Abstract
Type 1 diabetes (T1D) is mediated by destruction of pancreatic β cells by autoantigen-specific CD4+ and CD8+ T cells, thus the ideal solution for T1D is the restoration of immune tolerance to β cell antigens. We demonstrate the ability of carboxylated 500nm biodegradable poly(lactide-co-glycolide) (PLG) nanoparticles PLG nanoparticles (either surface coupled with or encapsulating the cognate diabetogenic peptides) to rapidly and efficiently restore tolerance in NOD.SCID recipients of both activated diabetogenic CD4+ BDC2.5 chromagranin A-specific and CD8+ NY8.3 islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)-specific TCR transgenic T cells in an antigen-specific manner. Further, initiation and maintenance of Ag-PLG tolerance operates via several overlapping, but independent, pathways including regulation via negative-co-stimulatory molecules (CTLA-4 and PD-1) and the systemic induction of peptide-specific Tregs which were critical for long-term maintenance of tolerance by controlling both trafficking of effector T cells to, and their release of pro-inflammatory cytokines within the pancreas, concomitant with selective retention of effector cells in the spleens of recipient mice.
Keywords: type 1 diabetes, tolerance, PLG nanoparticles, regulatory T cells
1. Introduction
Type 1 diabetes (T1D), the most frequently observed chronic disorder found in children, is an autoimmune disease that develops as a consequence of failed peripheral immune tolerance, resulting in the specific destruction of insulin producing pancreatic β cells by autoreactive CD4+ and CD8+ T cells [1]. The loss of pancreatic β cells renders the host incapable of regulating normal glucose metabolism, which if left unchecked, culminates in death [1]. Current treatment therefore, focuses on the life saving provision of insulin, however insulin therapy is laborious, requiring constant patient blood glucose monitoring, life-long insulin administration, and is associated with serious complications. As such there is a need for more elegant therapeutic approaches in T1D.
The ideal solution to treat T1D is the restoration of immune tolerance, prior to significant β cell loss. Treatments employing broad-spectrum immune suppression have shown only moderate efficacy in preventing/treating T1D in animal models and human clinical trials [2,3]. In addition, these approaches lack long-term efficacy and are associated with numerous deleterious events including cytokine release syndrome, and increased incidence of infections and neoplasias. To date, therapeutic attempts to induce Ag-specific tolerance to overcome side effects associated with broad-based immune suppression, including the administration of soluble islet Ags such as insulin, glutamate decarboxylase 65 (GAD65), or heat shock protein 60 (HSP60) by various routes have failed to show efficacy in large clinical trials [3]. A complete understanding of why these therapies failed is unclear. However, it is significant that we and others have shown that the subcutaneous route of Ag administration primarily employed in T1D tolerance trials is not adequately armed to induce immune tolerance without add on immunotherapy. We previously demonstrated the ability of i.v., but not s.c., administered Ag-conjugated apoptotic leukocytes (Ag-SP) to induce protective tolerance for the prevention and treatment of established of murine Th1/Th17-mediated autoimmune disease models of MS [4] and T1D [5], as well as Th2-mediated murine models of allergic airway inflammation and food allergy [6]. Antigen-conjugated autologous leukocytes which induce specific tolerance by two synergistic mechanisms, T cell intrinsic anergy and the activation of Tregs [4], have interestingly shown initial promise in clinical trials of MS patients [7]. However, the need for autologous cells to be isolated and manipulated prior to each treatment is costly, complex, and therefore limits the broad clinical application this tolerogenic treatment especially for treating adolescents at risk for T1D.
The development of shelf-stable, tolerance-inducing Ag carriers manufactured under GMP conditions represents the next generation of Ag-specific medicine. To this end, we have recently pioneered the use of i.v. infusion of Ag-associated carboxylated biodegradable poly(lactide-co-glycolide) nanoparticles (Ag-PLG), also termed tolerogenic immune-modifying particles (TIMP) for the safe and efficient induction of tolerance to prevent and treat Th1/Th17 EAE and Th2 allergic airway disease [8–11]. Mechanistic studies have shown that Ag-PLG are internalized by splenic marginal zone macrophages and liver phagocytic cells via scavenger receptors, such as macrophage receptor with collagenous structure (MARCO) [8]. While we have determined that ligation of these scavenger receptors triggers particle uptake and tolerogenic re-presentation of the cargo antigen resulting in the upregulation of negative-co-stimulation pathways, release of regulatory cytokines and induction of Ag-specific regulatory T cells (Tregs), the precise contribution of each factor to tolerance induction remains to be fully defined.
Our previous report employing tolerance induced by Ag-SP in the spontaneously arising NOD mouse model of T1D [5], showed that T cell responses to the insulin B9–23 epitope were dominant in young mice, but that epitope spreading to additional epitopes occurred as mice progressed to overt hyperglycemia. Given that development and progression of T1D in NOD mice and humans has been attributed to immune responses to numerous CD4 and CD8 T cell epitopes expressed on insulin, proinsulin, GAD65/67, islet specific glucose 6 phosphatase catalytic subunit related protein (IGRP), Islet antigen-2 (IA-2), phogrin (IA-2β), chromogranin A (ChgA), zinc transporter 8 (ZnT8), and vasostatin-1 [12–15], as well as hybrid β cell protein epitopes [16], it is critical to determine the efficacy of Ag-PLG treatment to restore tolerance in activated effector diabetogenic CD4+ and CD8+ T cells and to define the precise effector mechanisms responsible for tolerance induction and maintenance. Employing adoptive transfer models of T1D induced by the transfer of activated diabetogenic CD4+ BDC2.5 chromagranin A-specific [17] and CD8+ NY8.3 IGRP-specific [18] TCR transgenic T cells, we demonstrate the ability of PLG nanoparticles (either surface coupled with or encapsulating the cognate diabetogenic peptides) to rapidly and efficiently restore tolerance in NOD.SCID recipients of both activated CD4+ and/or CD8+ T cells in an antigen-specific manner. Further, Ag-PLG-induced peripheral tolerance initiation and maintenance were demonstrated to operate via several overlapping, but independent pathways including regulation via negative-co-stimulatory molecules (namely CTLA-4 and PD-1) and the systemic induction of peptide-specific Tregs. The net result of the tolerance therapy was inhibition of both trafficking of effector diabetogenic cells to the pancreas, as well as the release of pro-inflammatory cytokines via selective retention of effector cells within the spleens of recipient mice. These results clearly demonstrate the unique ability of Ag-PLG-induced tolerance to halt/reverse β cell destruction in mice with high numbers of activated effector diabetogenic CD4+ and CD8+ T cells.
2. Materials and Methods
2.1 Mouse strains
Female NOD/MrkTac mice were purchased from Taconic Farms (Germantown, MD). BDC2.5 TCR transgenic and NOD.SCID mice were purchased from Jackson laboratories (Bar Harbor, ME). NY8.3 TCR transgenic mice were obtained from Dr. Pere Santamaria (Univ. of Calgary) and bred in the Northwestern University Center for Comparative Medicine. All mice were housed under SPF conditions and maintained according to protocols approved by the Northwestern University Animal Care and Use Committee.
2.2 Antibodies
Monoclonal antibodies anti-CD25 (PC61.5), anti-PDL-1 (10F.9G2), anti-CTLA-4 (CD152) and anti-ICOS (7E.17G9) and their respective isotype control antibodies were purchased from Bio X Cell (West Lebanon, NH). The following antibodies along with their respective isotope controls were purchased from BD Biosciences, eBiosciences and/or Biolegend: V500 conjugated anti-CD4, PEcy7 conjugated anti-CD90.2 (53-2-1), PE conjugated anti-ICOS (C398.4A), Brillliant Violet 421 conjugated anti-PD-1 (J43), APC conjugated anti- Foxp3 (FJK-1ba), Brillliant Violet 421 conjugated anti-TNF-α (MP6-XT22), AlexaFluor700 conjugated anti-IFN-γ (XMG1.2), PE conjugated anti-IL-2 (JES6-5H2), FITC conjugated anti- GM-CSF (MP1-22E9) and efluor 450 conjugated anti-Ki67 (SolA15).
2.3 Peptides
BDC2.5 mimetope 1040-31 (p31) (YVRPLWVRME), NY8.3 mimetope NRPA7 (KYNKANAFL), and the linked p31-NRPA7-InsB9–23 (YVRPLWVRMEGAVVRGAKYNKANAFLGAVVRGASHLVEALYLVCGERG) peptides were purchased from Anaspec (Fremont, CA). MOG35–55 (MEVGWYRSPSRVVHLYRNGK) was purchased from Genemed Synthesis (San Francisco, CA).
2.4. Activation and adoptive transfer of BDC2.5 and NY8.3 T cells
BDC2.5 and NY8.3 TCR Tg lymphocytes were harvested and pooled from spleen and brachial, axillary, mesenteric and pancreatic lymph nodes. The cells were activated in vitro with 0.5 μM of p31 or NRPA7 peptide in complete RPMI (Gibco) containing 5×10−5 M β-2-ME (Gibco), 2mM L-glutamine, 100 U/ml penicillin/streptomycin (Gibco), 0.1 M nonessential amino acids (Gibco), and 10% fetal bovine serum (FBS) at a final concentration of 1×106 cells/ml in 96 well, round bottom plates. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 and harvested after 96 hours and washed, and 5–10×106 viable T cells were transferred i.v. to 8–12 week old NOD.SCID recipients.
2.5. Preparation and tolerance induction with Ag ECDI-fixed splenocytes, Ag-PLG and PLG(Ag) Nanoparticles
For Ag-SP tolerance, single cell suspensions of erythrocyte-free splenocytes harvested from donor NOD mice in PBS were coupled with peptide (1 mg/ml) using ECDI (150 mg/ml) (Calbiochem) on ice for 1 hour with intermittent shaking [5]. Ag-SP were washed 3× with PBS and a total of 5×107 in 200 μl PBS were injected i.v in individual recipient mice. For antigen-coupled PLG nanoparticles, either carboxylated Degradex® PLG 500nm particles were purchased from (Phosphorex Hopkinton, MA) or 500 nm PEMA-modified PLG particles were synthesized in the laboratory as previously detailed [8,9]. The particles were resuspended and washed 3× in PBS, suspended at 50mg/ml and coupled with peptide/protein (4 mg/ml) using ECDI (16 mg/ml) at room temperature (21°C) for 1 ho ur with intermittent shaking. Ag-ECDI fixed particles were washed 2× with PBS. A total of 0.125mg of Ag-PLG/PEMA in 200 μl PBS were injected i.v in individual recipient mice. Unused Ag-PLG or Ag-PLG/PEMA particles were lyophilized by Analytical BioNanoTechnology Equipment Core at Northwestern University. Lyophilized Ag-PLG/PEMA particles were stored at −20°C for re-suspension and use at a later time. 500 nm PLG-PEMA nanoparticles encapsulating either p31, NRPA7, MOG35–55, or the p31-NRPA7-InsB9–23 linked peptide were synthesized using a double emulsion process as previously described [10,19]. Details on PLG nanoparticle size distribution, purity and efficiency of antigen coupling/encapsulation can be found in our previous publications [8–10,20].
2.6. Assessment of diabetes
Blood glucose levels were measured in female NOD mice with One Touch UltraSmart Blood Glucose Monitoring System weekly starting at the age of 10 weeks. Blood glucose levels in NOD.SCID recipients of activated BDC2.5 or NY8.3 T cells were monitored daily beginning 1–2 days after cell transfer. Mice with two consecutive readings at or above 250 mg/dL were considered to be diabetic.
2.7 Isolation of pancreatic lymphocytes
The pancreas was cannulated with HBSS containing digest buffer contained 2mg/ml collagenase V from Clostridium histolyticum (Sigma) in a buffered HBSS solution supplemented with 0.1% BSA (Sigma), 100 U/ml penicillin/streptomycin (Cell Gro), 10mM HEPES (Gibco) and 10μg/ml of DNase (Sigma) via the pancreatic duct. The distended pancreas was then removed and incubated at 37°C for 30 minutes with shaking. The digested tissue was resuspended in 50 ml of cold buffer HBSS to stop digest reaction. Digested pancreas was filtered through a 100μm nylon mesh strainer. The cells were then washed 2× with the buffered HBSS solution. The filter and washed cells were then layered on a 6 ml or 40% Percoll and centrifuged at 1000g for 20 minutes with no brake. The isolated pancreatic lymphocytes were collected, washed and counted for further assay.
2.8. Flow cytometry
Single cell suspensions from spleen, lymph nodes and pancreas were prepared as described above. To examine the expression of surface markers on T cells subsets, cells were incubated with 2.4G2 FcR blocking Ab and LIVE/DEAD Fixable Dead Cell Stain (Invitrogen) for 30 minutes. The cells were washed 2× in HBSS and then stained for 30 minutes on ice with a panel of specific monoclonal antibodies at the recommended concentrations. If required, cells were washed 2× before staining with secondary antibodies. Intracellular Foxp3 staining was done using kits from BD Pharmingen (San Jose, CA) according to the manufacturer’s guidelines. Briefly, following surface staining, cells were fixed and permeabilized overnight in BD Pharmingen Mouse Foxp3 Permeabilization buffer and washed 2× with BD Pharmingen Stain Buffer before intracellular staining. For intracellular cytokine staining, mixed lymphocyte cultures or T cells culture with APCs were pulsed with p31 or MOG35–55 peptide (2μg/ml) for 45min. Golgi-plug and Golgi-stop (both purchased from BD Biosciences) were added at a final concentration of 3μg/ml and 0.6μg/ml respectively and incubated for 9 hours. Cells were washed with HBSS and surface staining was performed as described above. Cells were then fixed with BD Cytofix/Cytoperm (BD Biosciences) for 20 minutes at 4°C, washed 2× with BD Permeabilization Buffer (BD Biosciences). Cells were then stained with antibodies to anti-IFN-γ, anti-IL-2, anti-GM-CSF for 30 minutes at 4°C and then washed 2× in BD Permeabi lization Buffer. Stained cells were resuspended in fluorescence activated cell sorter (FACS) buffer for analysis. Flow cytometry was performed using a BD LSRFortessa (BD Biosciences) at the Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility and data was analyzed using FlowJo software (Tree Star).
2.9. Luminex multiplex cytokines/chemokines assay
Splenic and pancreatic lymphocytes isolated from BDC2.5 reconstituted NOD.SCID and samples from individual mice were restimulated as described above but without the addition of Golgi-plug and Golgi-stop. Culture supernatant was then collected and cytokine concentrations were measured by Luminex xMAP technology (Milliplex Cytokine Mouse multiplex) according to the instructions provided by the manufacturer. The analyzed cytokines included: IL-2, TNF-α, GM-CSF and IL-10.
2.10. Antibody treatment
BDC2.5 reconstituted NOD.SCID were treated with PD-1 (500μg first treatment, 250μg following treatments), CTLA (500μg), or ICOS (500μg) blocking antibody or corresponding isotype control antibodies was intraperitoneally administered daily on day 0–10 or on day 10–20 post AT. NOD mice were injected intraperitoneally with anti-CD25 (500 mg) or rat IgG control on day 0 and 2 post toleration with Ag-ECDI-SP.
2.11. Cell sorting
After in vitro p31 activation of BDC2.5 lymphocytes, cultures were washed, counted and sorted using kits from Miltenyi Biotec (Auburn, CA, USA) according to the manufacturer’s guidelines. Briefly, activated BDC2.5 T cells were subjected to antibody labeling of non-CD4+ and CD25+ cell populations. Labeled cells were first depleted for non-T cell populations, then the enriched CD4+ T cell fraction were subject to positive selection and separation using an autoMACS column. These cells were then washed and 3×106 cells CD4+CD25− T cells were AT in NOD.SCID recipients.
2.12. Assessment of insulitis
For histological analysis, the pancreas was removed and fixed with 2% paraformaldehyde (PFA). Multiple 10 mm sections were stained with hematoxylin and eosin and scored blindly for insulitis (Score: 0, no infiltrate; 1, peri-insulitis present; 2, <25%; 3, >25% of the islet is infiltrated). Average insulitis percentages were determined from the total number of islets counted from each treatment group. Statistical significance for islets with no infiltrate and islets with the most severe insulitis was determined by Student’s t test for comparison of percentages between treatment groups.
2.13. Statistical analyses
Statistical significance of the differences of mean values was calculated using two-tailed Student’s t tests. The Mantel-Cox test (log-rank test) and the Gehan-Breslow-Wilcoxon test was used to compare diabetes incidence. P-values <0.05 were considered significant (*p<0.05). Statistical analyses were performed using PRISM software version 6.0h (GraphPad). Data shown are means + SEM.
3. Results
3.1. Treatment with p31-SP or p31-coupled PLG/PEMA nanoparticles prevents/delays onset of hyperglycemia in spontaneous T1D in NOD mice and in the BDC2.5 transfer model of T1D
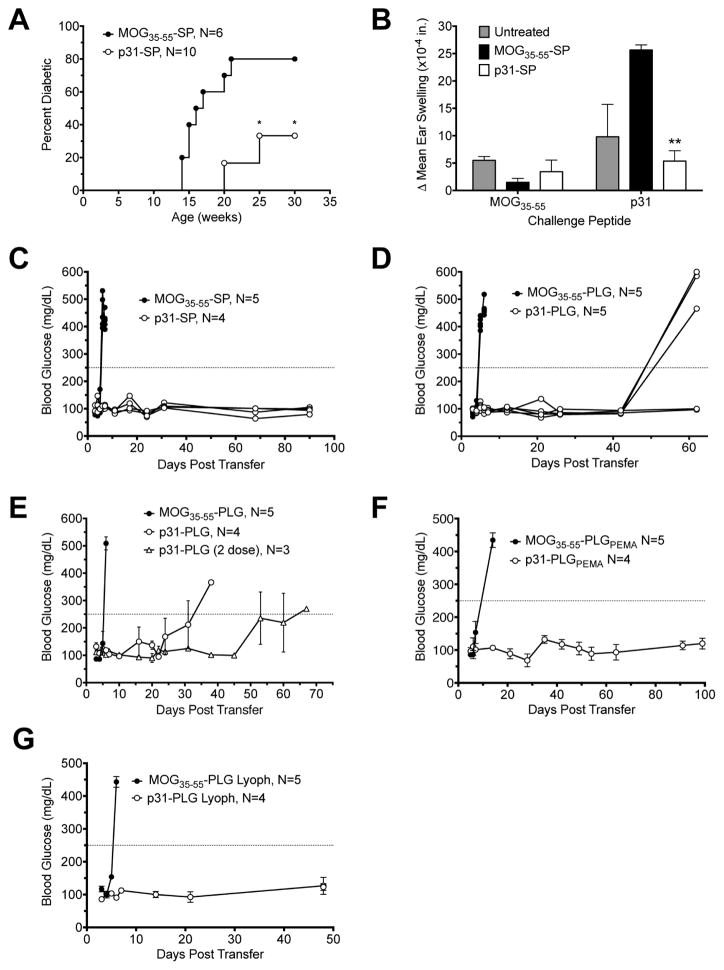
The p31 peptide is a mimetope for a natural T cell epitopes found on pancreatic Chromogranin A (ChgA) [14]. These epitopes include ChgA29–42 and ChgA358–371 [13,21]. Both native and transglutaminase-modified ChgA358–371 have been shown to be targets for autoreactive cells in new onset T1D patients [22]. While BDC2.5 TCR Tg T cells and the p31 mimetope have been widely used as a model for studying T1D, little is known about the role of ChgA reactive T cells in the development and pathogenesis in spontaneous disease. To determine whether ChgA can be utilized for antigen-specific immunotherapy, NOD mice were treated with syngeneic apoptotic NOD splenocytes, which were ECDI-coupled with p31 peptide (p31-SP) at 5 weeks of age, before onset of spontaneous T1D. Treatment with p31-SP reduced onset and incidence of increased blood glucose (Fig. 1A). In contrast, treatment with control MOG35–55-SP, a T1D irrelevant myelin peptide known to bind to NOD MHC II I-Ag7 [23], were not protected from T1D. Additionally, p31-SP reduced in vivo CD4+ T cell function as determined by the lack of delayed-type hypersensitivity (DTH) responses when compared to untreated or control MOG35–55-SP treated mice (Fig. 1B). This result indicates that ChgA is a potential viable target for Ag-specific tolerance therapy in spontaneous T1D in NOD mice.
Fig. 1. Treatment with p31-SP or p31-coupled PLG/PEMA nanoparticles prevents/delays onset of hyperglycemia in spontaneous T1D in NOD mice and in the BDC2.5 transfer model of T1D.
(A) Wildtype NOD mice were treated with p31-SP at 5 weeks of age. Percentage of spontaneous diabetes in p31-SP treated mice is compared to the control MOG35–55-SP treated mice. (B) Ear swelling, as a measure of DTH, was measured 24 h after ear challenge with the p31 and MOG35–55 peptides in treated and untreated NOD mice at 31 weeks of age. (C–G) Diabetes was induced by the adoptive transfer of 5×106 p31 mimetope peptide-activated MHC II-restricted transgenic BDC2.5 T cells into NOD.SCID recipients. Mice were treated at day 0 or 1 post transfer by i.v. infusion of p31-SP (C), p31-coupled PLG nanoparticles (D), p31-PLG nanoparticles with a 2nd dose administered at day 20 post transfer (E), p31-coupled PLG/PEMA nanoparticles (F), or by i.v. infusion of freshly prepared vs. lyophilized p31-coupled PLG nanoparticles (p31-PLG) (G). Individual (C,D,G) or grouped (E–F) recipient blood glucose measurements are shown. Data in each panel is representative of 2–5 individual experiments. *p<0.05, **p<0.01 vs. MOG35–55 control peptide tolerized mice.
We have recently demonstrated that carboxylated, biodegradable PLG nanoparticles can be effectively used as a surrogate Ag carrier for the induction of tolerance in Th1/Th17 autoimmune and Th2-mediated allergic diseases [8–11,19]. These inert particles offer many benefits compared to cell-based therapies including extended shelf-life and the ease and reduced costs of large scale production under GMP conditions. The BDC2.5 model for T1D was used to determine whether splenocytes (p31-SP) and PLG nanoparticles ECDI-coupled with the p31 mimetope peptide (p31-PLG) could prevent onset of T1D in NOD.SCID recipients induced by the transfer of p31 peptide-activated CD4+ BDC2.5 Tg T cells. Adoptive transfer of activated BDC2.5 Tg cells results in rapid onset of hyperglycemia within 5–7 days with 100% incidence in mice tolerized with control MOG35–55-SP or commercially available (Phosphorex) 500 nm PLG nanoparticles. In contrast, the i.v. infusion of either p31-SP or p31-PLG particles provided significant protection from disease onset (Fig 1. C&D) with p31-SP appearing to be more efficient for tolerance induction. Additionally, two infusions of p31-PLG (Phosphorex) prolonged the time to development of T1D (Fig. 1E). We next tested p31-coupled to PLG nanoparticles which we prepared in the lab using PEMA to increase the density of surface carboxyl groups thus lowering the ζ potential. p31-PLG/PEMA particles worked much more efficiently than the Phosphorex particles inducing protection that lasted at least 100 days post BDC2.5 T cell transfer (Fig. 1F). Interestingly, tolerization with p31-PLG that had been lyophilized and stored for 3 months worked as effectively as freshly prepared particles (Fig. 1G) demonstrating that peptide cross-linking remains stable and is ideal for use in a clinical setting.
3.2 Tolerance induced by peptide-encapsulating PLG/PEMA nanoparticles specifically prevents adoptive T1D mediated by both diabetogenic BDC2.5 CD4+ and NY8.3 CD8+ T cells
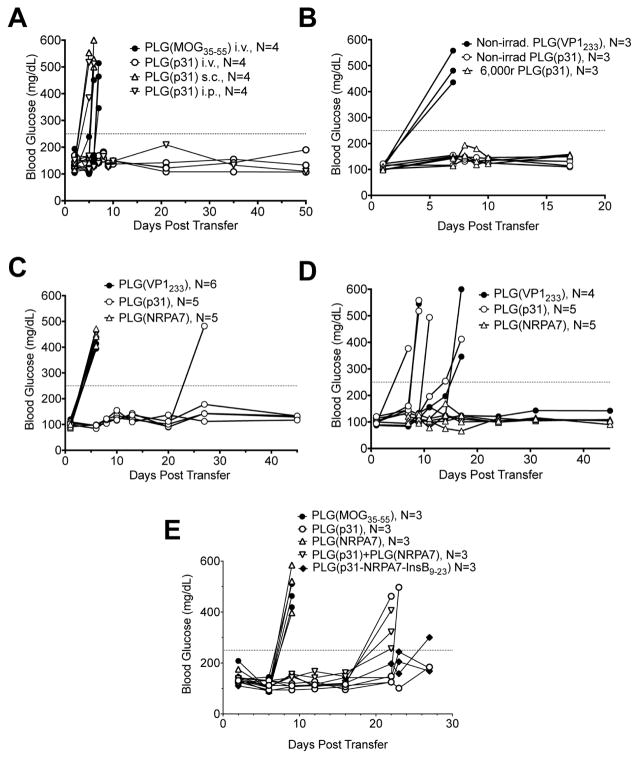
We next wished to determine the route of administration required and antigen specificity of tolerance induction, as well as the ability of Ag-encapsulating PLG nanoparticles to regulate autoimmune responses mediated by both activated diabetogenic CD4+ and CD8+ T cells. We recently showed that Ag-encapsulating PLG nanoparticles [PLG(Ag)] were effective for treatment of both Th1/Th17-mediated EAE [19] and Th2-mediated allergic airway disease avoiding anaphylactic responses in pre-primed mice [10]. Protection against onset of T1D was only observed in mice treated via the i.v. route, not following s.c. or i.p. infusion (Fig. 2A). Combining repeat experiments, i.p. infusion was effective in only 2/12 treated mice, while i.v. treatment protected 11/12 mice. In addition, p31 encapsulated PLG nanoparticles could induce protection following irradiation (6,000r, 60Co) (Fig. 2B) again demonstrating their clinical translation potential. To assess the Ag-specificity of tolerance induction, we compared the tolerogenic potential of PLG nanoparticles encapsulating the MHC II-restricted ChgA p31 mimetope or the MHC I-restricted IGRP NRPA7 mimetope in protecting from T1D induced by the transfer of p31-specific BDC2.5 CD4+ Tg T cells and/or the transfer of NRPA7-specific NY8.3 CD8+ Tg T cells. Tolerance in both CD4+ and CD8+ T cells proved to be exquisitely peptide-specific as T1D transfer by activated BDC2.5 CD4+ T cells was only inhibited by i.v. infusion of PLG(p31) particles (Fig. 2C), while T1D transfer by activated NY8.3 CD8+ T cells was only inhibited by treatment with PLG(NRPA7) encapsulating particles (Fig. 2D). In a more stringent test of tolerance, T1D in NOD.SCID recipients was induced by transfer of a mixture of 2×106 activated CD4+ BDC2.5 T cells + 2×106 CD8+ NY8.3 T cells. The recipients were treated with PLG (MOG35–55) as a control, PLG(p31), PLG (NRPA7), a mixture of PLG(p31) + PLG (NRPA7), or with PLG particles encapsulating a tri-peptide composed of p31, NRPA7 and InsB9–23 separated by cathepsin S sites to allow correct processing. Treatment with the CD8-targeting PLG(NRPA7) particles provided no protection in this model, while treatment with the CD4-targeting PLG(p31) or the mixture of these two particles provided partial protection delaying disease onset. Most interestingly, treatment with PLG(p31-NRP-A7-InsB9–23) provided the most significant protection (Fig. 2E) which was borne out in repeat experiments (not shown). Thus, tolerance appears to be exquisitely peptide-specific, can be conferred on both diabetogenic CD4+ and CD8+ T cells, and can be induced most efficiently in a complex disease setting by PLG particles encapsulating multiple diabetogenic epitopes providing a potential platform for treatment of autoimmune diseases mediated by responses to numerous autoepitopes.
Fig. 2. Tolerance induced by peptide-encapsulating PLG/PEMA nanoparticles prevents T1D in both the BDC2.5 CD4+ and NY8.3 CD8+ adoptive transfer models in an antigen-specific fashion.
(A–B) Diabetes was induced by adoptive transfer of 5×106 p31 mimetope peptide-activated BDC2.5 MHC II-restricted transgenic T cells into NOD.SCID recipients. Mice were treated within 2 hours post transfer with PLG/PEMA nanoparticles encapsulating control peptide (MOG35–55 or VP1233–241) by the i.v. route or by particles encapsulating the p31 peptide [PLG(p31)] by i.v., i.p. or s.c. routes (A) or non-irradiated vs. 6,000r-irradiated p31-encapsulating nanoparticles (B). (C–D) Diabetes was induced by adoptive transfer of 5×106 p31 mimetope peptide-activated MHC II-restricted BDC2.5 CD4+ T cells (C) or 5×106 NRPA7 mimetope peptide-activated MHC I-restricted NY8.3 CD8+ T cells (D) into NOD.SCID recipients. Mice were treated within 2 hours post transfer with PLG(VP1233), PLG(p31), or PLG(NRPA7). Data in each panel is representative of 3–4 individual experiments. (E) Diabetes was induced by adoptive transfer of 2×106 NRPA7-activated NY8.3 CD8+ T cells + 2×106 p31-activated BDC2.5 CD4+ T cells into NOD.SCID recipients. Mice were treated on day-1 and day+1 relative to transfer with PLG nanoparticles encapsulating MOG35–55, p31, NRPA7, both p31 and NRPA7, or a linked tripeptide containing p31, NRPA7 and InsB9–23. Individual blood glucose measurements are shown. This data is representative of 3 individual experiments.
3.3. Reduced infiltration of effector T cells and preservation of islets in p31-PLG tolerized mice
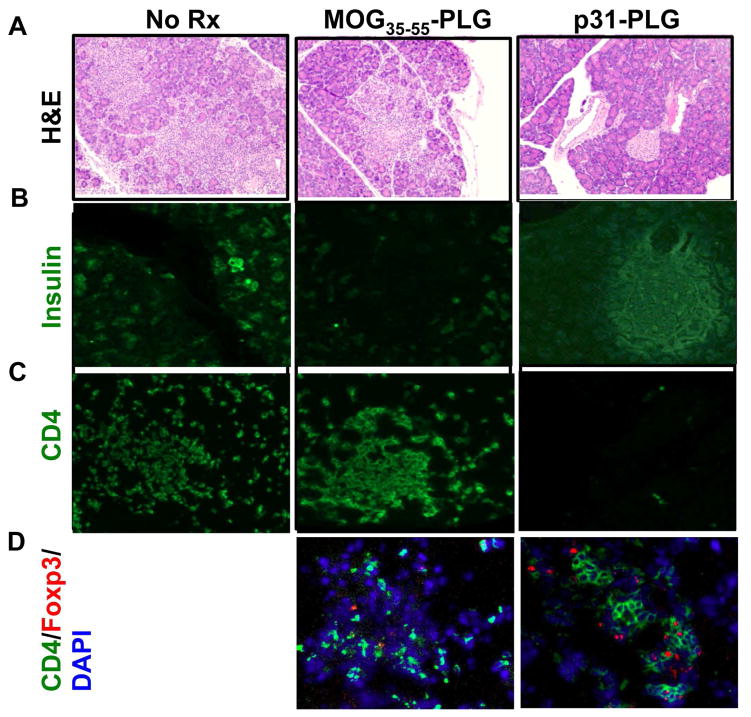
We next performed histology on the pancreata of NOD.SCID recipients of activated BDC2.5 T cells which has been treated with control MOG35–55-coupled PLG particles (MOG35–55-PLG) or p31-PLG. The pancreata of both untreated and MOG35–55-PLG treated mice showed totally disrupted islets which were infiltrated with large numbers of donor BDC2.5 T cells while normal islet architecture and only a few CD4+ T cells were observed in p31-PLG treated mice (Fig. 3A, B & C). The few areas where T cell infiltrates were observed in the p31-PLG-treated mice also had significant numbers of Foxp3+ donor BDC2.5 cells (Fig. 3D-right panel), while there were only very few Foxp3+ Tregs in the control MOG35–55-PLG treated mice (Fig. 3D-center panel).
Fig. 3. NOD.SCID recipients of activated BDC2.5 T cells tolerized with p31-PLG nanoparticles exhibit reduced pancreas infiltration of effector CD4+ T cells and preservation of insulin-producing islets.
Representative H&E staining (A) or FITC staining of insulin (B), CD4+ T cells (C), and CD4+Foxp3+ Treg cells (D) of pancreata isolated from untreated NOD.SCID recipients (left panels) and from NOD.SCID recipients of p31 activated BDC2.5 T cells 5 days post-treatment with PLG(MOG35–55) (middle panels) or PLG(p31) nanoparticles (right panels).
3.4. Treatment with p31-PLG causes retention of inflammatory BDC2.5 Tg cells in the spleen while reducing effector cell infiltrates and cytokine production in the pancreas
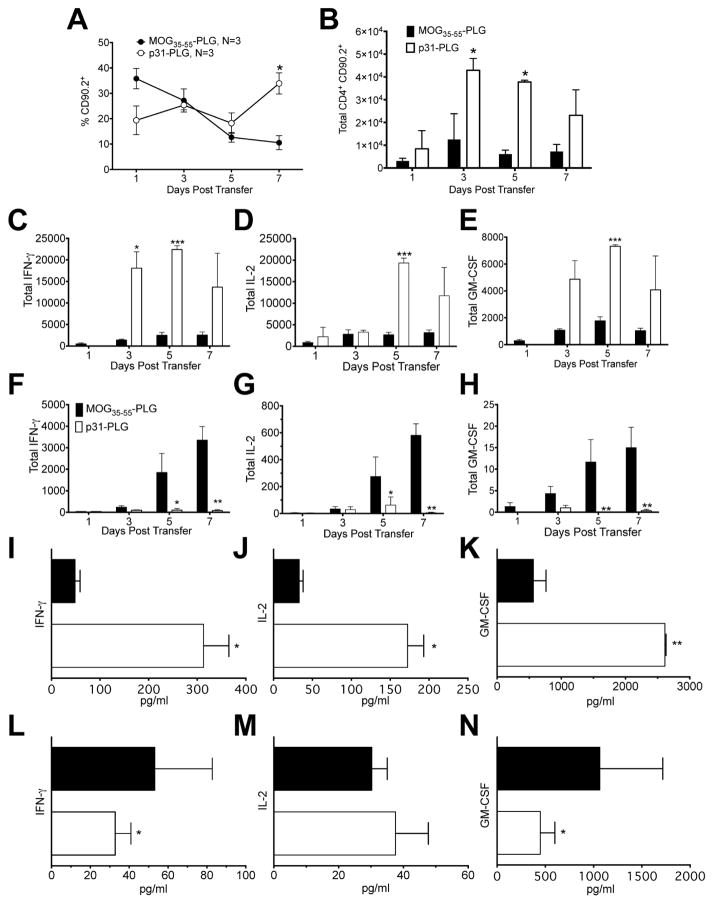
To determine how p31-PLG modulates the autoimmune response, CD4+ T cell populations and their effector functions in the spleen and pancreas were examined. Treatment with p31-PLG results in increased percentage and total numbers of donor BDC2.5 CD90.2+ Tg CD4+ T cells in the NOD.SCID recipient spleen compared to control MOG35–55-PLG treated animals (Fig. 4A, B). Increased accumulation of effector cells in the spleen of mice protected from development of T1D suggests that Ag-PLG tolerance induces alterations in effector T cell trafficking. Correspondingly, FACS analysis of p31-specific recall responses of T cells recovered from the spleens of p31-PLG treated mice showed significantly enhanced number of donor cells producing of inflammatory cytokines including IFN-γ, IL-2, and GM-CSF compared to MOG35–55-PLG treated controls (Fig. 4C–E), while p31-induced proinflammatory cytokine producing cells in the pancreata of p31-PLG treated animals were essentially absent (Fig. 4F–G). On day+5 following Ag-PLG treatment, cytokine secretion from pancreas-infiltrating and splenic T cells was determined following stimulation for 24 hours with p31 peptide. Corresponding to the numbers of cytokine producing cells as determined by intracellular staining, peptide-induced secretion of IFN-γ, IL-2, and GM-CSF was increased in splenic T cell cultures from p31-PLG treated mice as compared to mice treated with MOG35–55-PLG (Fig. 4I–K), while production of IFN-γ and GM-CSF from pancreas-infiltrating BDC2.5 T cells was significantly reduced (Fig. 4L–N).
Fig. 4. Treatment with p31-ECDI-PLG causes retention of inflammatory BDC2.5 Tg cells in the spleen while reducing cell infiltrates and cytokine production in the pancreas.
(A–B) Diabetes was induced by adoptive transfer of 5×106 p31-activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated i.v. within 2 hours of transfer with MOG35–55-PLG/PEMA or p31-PLG/PEMA nanoparticles. The percentage (A) and total numbers (B) of BDC2.5 Tg T cells in the spleen of NOD.SCID recipients were determined by flow cytometric analysis on days 1, 3, 5, and 7 post transfer. Compiled data shown on day 5 (B) are representative of 5 independent experiments with 3–5 mice per group. (C–H) Diabetes was induced by adoptive transfer of 5×106 p31 activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated at within 2 hours of transfer with MOG35–55-PLG or p31-PLG. Spleens (C–E) and pancreata (F–H) were isolated on days 1, 3, 5, and 7 and stimulated for 24h with p31 peptide. FACS analysis was used to determine numbers of transferred BDC.25 T cells producing IFN-γ (C,F), IL-2 (D,G) and GM-CSF (E,H) in MOG35–55-PLG (N=3) and p31-PLG (N=3) treated mice at each time point. Splenic (I–K) and pancreas infiltrating lymphocytes (L–N) were isolated on day 5 and stimulated for 24h with p31 peptide. Levels of cytokine secretion from cells from MOG35–55-PLG (N=3) and p31-PLG (N=3) treated mice were measured in culture supernatants using a cytometric bead array. Data is representative of three separate experiments. *p<0.05, **p<0.01, ***p<0.001.
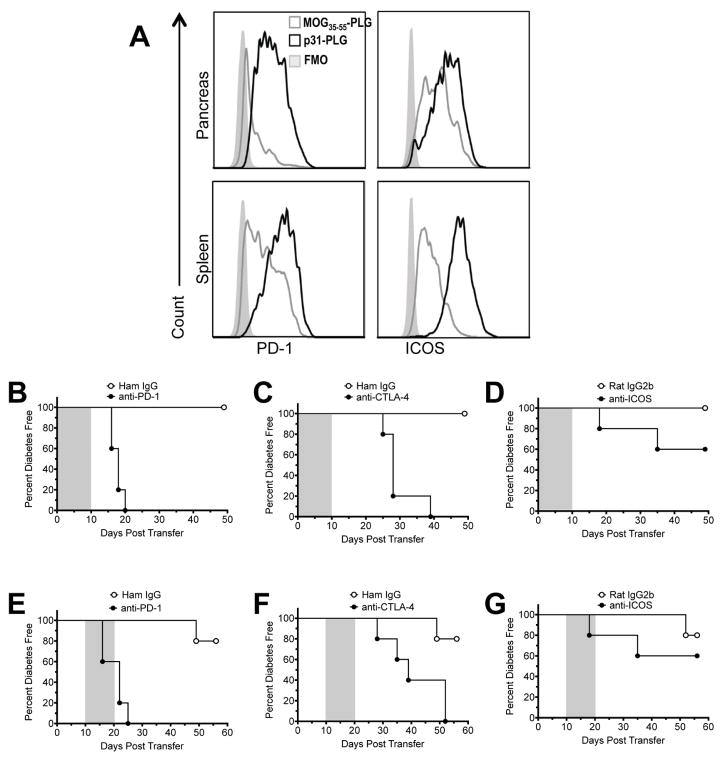
3.5 Co-inhibitory signals are required for both induction and maintenance of Ag-PLG-induced tolerance
Treatment with Ag-SP can mediate immune tolerance via a number of cell-intrinsic (anergy, negative costimulation, clonal deletion), and/or cell-extrinsic (Treg cells, immune deviation) mechanisms [4,5,24–26]. Both CTLA-4/CD80-CD86 and PD-1/PD-L1 pathways have been shown to be required for tolerance induction using cell-based treatments including p31-SP and Insulin-SP in the BDC2.5 transfer and NOD models of T1D, respectively, however only the PD-1/PD-L1 pathway was required for the maintenance of long-term tolerance [25]. Additionally, activation of the ICOS pathway has been shown to be involved in the development of Treg function and tolerance within mucosal tissues [27,28]. Therefore, to determine whether negative costimulatory pathways plays a similar role in the Ag-PLG tolerance the expression of CD4+ T cell co-inhibitory molecules, PD-1 and ICOS was examined in p31-PLG treated animals. Both molecules were upregulated on splenic and pancreatic T cells in p31- PLG treated animals (Fig. 5A). Mechanistically, expression of negative costimulation was required for tolerance induction as antibody blockage of PD-1, CTLA-4 and to a lesser extent ICOS during the period immediately following p31-PLG treatment (days 0–10) resulted in a marked reduction in the efficacy of tolerance induction (Fig. 5B–D). Further, antibody blockade of PD-1 (Fig. 5E) and CTLA-4 (Fig. 5F), but not ICOS (Fig. 5G), in mice with established tolerance (days 11–20) led to reversal of protection and onset of T1D. This demonstrates that PD-1 and CTLA-4 are crucial for long-term maintenance of Ag-PLG induced tolerance.
Fig. 5. Co-inhibitory signals are required for both induction and maintenance of Ag-PLG-induced tolerance.
(A) NOD.SCID recipients of 5×106 p31 activated BDC2.5 T cells were tolerized with either MOG35–55-PLG/PEMA (N=3) or p31-PLG/PEMA (N=3) and expression of PD-1 and ICOS on T cells in the pancreas and SP was determined by FACs analysis on day +5. (B–G) NOD.SCID recipients of p31 activated BDC2.5 T cells were tolerized with p31-PLG/PEMA within 2 hours of cell transfer. Mice (N=5 per group) were then treated with anti-PD-1 (500μg first treatment, 250μg following treatments), anti-CTLA-4 (500μg), or anti-ICOS (500μg) blocking antibodies administered daily on days 0–10 (grey shading - B–D) or on days 10–20 (grey shading - E–G) post transfer and tolerization as indicated by the shaded area. Blood glucose levels were monitored weekly to determine diabetes incidence. Data is representative of two separate experiments.
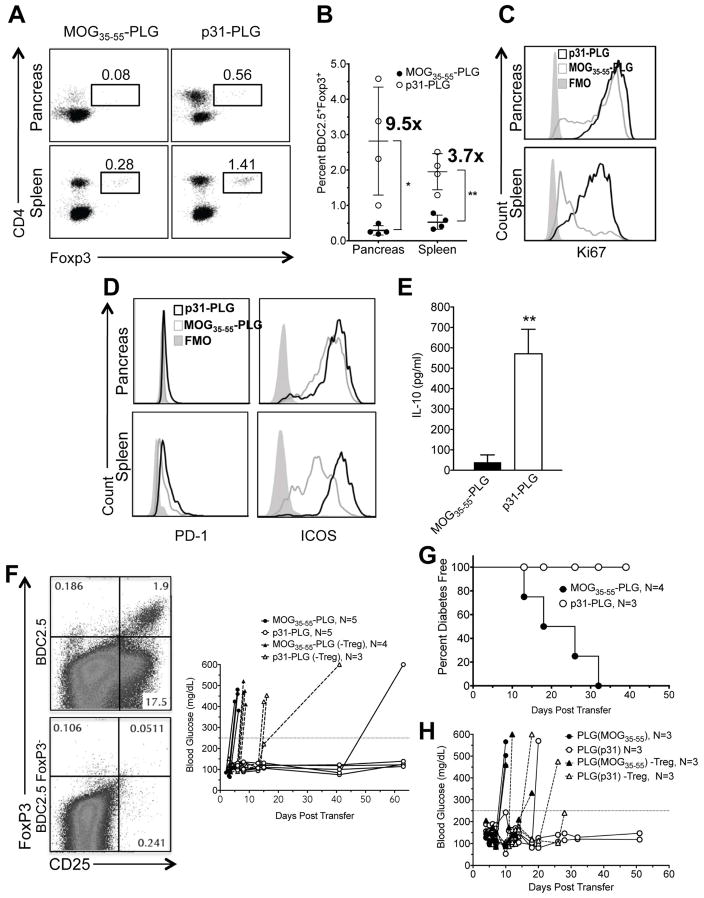
3.6. p31-PLG treatment induces expansion of Tregs which are required for maintenance of nanoparticle-induced tolerance
Regulatory T cells play a crucial role in Ag-specific tolerance [29]. The role of Tregs in the initiation and maintenance of Ag-PLG tolerance has yet to be established. FACS analysis of BDC2.5 T cells isolated from the spleen and pancreas of NOD.SCID recipients of BDC2.5 T cells 5 days post treatment with p31-PLG (Fig. 6A,B) showed significant expansion of Treg populations. The fold increase in p31-PLG treated mice as compared to mice treated with control MOG35–55-PLG particles was higher in the pancreas (9.5x) vs. the spleen (3.7x). Additionally, Tregs in the spleens of p31-PLG treated animals expressed high levels of Ki67 (Fig. 6C). Both ICOS and PD-1 expression were upregulated on Foxp3+ BDC2.5 T cells in p31-PLG as compared to MOG35–55 tolerized mice (Fig. 6D) and splenocytes from p31-PLG treated mice produced significant levels of IL-10 in response to overnight stimulation with p31 peptide (Fig. 6E). Together these results suggest that Tregs may utilize both negative costimulation and inhibitory cytokines to modulate the pathogenic BDC2.5 T cells.
Fig. 6. Ag-PLG treatment induces expansion of Tregs which are required for maintenance of p31-PLG nanoparticle-induced tolerance.
(A–C) Proliferation and expansion of Tregs in p31-PLG treated mice. Diabetes was induced by adoptive transfer of 5×106 p31 activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated 2 hours post transfer with either i.v. MOG35–55-PLG or p31-PLG. Pancreas infiltrating lymphocytes and splenocytes were isolated on day 5 and FACs analysis was used to determine the percentage of CD90.2+CD4+Foxp3+ shown in a representative plot from pooled samples from three mice (A) and numbers or Foxp3+ cells quantitated from 4 individual experiments (B). Ki67 expression on CD90.2+CD4+Foxp3+ BDC2.5 T cells in spleen and pancreas pooled from 3 mice was also determined (C). (D–E) Tregs in p31-PLG treated animals show increased expression of negative costimulatory molecules and production of IL-10 production. Diabetes was induced by adoptive transfer of 5×106 p31-activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated i.v. 2 hours post transfer with either MOG35–55-PLG or p31-PLG. Pancreas infiltrating lymphocytes and splenocytes were isolated on day 5. FACs analysis was used to determine expression of PD-1 and ICOS in p31-PLG (N=3) and MOG35–55-PLG (N=3) treated mice (D). Splenocytes from MOG35–55-PLG (N=3) and p31-PLG (N=3) treated mice were cultured in vitro overnight with p31 peptide and levels of IL-10 secretion in culture supernatants determined by a cytometric bead array (E). (F–H) Tregs are responsible for long-term tolerance maintenance. (F) BDC2.5 CD4+CD25+ T cells were either not depleted or depleted via MACs cell sorting following three days of culture with p31. NOD.SCID mice were infused with activated BDC2.5 cells either containing or not containing CD4+CD25+ T cells and were then treated within two hours of transfer with MOG35–55-PLG or p31-PLG. Individual blood glucose measurements are shown. Results are representative of three separate experiments. (G) Diabetes was induced by adoptive transfer of 5×106 p31-activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated within 2 hours of transfer with either MOG35–55-PLG or p31-PLG nanoparticles. On day 5, splenocytes harvested from both MOG35–55-PLG and p31-PLG treated animals were adoptively transferred into secondary NOD.SCID recipients and blood glucose levels were monitored to determine the incidence of diabetes in the secondary recipient animals. (H) A similar secondary transfer was performed using splenocytes from BDC2.5 NOD.SCID recipients tolerized MOG35–55-PLG or p31-PLG nanoparticles from which CD25+ cells were either not removed or depleted using MACS column sorting. Data from Panels G and H are each representative of two separate experiments.
To determine whether Tregs were important for the induction and/or maintenance of tolerance in Ag-PLG treated mice, we compared the effects of p31-PLG tolerance induction in NOD.SCID recipients of activated BDC2.5 T cells which either contained or were depleted of CD4+CD25+ Tregs (Fig. 6F, Left Panels). As CD25 is also highly upregulated on CD25+Foxp3− effector T cells, depletion of pathogenic BDC2.5 CD4+CD25+ T cells delayed onset of disease by one week when compared to non-depleted controls (Fig. 6F, Right Panel). Strikingly, unlike the long-term protection normally observed in recipients of activated BDC2.5 T cells containing Tregs, p31-PLG treatment only briefly delayed development of T1D in recipients of BDC2.5 T cells depleted of Tregs (Fig. 6F, Right Panel).
The critical role for Tregs in Ag-PLG tolerance maintenance along with the observation that effector BDC2.5 T cells are sequestered in the spleens and largely absent from the pancreata of p31-PLG tolerized mice (Fig. 4), led us to hypothesize that BDC2.5 Tregs may act by regulating the trafficking of transferred BDC2.5 effector cells preventing them from infiltrating the pancreas. To test this hypothesis, we asked if tolerance would be maintained upon transfer of splenic leukocytes from the initial p31-PLG treated NOD.SCID recipients of activated BDC2.5 T cells to a secondary NOD.SCID recipient. Interestingly tolerance was maintained in secondary recipients of splenocytes from p31-PLG tolerized donors, despite the higher numbers of diabetogenic effector cells in the population, while recipients of splenocytes from donors tolerized with the control MOG35–55-PLG developed T1D (Fig. 6G). This suggested that the p31-specific Tregs present in the spleens of tolerant donors were responsible for maintaining tolerance in the secondary recipient mice. Indeed, tolerance was not maintained in secondary recipients of spleen cells from tolerant donors depleted of CD25+ T cells (Fig. 6H).
4. Discussion
Currently the only treatment option for T1D patients is insulin replacement therapy. The studies completed herein confirm the potential for PLG particles, coupled to or encapsulating diabetogenic T cell Ags, to prevent the onset of autoimmune diabetes mediated by previously activated diabetogenic T cells. These results indicate that tolerance may be an effective therapy in patients at high risk for development of T1D in which autoreactive diabetogenic T cells have already begun the process of destroying pancreatic β cells. Mechanistically, Ag, either coupled to or encapsulated within highly negatively charged PLG nanoparticles is taken up by host APCs, processed and subsequently cross-presented in the context of MHC class I or II. These APCs also upregulate negative co-stimulatory molecules, e.g. PD-L1, as well as secrete regulatory cytokines, e.g. IL-10 and TGF-β (manuscript in preparation) culminating in the downstream regulation of autoreactive T cells. The regulatory process includes the systemic expansion of Tregs, which were observed to express high levels of negative co-stimulatory molecules (CTLA-4 and PD-1) and are essential in the sequestration of effector CD4+ T cells in the spleen. The functional inactivation of Tregs, using anti-CD25 (PC-61 antibody), resulted in the breakdown of established tolerance, confirming the importance of these cells in tolerance maintenance.
The utilization of Ag-SP to induce tolerance in rodents for the treatment of autoimmune disease and allergy has been well described [4–6] and translated to a successful phase I trial in MS patients showing safety and efficacy for inducing tolerance to myelin autoantigens [7]. The current studies further demonstrate that Ag-SP can be utilized to induce tolerance in the NOD model of T1D. Treatment with p31-ECDI-SP reduced onset and incidence of T1D in NOD mice. Importantly, the ability of targeted immunotherapy towards ChgA provides a viable potential target for antigen specific immunotherapy in T1D. However, the application of autologous apoptotic cells for clinical tolerance is associated with numerous manufacturing and translational hurdles. As such, we set out to demonstrate the utility of inert biodegradable PLG nanoparticles coupled to or encapsulating p31 to induce tolerance in a more robust model of T1D induced by the transfer of in vitro activated diabetogenic transgenic T cells to NOD.SCID recipients. While slight modifications of commercially available (Phosphorex) PLG nanoparticles, including the use of highly carboxylated nanoparticles and increasing Ag exposure, were found to be necessary, treatment with p31 coupled PLG particles (p31-PLG) was capable of preventing the rapid onset of hyperglycemia normally observed in recipients of activated CD4+ and CD8+ diabetogenic transgenic T cells.
Recently we reported in a model of Th2-mediated allergic airway disease that the encapsulation of Ag within PLG nanoparticles can be more efficacious and proved to be safer in a clinical therapeutic tolerance setting [10]. Therefore, the ability of PLG nanoparticles encapsulating diabetogenic peptides to induce immune tolerance was also examined. Using the adoptive transfer of activated BDC2.5 CD4+ T cells and/or NY8.3 CD8+ T cells to induce T1D, we observed that the encapsulation of one or multiple T cell epitopes can be deployed to target and tolerize both CD4+ and/or CD8+ diabetogenic T cells. These observations confirm previous studies showing the ability of antigen delivered in the context of nanoparticles not only to induce tolerance in MHC II-restricted T cells [4–6,19,30,31], but also that Ag delivered to APCs in the context of negatively charged nanoparticles can be cross-presented to regulate CD8+ T cells responses as illustrated in PLG(NRPA7)-treated recipients of CD8+ NY8.3 T cells. Significantly, tolerance was found to be exquisitely peptide-specific as tolerance in recipients of MHC II-restricted BDC2.5 T cells was only induced with PLG particles encapsulating the cognate ChgA p31 mimetope peptide, not with the MHC I-restricted IGRP NRPA7 mimetope peptide, and vice versa in recipients of NY8.3 transgenic CD8+ T cells. More significantly, tolerance in NOD.SCID recipients of a mixture of activated BDC2.5 and NY8.3 T cells was only maintained upon infusion of PLG nanoparticles encapsulating a polypeptide containing linked p31, NRPA7, and InsB9–23 epitopes, i.e. targeting both the CD4+ an CD8+ T cell epitopes. The use of the linked peptide tolerance approach has the advantage of assuring equimolar loading of a variety of autoepitopes within the PLG nanoparticles and the ability to simultaneously target multiple pathogenic autoreactive CD4+ and CD8+ T cells which will invariably be activated in established T1D via the epitope spreading process [5,32].
The induction of tolerance using Ag-PLG appears to be largely similar to, but to differ slightly than tolerance induced by Ag-SP. We previously have shown that while the scavenger receptor MARCO, was largely indispensable for Ag-PLG-induced tolerance, this was not the case for Ag-SP [4,8]. Further investigation is necessary to delineate these nuances in tolerance induction when using Ag-loaded apoptotic cells vs. PLG nanoparticles, although we propose that unlike carboxylated engineered nanoparticles that are designed to target specific scavenger receptor pathways, e.g. MARCO which binds polyanionic surfaces [33], apoptotic cells have the potential to interact with numerous apoptotic uptake receptor pathways thereby exhibiting some redundancy. We have previously published extensive biodistribution studies of 500nm nanoparticles with a charge of less than −30mv [34]. The exquisite antigen specificity of the tolerance shown in Figs. 2C and 2D and the critical role of Tregs in tolerance induction and maintenance (Figs. 4 and 6) indicate that the ultimate effect of Ag-PLG tolerance is on pathologic T cells and not on APCs. However, as we have previously published, the specific effect on T cells is secondary to uptake of PLG nanoparticles by APCs, including macrophages and DCs mainly in the spleen and liver, following i.v. infusion [19] which induce anergy and Tregs via processes dependent on upregulation of PD-L1 and IL-10 (Figs. 5 and 6) [35]. Here, Ag-PLG was demonstrated to have similar efficacy as Ag-SP, although antigen dosing regimen modifications were necessary. Tolerance using p31-PLG required the use of highly negatively charged nanoparticles as well as increased antigen delivery. Mechanistically, minor differences in negative co-stimulatory requirements were observed for Ag-PLG compared to Ag-SP. For instance, the induction of p31-SP tolerance was shown by Fife, et al. to be dependent on PD-1/PD-L1 pathways, whereas CTLA-4 signaling was seen to be dispensable for long term tolerance maintenance [25]. Conversely, induction and maintenance of p31-PLG tolerance requires both CTLA-4 and PD-1, although tolerance was much more readily reversed using anti-PD-1 blockade.
Notwithstanding alterations in the underlying co-stimulatory signals needed for tolerance induction, the importance of Tregs is shared a characteristic of both Ag-SP and Ag-PLG. It is well defined that Tregs are essential for tolerance when using Ag-SP [4], and the data presented here clearly show that p31-PLG tolerance is also dependent on Tregs. The administration of p31-PLG resulted in significant increased Treg numbers in the spleens and pancreata of treated animals, increased production of IL-10, and increased expression of PD-1 and ICOS on Tregs. The expansion of Tregs correlated with the accumulation of IFN-γ producing effector CD4+ T cells in the spleen. The precise mechanism(s) though which these effector cells are sequestered in the spleen is not yet known, but appears to be an important component of tolerance maintenance. While splenic sequestration of Ag-specific effector cells may be a result of antigen accumulation in spleen resulting from particle infusion, we propose an alternative hypothesis. Specifically, we suggest that treatment-induced Tregs are capable of regulating the trafficking of effector T cells. In this context Tregs sequester effector T cells in the spleen preventing them from trafficking to sites of inflammation. This is supported by the maintenance of tolerance upon transfer of splenic T cells from tolerant donors to a secondary NOD.SCID recipient despite the transfer of large numbers of effector T cells into a lymphopenic host in which homeostatic expansion has been shown to reverse anergy [36], and the reversal of tolerance by removal of CD25+ T cells prior to secondary transfer. The ability of Tregs to modulate effector T cell trafficking has previously been described by others [37]. It is also relevant to mention that the exquisite peptide specificity and lack of bystander suppression observed in the context of the Ag-PLG Treg-inducing tolerogenic regimen was previously observed by our group in Treg-mediated tolerance to myelin antigens in induced and spontaneous EAE models [38].
Tregs induced by p31-PLG treatment also express high levels of PD-1 and CTLA-4, with interference of these molecules, through antibody-mediated blockade, resulting in the loss of tolerance. Further experimentation is necessary to delineate the importance of Treg expressed CTLA-4 and PD-1 expression. However, it is likely that Tregs utilize these molecules to regulate effector T cells that have accumulated in the spleen and the pancreas. Recently, using mice with partial Foxp3 insufficiency and deficient for PD-1 expression, it was found that PD-1 plays an important role in regulating the activation thresholds of effector T cells, whereas Foxp3 expression was important for regulating the balance between regulatory and effector T cells [39]. Taken together, p31-PLG treatment appears to harness similar immune regulatory mechanisms to prevent the pathogenic activity of autoreactive T cells.
Numerous nanoparticle therapies focused on inducing immune tolerance in autoimmune conditions have recently emerged in the literature [40]. These include non-antigen specific approaches delivering immunosuppressants such as microencapsulated rapamycin [41]. Antigen-specific approaches include delivery of antigen on/in nanoparticles targeting MARCO or scavenger receptor class F member 1 (SCARF1) scavenger receptors [42,43], antigen loaded particles containing rapamycin [41,44,45] or arylhydrocarbon receptor agonists ligands [46,47] to condition tolerogenic antigen presentation, or delivery of iron nanoparticles coated with antigen-loaded MHC II to expand regulatory T cell populations [48]. While some of these other approaches are elegant, successful treatment in humans may require continuous administration of nanoparticle formulations containing immunosuppressive or immune modulating agents. Therefore, questions surrounding nanoparticle toxicity remain to be addressed. Significantly, our data demonstrate that a single i.v. infusion of carboxylated Ag-PLG or PLG(Ag) nanoparticles results in essentially indefinite protection from T1D induced by highly activated diabetogenic CD4+ or CD8+ T cells. Furthermore, the scale-up of many of these nanoparticle formulations will require advances in technological know-how not currently available. It is thus highly relevant that the highly negatively charged nanoparticles utilized in our studies maintain their tolerogenic capacity following lyophilization and sterilization using gamma irradiation. GMP manufacture of PLG(Ag) nanoparticles has recently been successfully advanced to clinical scale (Getts, personal communication). Furthermore, clinical trials testing carboxylated PLG nanoparticles encapsulating gliadin are on-going in celiac disease, sponsored by Cour Pharmaceutical Development Company (www.courpharma.com/).
A number of challenges remain for the translation of this treatment of T1D. These include identification of appropriate human T1D T cell epitopes required to be targeted. In addition, the timing of administration to induce tolerance in T1D patients remains controversial. Prevailing dogma suggests that type 1 diabetics have limited residual islet cell mass at the time of disease diagnosis, suggesting a tolerance induction agent may have limited clinical utility. Therefore, the use of negatively charged PLG particles, loaded with T1D antigen, will need to be deployed either in conjunction with some form of islet cell replacement strategy (transplant for example) or before islet cell mass is lost in patients at high risk for disease development [3]. In either case, this work further confirms the potential of using highly negatively charged PLG nanoparticles coupled with or encapsulating known diabetogenic CD4+ and CD8+ T cell epitopes to safely and effectively induce tolerance for prevention of the onset or reversal of new onset T1D.
Supplementary Material
Highlights.
Antigen-specific tolerance can be induced to indefinitely prevent the induction of type 1 diabetes mediated by the transfer of activated diabetogenic CD4 and CD8 transgenic T cells using antigen encapsulating biodegradable PLG nanoparticles [PLG(Ag)]
PLG(Ag)-induced tolerance results in the systemic increase of β cell antigen-specific regulatory T cells (Tregs) resulting in sequestration of effector cells in the spleen
Long-term tolerance is dependent on the activation of antigen-specific FoxP3+ Tregs
Induction and maintenance of tolerance induced by PLG(Ag)-induced Tregs is dependent on PD-1 and CTLA-4
Acknowledgments
This work was supported by JDRF Grant 2-SRA-2014-279-Q-R and NIH grant R01 EB013198 to SDM and LDS. We thank the Miller and Shea lab members for their support and insightful comments.
Abbreviations
- Ag-PLG
antigen-coupled PLG nanoparticles
- Ag-SP
antigen-coupled apoptotic splenocytes
- APCs
antigen presenting cells
- ChgA
chromogranin A
- IGRP
islet specific glucose 6 phosphatase catalytic subunit related protein
- PEMA
2-ethyl-2-phenylmalonamide monohydrate
- PLG
poly(lactide-co-glycolide) nanoparticles
- PLG(Ag)
antigen-encapsulating PLG nanoparticles
- TCR
T cell receptor
- T1D
type 1 diabetes
- TIMP
tolerogenic immune-modifying particles
- Treg
regulatory T cells
Footnotes
The authors have declared that no conflict of interest exists
Author contributions: SP and TN designed research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript. DX conducted experiments and acquired and analyzed data. SDM, JRP, DRG and LDS designed research studies and wrote the manuscript.
Conflict of interest: SDM, DRG, and LDS are co-founders of Cour Pharmaceutical Development Co. which is partnering with Takeda Pharmaceutical Co. for clinical translation of the Ag-PLG tolerance technology for the treatment of celiac disease. SDM, DRG, LDS, and JRP are members of the scientific advisory board and consultants for Cour.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52:1553–78. doi: 10.1016/j.pcl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–99. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9–23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39:347–53. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol. 2011;187:5090–98. doi: 10.4049/jimmunol.1100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutterotti A, Yusef S, Sputtek A, Sturner K, Stellmann J-P, Breiden P, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(5):188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–60. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smarr CB, Yap WT, Neef TP, Pearson RM, Hunter ZN, Ifergan I, et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. Proc Natl Acad Sci USA. 2016;113:5059–64. doi: 10.1073/pnas.1505782113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy DP, Hunter ZN, Chackerian B, Shea LD, Miller SD. Targeted immunomodulation using protein coated nanoparticles. WIRES Nanomed Nanobiotechnol. 2014;8:2148–60. doi: 10.1002/wnan.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikoopour E, Sandrock C, Huszarik K, Krougly O, Lee-Chan E, Masteller EL, et al. Cutting Edge: Vasostatin-1-derived peptide ChgA29–42 is an antigenic epitope of diabetogenic BDC2. T cells in nonobese diabetic mice. J Immunol. 2011;186:3831–5. doi: 10.4049/jimmunol.1003617. [DOI] [PubMed] [Google Scholar]
- 14.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–31. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–4. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 18.Verdaguer J, Yoon JW, Anderson B, Averill N, Utsugi T, Park BJ, et al. Acceleration of spontaneous diabetes in TCR-beta-transgenic nonobese diabetic mice by beta-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-alpha chains. J Immunol. 1996;157:4726–35. [PubMed] [Google Scholar]
- 19.McCarthy DP, Yap JW, Harp CT, Song WK, Chen J, Pearson RM, et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomed. 2017;13:191–200. doi: 10.1016/j.nano.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap WT, Song WK, Chauhan N, Scalise PN, Agarwal R, Miller SD, et al. Quantification of particle-conjugated or particle-encapsulated peptides on interfering reagent backgrounds. BioTechniques. 2014;57:39–44. doi: 10.2144/000114190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes. 2012;61:3239–46. doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb PA, Delong T, Baker RL, Fitzgerald-Miller L, Wagner R, Cook G, et al. Chromogranin A is a T cell antigen in human type 1 diabetes. J Autoimmun. 2014;50:38–41. doi: 10.1016/j.jaut.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, et al. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–43. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 24.Daniel C, Wennhold K, Kim HJ, von Boehmer H. Enhancement of antigen-specific Treg vaccination in vivo. Proc Natl Acad Sci USA. 2010;107:16246–51. doi: 10.1073/pnas.1007422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–47. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–20. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto K, Kingsley CI, Zhang X, Jabs C, Izikson L, Sobel RA, et al. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 28.Busse M, Krech M, Meyer-Bahlburg A, Hennig C, Hansen G. ICOS mediates the generation and function of CD4+CD25+Foxp3+ regulatory T cells conveying respiratory tolerance. J Immunol. 2012;189:1975–82. doi: 10.4049/jimmunol.1103581. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hlavaty KA, Luo X, Shea LD, Miller SD. Cellular and molecular targeting for nanotherapeutics in transplantation tolerance. Clin Immunol. 2015;160:14–23. doi: 10.1016/j.clim.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hlavaty KA, McCarthy DP, Saito E, Yap WT, Miller SD, Shea LD. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2016;76:1–10. doi: 10.1016/j.biomaterials.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–77. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 33.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci. 2007;97:398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- 34.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo R, Saito E, Miller SD, Shea LD. Peptide-Conjugated Nanoparticles Reduce Positive Co-stimulatory Expression and T Cell Activity to Induce Tolerance. Mol Ther. 2017;25:1676–85. doi: 10.1016/j.ymthe.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, Sarvetnick N. Lymphocyte proliferation in immune-mediated diseases. Trends Immunol. 2009;30:430–8. doi: 10.1016/j.it.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Davidson TS, Shevach EM. Polyclonal Treg cells modulate T effector cell trafficking. Eur J Immunol. 2011;41:2862–70. doi: 10.1002/eji.201141503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Podojil JR, Luo X, Chang J, Miller SD. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J Immmunol. 2010;184:6629–36. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci USA. 2016;113:8490–5. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Getts DR, Shea LD, Miller SD, King NJ. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36:419–27. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kishimoto TK, Ferrari JD, LaMothe RA, Kolte PN, Griset AP, O’Neil C, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol. 2016;11:890–99. doi: 10.1038/nnano.2016.135. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez-Ortiz ZG, Pendergraft WF, 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14:917–26. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shannahan JH, Podila R, Aldossari AA, Emerson H, Powell BA, Ke PC, et al. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci. 2015;143:136–46. doi: 10.1093/toxsci/kfu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112:E156–65. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang AH, Rossi RJ, Yoon J, Wang H, Scott DW. Tolerogenic nanoparticles to induce immunologic tolerance: Prevention and reversal of FVIII inhibitor formation. Cell Immunol. 2016;301:74–81. doi: 10.1016/j.cellimm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109:11270–5. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal. 2016;9:ra61. doi: 10.1126/scisignal.aad0612. [DOI] [PubMed] [Google Scholar]
- 48.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–40. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.