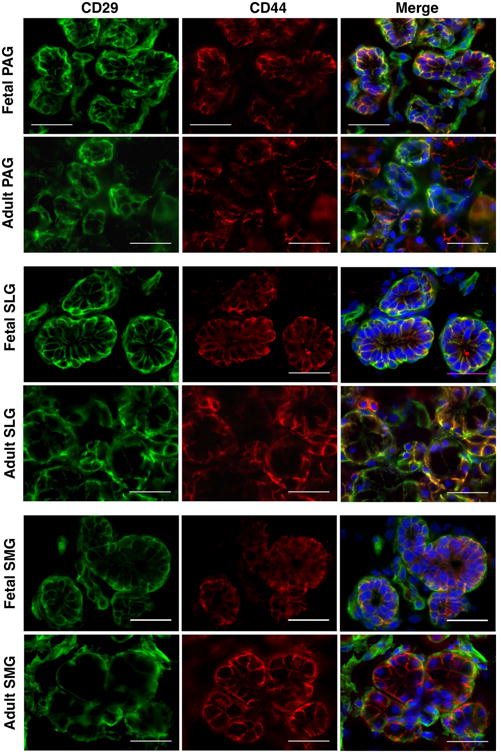
In situ localization of CD29 (green) and CD44 (red) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Recent data from our group showed that CD34 is widely expressed by the MSCs of human SGs (Togarrati et al., 2017). Hence, we intended to confirm mesenchymal cell lineage specific expression of CD29+ cells through co-staining with CD34 and vimentin markers. Our present data showed elegant co-localization of CD29+ cells with CD34+ and vimentin+ cells at the stromal regions (Figure 6, 7), further confirming that CD29 is expressed by the mesenchymal cells of SGs.
Figure 6.
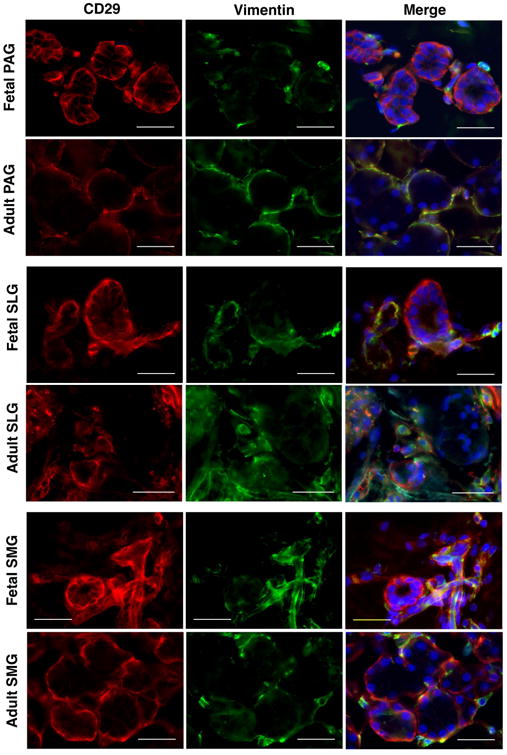
In situ localization of CD29 (red) and vimentin (green) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Figure 7.
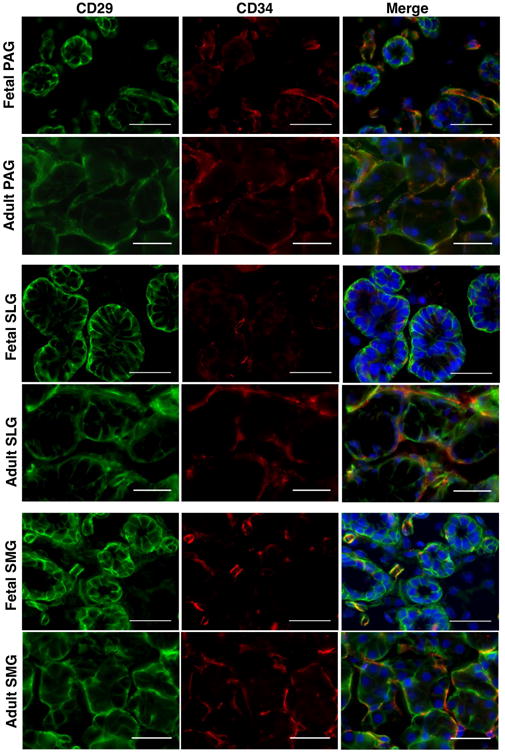
In situ localization of CD29 (green) and CD34 (red) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Variations in the immunohistochemical findings could result from the usage of different antibody clone types of CD29 in human SGs
Intriguingly, immunohistochemical assay results in fetal and adult SGs revealed clone-specific variations in the expression patterns of CD29 when different clones of the antibody were used. Analysis of CD29 expression was carried out using D2E5, TS2/16 and EP1041Y clones of CD29. The TS2/16 clone showed evidence of epithelial as well as mesenchymal cell-specific staining of CD29 (Figure 8). Additionally, TS2/16 antibody also detected myoepithelial cell-specific expression of CD29 (Figure 8). However, expression of CD29 was restricted to only mesenchymal stromal regions when EP1041Y (Figure 8) and D2E5 antibodies were used in the histolocalization assays.
Figure 8.
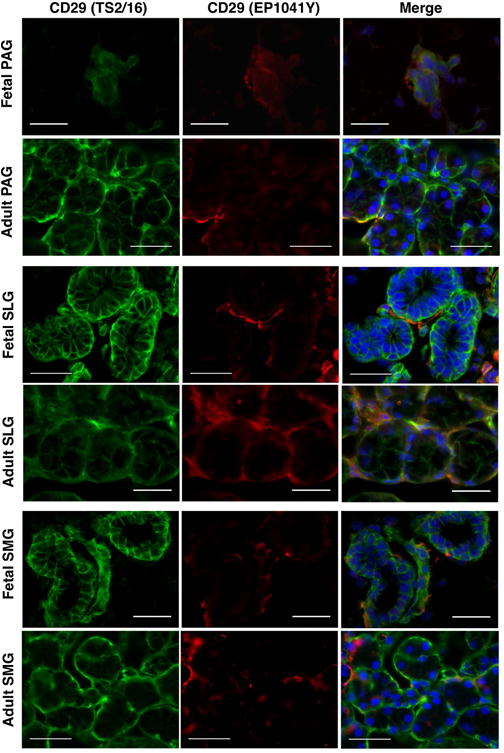
In situ co-localization of clones TS2/16 (green) and EP1041Y (red) of CD29 in human fetal and adult parotid, sublingual and submandibular glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Comparative quantitative expression analysis of CD29 using TS2/16 and EP1041Y clones revealed that, CD29 was highly expressed in both fetal as well as adult SGs. In fact significantly higher MSC specific expression of clone EP1041Y was observed in adult SLGs and SMGs when compared with the fetal SG counterparts (Figure S2 and Table S3). These findings indicated that different clones of CD29 antibody show variations in their ability to stain various cell types in SGs.
Discussion
Identification of the cell surface proteins that determine the phenotype and distribution of lineage-specific cell types across various structural regions of SGs is important in the field of diagnostics and therapeutics of SG disorders and malignancies. Our data revealed that CD29 is highly expressed in human SGs (Figure 1). Furthermore, a large fraction of CD29 expressing cells were found to be of mesenchymal and epithelial nature (Figure 1B). Majority of the putative CD29+CD73- mesenchymal cells expressed epithelial marker, CD326 in fetal SGs. Even though >79% of the CD29+CD90- mesenchymal cells expressed epithelial CD324 in SLGs and SMGs, only few of these cells expressed CD324 in fetal PAGs (Figure 1B). This observation might hint at the interglandular architectural differences, as fetal PAGs are mostly rich in mesenchymal connective tissue and have comparatively less developed acinar and ductal epithelial structures in comparison to the more branched SLGs and SMGs. In situ, expression of CD29 could be localized to the stromal, acinar and ductal epithelial, and myoepithelial cells of both fetal as well as adult PAGs, SLGs and SMGs (Figure 2). To the best of our knowledge, expression of CD29 has not been well characterized in all three major human SGs and a lot of variation exists in the documentation of specific lineage of CD29+ cells in SGs.
Some of the existing reports focused on the histolocalization of CD29 in the developing fetal and adult SGs have shown that, it is mostly expressed by the epithelial progenitors of acini and ducts (Lourenco & Kapas, 2005; Lourenco et al., 2007). It has been shown to be expressed by both mucous and serous acinar cells at the basolateral regions (Lourenco & Kapas, 2005). Lourenco and group had performed a detailed analysis on the expression of CD29 at different stages of SG development, namely, bud, proliferation, canalization, branching and cytodifferentiation, and found it to be present at all stages development except at the bud stage (Lourenco & Kapas, 2005). Our results showed strong CD29+ expression in both mucous and serous types of acinar cells of PAG, SLGs and SMGs (Figures 2 and 5). Expression of CD29 on acinar cells was further confirmed by its co-localization with pan-acinar cell differentiation marker NKCC1 (Figure 4). Dominant expression of CD29 was found to be uniformly distributed on the acinar and ductal cells whereas, NKCC1 expression was more confined to the basolateral acinar and ductal cells. We also investigated expression of CD29 with respect to another transmembrane glycoprotein CD44 in SGs (Figure 5). Distribution of three isoforms of CD44 (CD44v3, CD44v4/5, CD44v6) has been characterized through immunohistochemistry in human SLGs and SMGs and it was found that CD44 was strongly localized in serous acinar and myoepithelial cells (Fonseca, Mouna Nunes & Soares, 2000). However, Maria, et al, later observed CD44 staining to be confined only to serous acinar cells in human PAGs and SMGs, and they further reported absence of CD44 in myoepithelial cells (Maria et al., 2012). Characterization of CD44 in human fetal and adult PAGs, SLGs and SMGs in our study revealed that CD44 expression was strongly positive in both serous and mucous type of acinar cells. Additionally we observed dominant CD44 staining in striated, intercalated ductal and stromal cells. These conflicting findings on the distribution pattern of CD44 in SGs might be due to the variations in the clones of the antibodies employed in different studies. Interestingly, we found that CD29+ expression on epithelial and stromal cells strongly co-localized with CD44+ expression in both fetal and adult SGs.
Furthermore, we did observe strong expression of CD29 in the intercalated, striated and interlobular (excretory ducts) ducts of SGs (Figure 2). In accordance a previous study, we observed ubiquitous expression of CD29 in the myoepithelial cells surrounding acinar and ductal cells (Figure 2) (Ianez et al., 2010). CD29+ myoepithelial cells were also co-positive for α-SMA, suggesting role of CD29 as a putative myoepithelial progenitor marker (Figure S1). Moreover, co-localization of CD29+ cells with vimentin and CD34 expressing SG cells confirmed the expression of CD29 on mesenchymal cell lineage (Togarrati et al, 2017). These data indicated that CD29 is widely expressed in both fetal as well as adult human SGs, further highlighting its functional importance right from the stage of glandular development to the maintenance of homeostasis in mature glands.
Another interesting aspect of our study was to find clone specific variation in the expression patterns of CD29 in SGs. It was found that using clone TS2/16, CD29 expression could be detected in a wide range of cell types such as, myoepithelial, acinar, ductal and mesenchymal cells, whereas EP1041Y and D2E5 clones showed expressions only on mesenchymal cells (Figure 8). So far, five different isoforms of CD29 have been identified, namely β1A, β1B, β1C, β1C-2 and β1D (Fornaro & Languino, 1997). These variants have differential effects on the cell proliferation, receptor localization, cell adhesion, migration and downstream regulation of signaling pathways; for example β1C has been shown to strongly inhibit cell proliferation and its protein expression is reduced in epithelial, endothelial, fibroblast and prostate cancer cells, however, β1A supports cell proliferation and its levels remain unchanged in these cells (Belkin & Retta, 1998; Fornaro, Steger, Bennett, Wu & Lanuino, 2000; Perlino et al., 2000; Pfaff, Liu, Erle & Ginsberg, 1998). Transcriptional and post-transcriptional modification might account for the functional variations observed between various isoforms of CD29. Similar observations in the variations in the expression pattern of CD133 has been reported in glioblastoma cancer tissues when different clones were used (Hermansen, Christensen, Jensen & Kristensen, 2011). Nevertheless, these data might explain the wide variations observed in the reported expression patterns of CD29 on different cell lineage types of SGs by different research groups.
In summary, our study demonstrated ubiquitous expression of CD29 on various types of cell lineages. Strong CD29 expression in fetal as well as adult tissues might suggest that CD29 is expressed on primitive as well as differentiating progenitor cells of SGs. Therefore, CD29 could be used as a biomarker that might have prospective implications in the diagnostics and therapeutics of SG diseases and malignancies.
Supplementary Material
Figure S1. In situ localization of CD29 (green) and α-SMA (red) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Figure S2. Bar graphs representation of percentage CD29 expression using clones TS216 and EP1041Y in fetal and adult salivary glands. Error bars represent mean±SD. ** indicates p<0.001.
Table S1. List of all the antibodies used in flow cytometry.
Table S2. List of all the antibodies used in immunohistochemistry.
Acknowledgments
We thank the staff and faculty of the San Francisco General Hospital Women's Options Center and Department of Otolaryngology of the University of California San Francisco for their assistance in the collection of human fetal and adult SG tissues. We are indebted to the administrative staff at Blood Systems Research Institute for their tremendous support. This work was supported by the National Institutes of Health - grant number RO1 DE024188 and the RIVA foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research. Ms. Dinglasan was supported by a Bridges to Stem Cell Training grant TB1-01188 from the California Institute of Regenerative Medicine.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
References
- Baek H, Noh YH, Lee JH, Yeon SI, Jeong J, Kwon H. Autonomous isolation, long-term culture and differentiation potential of adult salivary gland-derived stem/progenitor cells. Journal of tissue engineering and regenerative medicine. 2014;8:717–27. doi: 10.1002/term.1572. [DOI] [PubMed] [Google Scholar]
- Banh A, Xiao N, Cao H, Chen CH, Kuo P, Krakow T, et al. Le QT. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7265–72. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Retta SF. beta1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. The Journal of biological chemistry. 1998;273:15234–40. doi: 10.1074/jbc.273.24.15234. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Molecular biology of the cell. 2005;16:1131–41. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Shai E, Aframian DJ, Palmon A. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue engineering Part A. 2008;14:331–7. doi: 10.1089/tea.2007.0122. [DOI] [PubMed] [Google Scholar]
- Davies JA. Do different branching epithelia use a conserved developmental mechanism? BioEssays : news and reviews in molecular, cellular and developmental biology. 2002;24:937–48. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- Denny PC, Chai Y, Klauser DK, Denny PA. Parenchymal cell proliferation and mechanisms for maintenance of granular duct and acinar cell populations in adult male mouse submandibular gland. The Anatomical record. 1993;235:475–85. doi: 10.1002/ar.1092350316. [DOI] [PubMed] [Google Scholar]
- Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:466–71. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Fonseca I, Moura Nunes JF, Soares J. Expression of CD44 isoforms in normal salivary gland tissue: an immunohistochemical and ultrastructural study. Histochemistry and cell biology. 2000;114:483–8. doi: 10.1007/s004180000220. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Languino LR. Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix biology : journal of the International Society for Matrix Biology. 1997;16:185–93. doi: 10.1016/s0945-053x(97)90007-x. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Steger CA, Bennett AM, Wu JJ, Languino LR. Differential role of beta(1C) and beta(1A) integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated protein kinase pathways. Molecular biology of the cell. 2000;11:2235–49. doi: 10.1091/mbc.11.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hormann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. International journal of molecular medicine. 2008;21:271–9. [PubMed] [Google Scholar]
- Hermansen SK, Christensen KG, Jensen SS, Kristensen BW. Inconsistent immunohistochemical expression patterns of four different CD133 antibody clones in glioblastoma. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59:391–407. doi: 10.1369/0022155411400867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, Larsen M. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–78. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–54. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ianez RF, Buim ME, Coutinho-Camillo CM, Schultz R, Soares FA, Lourenco SV. Human salivary gland morphogenesis: myoepithelial cell maturation assessed by immunohistochemical markers. Histopathology. 2010;57:410–7. doi: 10.1111/j.1365-2559.2010.03645.x. [DOI] [PubMed] [Google Scholar]
- Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Lohrs U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Archiv : an international journal of pathology. 2002;440:519–26. doi: 10.1007/s004280100537. [DOI] [PubMed] [Google Scholar]
- Jeong J, Baek H, Kim YJ, Choi Y, Lee H, Lee E, et al. Kwon H. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Experimental & molecular medicine. 2013;45:e58. doi: 10.1038/emm.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K, Zhang L, Bewick M, Lafrenie RM. HSG cells differentiated by culture on extracellular matrix involves induction of S-adenosylmethione decarboxylase and ornithine decarboxylase. Journal of cellular physiology. 2005;203:353–61. doi: 10.1002/jcp.20247. [DOI] [PubMed] [Google Scholar]
- Lee JL, Streuli CH. Integrins and epithelial cell polarity. J Cell Sci. 2014;127:3217–25. doi: 10.1242/jcs.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco SV, Kapas S. Integrin expression in developing human salivary glands. Histochemistry and cell biology. 2005;124:391–9. doi: 10.1007/s00418-005-0784-3. [DOI] [PubMed] [Google Scholar]
- Lourenco SV, Lima DM, Uyekita SH, Schultz R, de Brito T. Expression of beta-1 integrin in human developing salivary glands and its parallel relation with maturation markers: in situ hybridisation and immunofluorescence study. Archives of oral biology. 2007;52:1064–71. doi: 10.1016/j.archoralbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN, et al. Coppes RP. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem cell reports. 2016;6:150–62. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. The Anatomical record. 2001;263:202–14. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- Maria OM, Maria AM, Cai Y, Tran SD. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral diseases. 2012;18:162–8. doi: 10.1111/j.1601-0825.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Okumura K, Ogata A, Hisatomi Y, Sato A, Hattori K, et al. Endo F. Isolation of tissue progenitor cells from duct-ligated salivary glands of swine. Cloning and stem cells. 2007;9:176–90. doi: 10.1089/clo.2006.0022. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104:51–9. doi: 10.1242/dev.104.1.51. [DOI] [PubMed] [Google Scholar]
- Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, et al. Coppes RP. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem cell reports. 2014;3:957–64. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmon A, David R, Neumann Y, Stiubea-Cohen R, Krief G, Aframian DJ. High-efficiency immunomagnetic isolation of solid tissue-originated integrin-expressing adult stem cells. Methods. 2012;56:305–9. doi: 10.1016/j.ymeth.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Perlino E, Lovecchio M, Vacca RA, Fornaro M, Moro L, Ditonno P, et al. Languino LR. Regulation of mRNA and protein levels of beta1 integrin variants in human prostate carcinoma. The American journal of pathology. 2000;157:1727–34. doi: 10.1016/s0002-9440(10)64809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. The Journal of biological chemistry. 1998;273:6104–9. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Rezvani M, Espanol-Suner R, Malato Y, Dumont L, Grimm AA, Kienle E, et al. Willenbring H. In Vivo Hepatic Reprogramming of Myofibroblasts with AAV Vectors as a Therapeutic Strategy for Liver Fibrosis. Cell stem cell. 2016;18:809–16. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, Endo F. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning and stem cells. 2007;9:191–205. doi: 10.1089/clo.2006.0054. [DOI] [PubMed] [Google Scholar]
- Spooner BS, Faubion JM. Collagen involvement in branching morphogenesis of embryonic lung and salivary gland. Developmental biology. 1980;77:84–102. doi: 10.1016/0012-1606(80)90458-3. [DOI] [PubMed] [Google Scholar]
- Spooner BS, Thompson-Pletscher HA, Stokes B, Bassett KE. Extracellular matrix involvement in epithelial branching morphogenesis. Dev Biol (N Y 1985) 1986;3:225–60. doi: 10.1007/978-1-4684-5050-7_12. [DOI] [PubMed] [Google Scholar]
- Togarrati PP, Sasaki RT, Abdel-Mohsen M, Dinglasan N, Deng X, Desai S, et al. Muench MO. Identification and characterization of a rich population of CD34+ mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Scientific reports. 2017;7:3484. doi: 10.1038/s41598-017-03681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, et al. Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93–7. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. In situ localization of CD29 (green) and α-SMA (red) in human fetal and adult salivary glands. Nuclei was stained in DAPI (blue). All scale bars represent 50 μM.
Figure S2. Bar graphs representation of percentage CD29 expression using clones TS216 and EP1041Y in fetal and adult salivary glands. Error bars represent mean±SD. ** indicates p<0.001.
Table S1. List of all the antibodies used in flow cytometry.
Table S2. List of all the antibodies used in immunohistochemistry.


