Abstract
Background
Glycosaminoglycan (GAG), a major component of the endothelial glycocalyx, is severely perturbed in diabetic vasculature leading to endothelial inflammation and vascular disease in diabetes. We tested the hypothesis that blueberry metabolites (BBM) ameliorate endothelial inflammation in diabetic endothelial cells (ECs) by restoring cell surface GAGs.
Methods
ECs isolated from healthy individuals [human aortic ECs (HAECs)] and diabetic patients (diabetic-HAECs) were treated with ± BBM (benzoic acid-4-sulfate, hippuric acid, hydroxyhippuric acid, isovanillic acid-3-sulfate, and vanillic acid-4-sulfate at concentrations known to circulate in human plasma following blueberry consumption) for 3 days and indices for endothelial inflammation were measured. To analyze GAGs, ECs were incubated with sulfate-free medium supplemented with [35S] Na2SO4 ± BBM. Total GAGs in ECs and medium were purified using DEAE-Sepharose column and were analyzed with high-pressure liquid chromatography coupled to an inline flow scintillation analyzer. Heparan sulfate/chondroitin sulfate ratio and disaccharide composition of GAGs from the medium were analyzed using DEAE-3SW column and Dionex CarboPac PA1 column, respectively.
Results
BBM suppressed diabetes-induced monocyte binding to ECs, and reduced the expression of inflammatory markers in diabetic-HAECs. Diabetic-HAECs displayed a decrease in [35S] sulfate incorporation into the cell surface GAGs indicating the dysregulation of sulfated GAGs. However, treatment with BBM restored the levels of GAGs in diabetic-HAECs. The composition, heparan sulfate/chondroitin sulfate ratio, and disaccharide composition of GAGs from medium were similar among groups.
Conclusions
BBM restored cell surface GAGs and attenuated endothelial inflammation in diabetic-HAECs. Blueberry might complement conventional therapies to improve vascular complications in diabetes.
Keywords: blueberry metabolites, endothelial inflammation, diabetes, glycosaminoglycans
1. Introduction
Diabetes greatly increases the risk of cardiovascular disease such as atherosclerosis that accounts for the largest number of deaths among diabetic patients [1]. In diabetes, high glucose induced endothelial inflammation, resulting in the binding of monocytes to vascular endothelial cells (ECs) and their transmigration into the sub-endothelial space, plays a pivotal role in the pathogenesis of vascular disease [1]. The glycocalyx is a carbohydrate-rich layer that covers the luminal side of the vascular endothelium which is comprised of proteoglycans [core proteins with glycosaminoglycans (GAG) side chains], glycoproteins, and plasma proteins [2, 3]. An intact endothelial glycocalyx in healthy vasculature acts as a protective barrier by reducing vascular inflammation and maintaining a selectively permeable barrier [2]. The structure and function of the glycocalyx, particularly that of GAGs, are severely compromised in diabetes leading to enhanced sensitivity of the vasculature towards inflammatory stimuli [3, 4]. Hence, the preservation and restoration of GAGs may be a novel strategy to ameliorate vascular complications in diabetes.
Clinical studies support the vascular benefits of berry anthocyanins. For example, intake of blueberry anthocyanins increases vasodilation in healthy men and improves endothelial dysfunction in humans with metabolic syndrome [5, 6]. Anthocyanins are extensively metabolized by the intestinal microbiota and digestive enzymes in humans, suggesting that their vascular benefits may be mediated by their circulating metabolites [5, 7, 8]. Nevertheless, the bioactivities of anthocyanin metabolites remain poorly understood because many of these compounds are not commercially available. Recently we synthesized the key metabolites of blueberry anthocyanins and showed that blueberry metabolites (that circulate in human plasma at the time of the observed biological effect following blueberry consumption) attenuate palmitate-induced endothelial dysfunction in ECs and arteries [9]. However, it is unknown whether blueberry metabolites modulate GAGs and attenuate endothelial inflammation in diabetic vasculature. In the present study, we tested the hypothesis that blueberry metabolites improve endothelial inflammation by restoring the cell surface GAGs in diabetic human vascular endothelial cells.
2. Materials and Methods
2.1. Materials
ECs isolated from the aortae of healthy individuals [human aortic endothelial cells (HAECs)] and type 2 diabetic patients (diabetic-HAECs) were purchased from Lonza. The radiochemical [35S]Na2SO4 was from PerkinElmer Life Sciences, sulfate-free DMEM/F12 medium and Dionex CarboPac PA1 column were from Thermofisher.
2.2. Cell culture
HAECs and diabetic-HAECs were cultured in EBM-2 supplemented with EGM-2 growth supplements and 2% heat inactivated FBS (Lonza) [1, 9]. Human monocytic THP1 cells (American Type Culture Collection) were cultured in RPMI-1640 medium with 10% FBS [1, 9].
2.3. Blueberry metabolites
Benzoic acid-4-sulfate, isovanillic acid-3-sulfate, and vanillic acid-4-sulfate were synthesized as we described [9]. Hippuric acid and hydroxyhippuric acid were purchased from Sigma-Aldrich. Blueberry metabolites mixture used in the present study contained 75 nM vanillic acid-4-sulfate, 75 nM isovanillic acid-3-sulfate, 700 nM benzoic acid-4-sulfate, 3 μM hydroxyl hippuric acid, and 5 μM hippuric acid. The physiologically relevant dosage of metabolites was based on our previous study that closely represents the concentrations of metabolites that circulate in the human plasma at the time of the observed biological effect following the consumption of ~240 g of blueberries [9].
2.4. Monocyte adhesion assay and gene expression analysis
HAECs and diabetic-HAECs (passages 4–5) were incubated with ± blueberry metabolites mixture or the solubilizing vehicle DMSO for 3 days under physiological shear. Upon completing the treatments, monocyte binding to ECs, and mRNA expression of inflammatory markers [vascular adhesion molecule-1 (VCAM1), intercellular adhesion molecule-1 (ICAM1), E-selectin, interleukin-8 (IL8) and monocyte chemotactic protein-1 (MCP1)] were assessed as we described [9].
2.5. Analysis of GAGs
HAECs and diabetic-HAECs (passage 4) were grown to 70% confluency in EGM-2 complete medium in 150 cm2 cell culture flask. Then, medium was replaced with sulfate-free DMEM/F12 medium supplemented with 200 μCi [35S] Na2SO4 ± blueberry metabolites mixture or DMSO. ECs were then incubated for 3 days under physiological shear to mimic the shear stress of blood flowing over ECs, which is a major regulator of glycocalyx [10, 11]. Blueberry metabolites and additional medium were added each day. Upon completing the respective treatments, the medium was collected for the analysis of shed GAGs. Following one day of incubation with Pronase, EC components were collected and used to analyze cell surface GAGs. Total GAGs in ECs and medium were purified using DEAE-Sepharose column and were analyzed with high-pressure liquid chromatography coupled to an inline flow scintillation analyzer as we described [12]. The amount of GAG chains was determined by quantifying the radioactivity [35S] incorporated in the purified GAGs and the counts were normalized with protein concentration. The relative amount of heparan sulfate (HS) and chondroitin sulfate (CS), and disaccharide composition of GAGs from the medium were analyzed using DEAE-3SW column and Dionex CarboPac PA1 column, respectively [12].
2.6. Data analyses
Data are presented as mean ± SE, and P < 0.05 was considered significantly different. Comparison was made using one-way ANOVA with SPSS/10. Tukey post hoc tests were performed when significant main effects were obtained.
3. Results
3.1. Blueberry metabolites suppress diabetes-induced endothelial inflammation
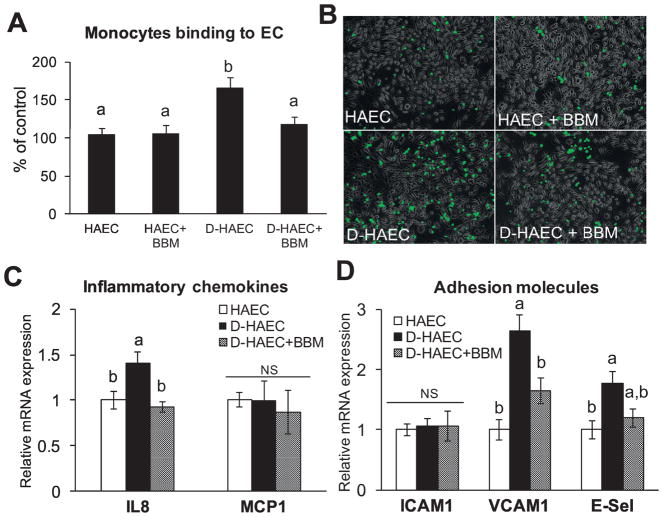
We tested whether blueberry metabolites ameliorate endothelial inflammation in diabetic-HAECs. As expected, there was an increased monocyte binding to diabetic-HAECs vs. HAECs (Fig. 1A and 1B). Further, diabetic-HAECs exhibited an increased mRNA expression of IL-8, VCAM1 and E-Selectin (Fig. 1C and 1D). In support to our hypothesis, blueberry metabolites suppressed monocyte binding and reduced IL8 and VCAM1 in diabetic-HAECs (Fig. 1A–D).
Fig. 1. Blueberry metabolites suppress diabetes-induced endothelial inflammation.
HAECs or diabetic-HAECs (D-HAEC) were treated ± blueberry metabolites (BBM) for 3 days. (A) The binding of monocyte to HAECs was determined by using fluorescent labelled THP-1 human monocytic cells. Values are mean ± SEM, n=6 (means of triplicate). (B) Representative images for fluorescent labelled THP-1 human monocytic cells binding to ECs. mRNA expression of IL8 and MCP1 (C), and ICAM1, VCAM1 and E-selectin (D) were analyzed by qPCR using SYBR green. Values are mean ± SEM, n=6 (means of duplicate). Means without a common letter differ, P < 0.05, and NS – Non-significant.
3.2. Blueberry metabolites restore cell surface GAGs in diabetic-HAEC
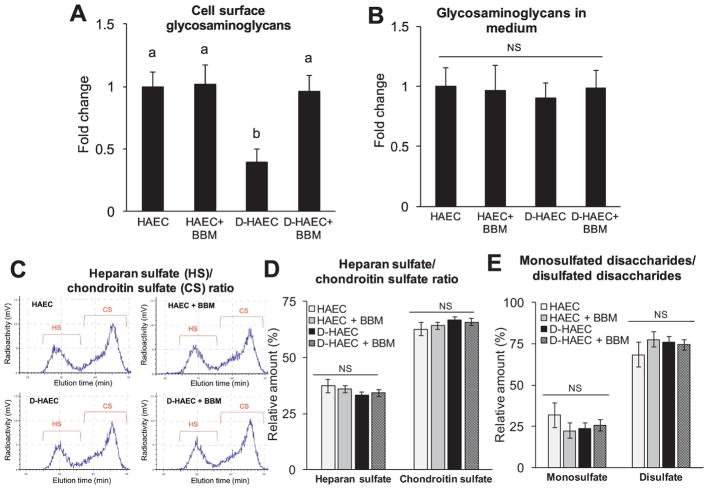
Next, we analyzed whether the effect of metabolites observed on endothelial inflammation might result from their ability to restore GAGs in diabetic-HAECs. Metabolic radiolabeling showed a decrease in [35S] sulfate incorporation into the cell surface GAGs produced by diabetic-HAECs vs. HAECs (Fig. 2A) indicating a reduced cell surface GAGs in diabetic ECs. However, blueberry metabolites restored the levels of sulfated GAGs in diabetic-HAECs (Fig. 2A).
Fig. 2. Blueberry metabolites restore cell surface GAGs in diabetic-HAEC.
HAECs or diabetic-HAECs were treated ± blueberry metabolites (BBM) for 3 days. The amount of GAG chains in cell surface (A) and medium (B) was determined by quantifying the radioactivity [35S] incorporated in the purified GAG solution. Values are mean ± SEM, n=6. Means without a common letter differ, P < 0.05, and NS – Non-significant. Heparan sulfate (HS)/chondroitin sulfate (CS) ratio, and disaccharide composition in shed GAGs. Purified radiolabeled GAGs from the cell culture medium was used to analyze relative amount of HS and CS (C and D) and the relative amounts of monosulfated- and disulfated- disaccharides within shed HS (E). Values are mean ± SEM, n=3. NS – Non-significant.
3.3. The composition, HS/CS ratio, and disaccharide composition in shed GAGs
The amount of GAGs in the medium (Fig. 2B), and the relative amount of HS and CS (the most prominent endothelial GAGs [2, 3]) in shed GAGs were similar among all groups (Fig. 2C and 2D). HS function is known to be majorly dependent on the sulfation patterns and disaccharide compositions [13]. Hence we assessed the relative amounts of monosulfated- and disulfated- disaccharides within shed HS and no difference was observed among the groups (Fig. 2E).
4. Discussion
Cell surface GAGs act as a protective barrier by preventing endothelial inflammation and an impairment in GAGs lead to endothelial dysfunction in diabetes. Hence, counteracting the damage to GAGs is one of the novel strategies to improve vascular complications in diabetes. In this study, we investigated the hypothesis that blueberry metabolites ameliorate endothelial inflammation in diabetic ECs through the restoration of glycocalyx GAGs.
Evidence shows that the disruption of the endothelial glycocalyx exacerbates vascular inflammation and atherosclerosis [2, 14, 15]. A compromised endothelial glycocalyx in diabetic vasculature was reported in both type 1 and 2 diabetic patients [15, 16]. Specifically, the GAG components of the glycocalyx undergo remodeling during diabetes and these alterations in aortic GAGs negatively impact the vascular function [17]. Cell surface GAGs regulate leukocyte adhesion and the removal of glycocalyx enhances leucocyte adhesion to ECs [18]. Consistent with these studies, we found a reduced amount of cell surface GAGs and increased monocyte adhesion in diabetic-HAECs. Interestingly, the factors that restore EC glycocalyx decrease leucocyte adhesion to ECs [18]. Similarly, in the present study, we demonstrate that blueberry metabolites restore the amount of cell surface GAGs and reduce monocyte adhesion in diabetic-HAECs. We further examined whether these metabolites have an influence on GAGs shedding from ECs. The amount, HS/CS ratio, and disaccharide composition of the shed GAGs were similar among all groups. This indicates that diabetes or metabolites have no influence on shed GAGs. Due to low counts in ECs (less than 20,000 CPM, the amount insufficient for extensive analysis) we were unable to analyze the HS/CS ratio, and disaccharide composition of the cell surface GAGs.
Animal studies showed that dietary supplementation of blueberries modulate GAGs in the aortic vessels of Sprague-Dawley rats and spontaneously hypertensive rats [19, 20]. Blueberry anthocyanins are extensively metabolized in humans suggesting the bioactivities of blueberries are mediated through their circulating metabolites [5]. To our knowledge, this is the first study to show that blueberry metabolites suppress endothelial inflammation in diabetic ECs through the regulation of cell surface GAGs. In addition, blueberry metabolites reduced the expression of inflammatory markers in diabetic-HAECs indicating metabolites act on multiple targets to improve endothelial inflammation.
In conclusion, blueberry metabolites restore normal levels of GAGs and attenuate endothelial inflammation in diabetic ECs. These data indicate that the benefits of blueberry consumption in ameliorating endothelial inflammation, at least in part, are mediated through the effects of metabolites on GAGs. Blueberry consumption may be a potential adjunct therapy to prevent the severity of vascular complications in diabetes. However, future research is warranted to better understand and validate the bioactivities of blueberries. Our ongoing studies are focused on identifying the effect of blueberries on vascular GAGs in diabetic animals and elucidating the molecular mechanisms involved.
Highlights.
Diabetic endothelial cells displayed an increased inflammation and reduced glycosaminoglycans
Blueberry metabolites reduced diabetes-induced endothelial inflammation
Blueberry metabolites restored cell surface glycosaminoglycans in diabetic endothelial cells
Acknowledgments
Funding
This work was supported by the University of Utah Seed Grant, research start-up fund and College of Health Pilot grant (to P.V.A.B.); NHLBI sponsored Programs of Excellence in Glycosciences Grant, HL107152; and Undergraduate Research Opportunities Program award (to B.R.C., and S.G.).
Footnotes
All above authors takes responsibility of all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. Authors declare no potential conflict of interest.
Conflict of interest
Authors declare no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babu PV, Si H, Fu Z, Zhen W, Liu D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr. 2012;142:724–30. doi: 10.3945/jn.111.152322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolsen-Petersen JA. The endothelial glycocalyx: the great luminal barrier. Acta anaesthesiologica Scandinavica. 2015;59:137–9. doi: 10.1111/aas.12440. [DOI] [PubMed] [Google Scholar]
- 3.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators of inflammation. 2014;2014:694312. doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiebert LM, Han J, Mandal AK. Glycosaminoglycans, hyperglycemia, and disease. Antioxidants & redox signaling. 2014;21:1032–43. doi: 10.1089/ars.2013.5695. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, et al. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. The American journal of clinical nutrition. 2013;98:1179–91. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 6.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–7. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. British journal of pharmacology. 2014 doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Ferrars RM, Cassidy A, Curtis P, Kay CD. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Molecular nutrition & food research. 2014;58:490–502. doi: 10.1002/mnfr.201300322. [DOI] [PubMed] [Google Scholar]
- 9.Bharat D, Cavalcanti RRM, Petersen C, Begaye N, Cutler BR, Costa MMA, et al. Blueberry Metabolites Attenuate Lipotoxicity-Induced Endothelial Dysfunction. Molecular nutrition & food research. 2017 doi: 10.1002/mnfr.201700601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 2014;9:e86249. doi: 10.1371/journal.pone.0086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo A, Dewey CF, Jr, Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. American journal of physiology. 2013;304:C137–46. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua JS, Tran VM, Kalita M, Quintero MV, Antelope O, Muruganandam G, et al. A glycan-based approach to therapeutic angiogenesis. PloS one. 2017;12:e0182301. doi: 10.1371/journal.pone.0182301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu P, Victor XV, Nelsen E, Nguyen TK, Raman K, Kuberan B. Hydrogen/deuterium exchange-LC-MS approach to characterize the action of heparan sulfate C5-epimerase. Analytical and bioanalytical chemistry. 2011;401:237–44. doi: 10.1007/s00216-011-5087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. British journal of clinical pharmacology. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–55. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia. 1993;36:316–22. doi: 10.1007/BF00400234. [DOI] [PubMed] [Google Scholar]
- 17.Gowd V, Gurukar A, Chilkunda ND. Glycosaminoglycan remodeling during diabetes and the role of dietary factors in their modulation. World J Diabetes. 2016;7:67–73. doi: 10.4239/wjd.v7.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–74. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 19.Kalea AZ, Lamari FN, Theocharis AD, Cordopatis P, Schuschke DA, Karamanos NK, et al. Wild blueberry (Vaccinium angustifolium) consumption affects the composition and structure of glycosaminoglycans in Sprague-Dawley rat aorta. J Nutr Biochem. 2006;17:109–16. doi: 10.1016/j.jnutbio.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Kristo AS, Malavaki CJ, Lamari FN, Karamanos NK, Klimis-Zacas D. Wild blueberry (V. angustifolium)-enriched diets alter aortic glycosaminoglycan profile in the spontaneously hypertensive rat. J Nutr Biochem. 2012;23:961–5. doi: 10.1016/j.jnutbio.2011.05.002. [DOI] [PubMed] [Google Scholar]