Abstract
Background
Twin pregnancies are at increased risk for adverse outcomes and are associated with greater gestational weight gain compared to singleton pregnancies. Studies that disentangle the relationship between gestational duration, weight gain and adverse outcomes are needed to inform weight gain guidelines. We created charts of the mean, standard deviation and select percentiles of maternal weight gain-for-gestational age in twin pregnancies and compared them to singleton curves.
Methods
We abstracted serial prenatal weight measurements of women delivering uncomplicated twin pregnancies at Magee-Womens Hospital (Pittsburgh, PA, 1998–2013) and merged them with the hospital’s perinatal database. Hierarchical linear regression was used to express pregnancy weight gain as a smoothed function of gestational age according to pre-pregnancy BMI category. Charts of week- and day-specific values for the mean, standard deviation, and percentiles of maternal weight gain were created.
Results
Prenatal weight measurements (median: 11 [interquartile range: 9, 13] per woman) were available for 1109 women (573 normal weight, 287 overweight, and 249 obese). The slope of weight gain was most pronounced in normal weight women and flattened with increasing pre-pregnancy BMI (e.g., 50th percentiles of 6.8, 5.7, and 3.6 kg at 20 weeks and 19.8, 18.1, and 14.4 at 37 weeks in normal weight, overweight, and obese women, respectively). Weight gain patterns in twins diverged from singletons after 17–19 weeks.
Conclusions
Our charts provide a tool for the classification of maternal weight gain in twin pregnancies. Future work is needed to identify the range of weight gain associated with optimal pregnancy health outcomes.
Keywords: Gestational weight gain, Twins, Prenatal Nutrition, Reference values, Pregnancy
Introduction
Women carrying twin gestations are at increased risk of a number of adverse pregnancy outcomes linked with gestational weight gain, such as fetal growth restriction, gestational diabetes, and preeclampsia.1–4 Further, women carrying twins tend to gain more weight than women carrying singletons,5 potentially increasing their risk of excess post-partum weight retention and longer term obesity.6–9 In 1990 and again in 2009, the Institute of Medicine (IOM) recognized the importance of identifying optimal weight gain ranges for women with twin pregnancies.5 Yet, as highlighted in a recent systematic review, the evidence base to inform weight gain guidelines for twin pregnancies is extremely limited.10 The 2009 IOM Committee was only able to produce provisional weight gain guidelines for twin pregnancies, which were based on the observed interquartile range of weight gain observed in a single study of mothers who delivered twins weighing at least 2500 g at ≥36 weeks gestation.5
A major challenge in determining optimal gestational weight gain in twin pregnancies is that both pregnancy weight gain and adverse outcomes are strongly correlated with gestational duration.11 That is, many adverse outcomes (such as stillbirth or preterm birth) occur at younger gestational ages, and women delivering earlier have less time to gain weight. This makes it difficult to disentangle the contribution of low maternal weight gain to adverse outcomes from the contribution of preterm birth. That is, low weight gain among women delivering preterm may be a result of the preterm delivery, not the cause of it (reverse causation). In the study of fetal and pediatric growth, this methodological challenge has been overcome with the use of birthweight- or estimated fetal weight-for-gestational-age charts, where a fetus’ weight is expressed in relation to that of its peers of similar gestational age using fetal weight percentiles or z-scores.12,13 More recently, charts for maternal weight gain-for-gestational-age in singleton pregnancies have been proposed for global,14 Swedish,15 and United States populations.16,17 However, no weight gain charts are available for twin pregnancies.
In this report, we present charts of weight-gain-for-gestational-age in women with uncomplicated dichorionic twin pregnancies, providing a tool for studies seeking to establish weight gain ranges associated with lowest risks of adverse outcomes in twins.
Methods
Study population
Our cohort was drawn from all women delivering twin pregnancies from 1998 to 2013 at the Magee-Womens Hospital in Pittsburgh, Pennsylvania. Detailed data on these deliveries are maintained in the electronic Magee Obstetrics, Medical, and Infant Database. A validation study demonstrated excellent correlations between database content and medical record re-abstraction for key variables in this study such as pre-pregnancy weight, height, last measured weight, gestational age (all >0.93).18
We restricted our study population to twin pregnancies with no comorbid pre-pregnancy conditions [diabetes (type 1 or 2) or hypertension] resulting in two non-anomalous livebirths at or beyond 35 weeks’ gestation. We excluded monochorionic twins because the antenatal care and monitoring of these twins is markedly different than dichorionic twins, and they are at significantly higher risk of adverse outcomes. We further excluded twin pregnancies that were reduced (spontaneously or intentionally) from triplet pregnancies as well as women with missing pre-pregnancy body mass index (BMI, kg/m2), no prenatal weight measurements, or no ultrasound confirmed estimate of gestational age (<24 weeks).
Weight measurements
Self-reported pre-pregnancy height (m) and weight (kg) were used to calculate pre-pregnancy BMI (weight divided by height squared, kg/m2), which was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Serial prenatal weights measurements were abstracted from the medical record using a REDCap (Research Electronic Data Capture) database specifically designed to conduct electronic data entry for this study. Prenatal weight gain at each week of gestation was calculated as the difference between each measured prenatal visit weight minus pre-pregnancy weight. Total pregnancy weight gain (kg) was defined as last weight before delivery (measured) minus pre-pregnancy weight. The electronic forms were also used to abstract data on chorionicity and assisted reproductive technology.
Gestational age
Gestational age at Magee-Womens Hospital is estimated using last menstrual period confirmed or revised with early ultrasound. Based on American Congress of Obstetricians and Gynecologists guidelines, last menstrual period is revised with the ultrasound estimate if the discrepancy with first trimester ultrasound estimates is greater than 7 days (with a first trimester ultrasound), or 10 days (second trimester ultrasound).19
Statistical analysis
We used a hierarchical linear regression model to express the serial prenatal weight gain measurements as a function of gestational age. Separate charts were constructed for each pre-pregnancy BMI category, as the effect of pregnancy weight gain on adverse outcomes is known to differ by pre-pregnancy BMI, and current provisional IOM guidelines are BMI-specific.5 Weight gain measurements were log-transformed to ensure that the model’s assumptions of homoscedasticity of residual errors, and normality of distribution of response variable were not violated. A constant was added to all weight measurements to eliminate negative weight values, as negative values cannot be log transformed (7.59 for normal weight, 9.64 for overweight, and 11.45 for obese women).
We modeled gestational age using a restricted cubic spline 20 to allow the weight gain curve to vary across gestation in a smooth, non-linear manner. Random effects were added to the intercept and linear term of the restricted cubic spline, which ensured that our estimates accounted for the correlation between a woman’s repeated prenatal weight measurements. We used visual inspection of the fitted values and the Akaike Information Criteria to determine the number of knots in the spline. The final regression equations were used to produce a chart with smoothed estimates of the mean, standard deviation (SD), and selected percentiles of gestational weight gain at each week throughout gestation.
The goodness-of-fit of the estimated means and standard deviations of weight gain to the raw data was assessed using recommended approaches.21 We produced a scatter diagram superimposing the fitted means and standard deviation on the raw data to ensure that no systematic over- or under-estimation of weight gain was apparent. We checked that the appropriate proportion of observations falls below and above the estimated threshold for 1 and 2 standard deviations.
Sample size estimation
We based the sample size requirements for each weight gain chart on the number of women, rather than the number of weight gain observations.21 Based on preliminary data, we estimated that we would have a fixed sample size of 687 normal weight, 270 overweight, and 249 obese class women with uncomplicated twin pregnancies. We estimated the accuracy of our charts at the 16th and 84th percentiles, which are the percentiles corresponding to 1 SD (z=−1 and +1 for 16th and 84th percentiles, respectively). The standard error associated with a given percentile can be expressed as a multiple of the gestational age-specific SD through the equation: SE(percentile) = √[(1+z2/2)/n], where z is the appropriate value from the standard normal distribution. Based on BMI category-specific gestational weight gain means and standard deviations from deliveries in our population, we estimated that the 95% confidence interval for the 16th and 84th percentiles would be within 0.6kg in normal weight, 1.3kg in overweight, and 1.2 in obese women. Our initial plan to stratify by severity of obesity was not possible due to the relatively small numbers in these subgroups. The anticipated low number of underweight women carrying twin pregnancies precluded the creation of charts for this category.
Results
A total of 3143 twin pregnancies were delivered at Magee-Womens during our study period. We excluded 658 monochorionic twin pregnancies, 54 twin pregnancies reduced from higher order multiples, and 103 pregnancies for whom antenatal chart data were unavailable (typically because they received antenatal care elsewhere), 843 pregnancies delivered before 35 weeks, 9 pregnancies with a stillbirth, 41 anomalous pregnancies, 27 pregnancies with pre-gestational diabetes, 55 pregnancies with pre-pregnancy hypertension, 88 with no ultrasound-based estimate of gestational age, 23 without valid prenatal weights measurements, and 96 with missing pre-pregnancy weight or height data. There were too few underweight women (n=37) from which to produce a chart. This left an analysis sample of 1,109 pregnancies.
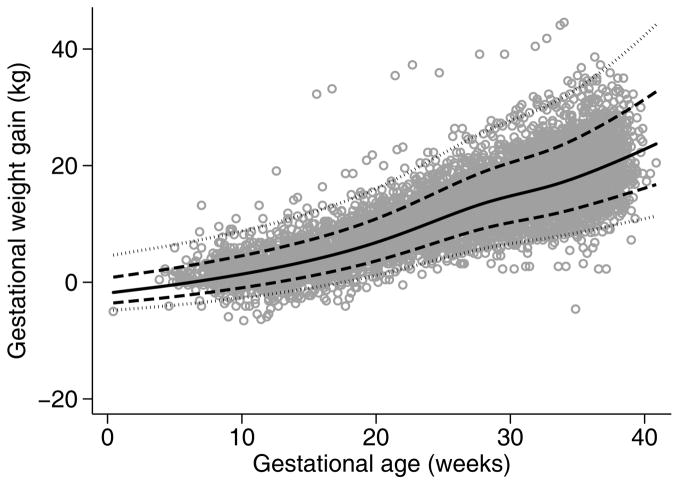
As shown in Table 1, women in our cohort tended to be older than 30 years of age, non-Hispanic White, and covered by private insurance. Approximately 45% of the cohort was nulliparous, and began pregnancy either overweight or obese. Thirty (30) percent of the cohort conceived using assisted reproductive technologies. Three-quarters of women had 11 or more prenatal weight measurements available. Total gestational weight gain decreased with increasing pre-pregnancy BMI, with normal weight, overweight, and obese women having median [interquartile range] weight gains at delivery of 20 [16–24], 19 [15–23], and 15 [10–20] kg, respectively. As shown in Figure 1 (for normal weight women) and Figure S1 (overweight and obese women), there were no pronounced inflection points in the pattern of weight gain at the end of the first trimester, but a slight flattening of the weight gain slope after 28 weeks.
Table 1.
Descriptive characteristics of the analytic sample of 1,109 women delivering twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.
| Characteristic | Median [Interquartile range] or n(%) |
|---|---|
| Maternal age (years) a | |
| <20 | 34 (3.1) |
| 20–29 | 391 (31.5) |
| ≥30 | 680 (61.4) |
| Parity | |
| 0 | 482 (43.5) |
| 1 | 523 (47.2) |
| 2+ | 104 (9.4) |
| Pre-pregnancy Body Mass Index (kg/m2) | |
| Normal weight (18.5–24.9) | 573 (50.0) |
| Overweight (25–29.9) | 287 (24.0) |
| Obese (≥30) | 249 (21.8) |
| Maternal height (cm) | 165 [160, 170] |
| Smoked during pregnancy | 111 (10.0) |
| Race | |
| Non-Hispanic White | 891 (80.3) |
| Non-Hispanic Black | 168 (15.2) |
| Hispanic | 11 (1.0) |
| Non-Hispanic Other | 39 (3.5) |
| Insurance status | |
| Private | 683 (61.6) |
| Medicaid | 405 (36.5) |
| Other | 21 (1.9) |
| Use of Assisted Reproductive Technology | |
| IVF | 232 (21.9) |
| Other or unspecified ART (includes Clomid, Gonadotropin, and/or intrauterine insemination) | 120 (10.8) |
| None | 757 (68.3) |
| Gestational age at first ultrasound | 12 [8, 18] |
| Gestational age at delivery | 37 [36, 38] |
| Prenatal weight measurements, number | 11 [10, 13] |
Maternal age missing in 4 women
Figure 1.
Observed vs predicted weight gain values in normal weight women delivering twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013. Grey dots indicate observed weight gain measurements, solid black line indicates predicted mean, dashed lines indicate predicted +/− 1 standard deviation, and dotted lines indicate predicted +/− 2 standard deviations.
The equations from our final models describing the mean and variance of weight gain as a function of gestational age are provided in Appendix S1. These estimates are shown in Figure 1 and Figure S1 superimposed on the observed weight gain measurements. We made a post-hoc decision to use restricted cubic splines with 5 knots in the default position after visual inspection of the model with the best fit of 7 knots (based on Akaike Information Criteria) suggested overfitting. The fitted means and standard deviations appeared to fit the crude data reasonably well, with 75.2, 75.3, and 76.2 percent of crude observations classified as within 1 standard deviation (68% expected) and 96.7, 95.7, and 95.3 within 2 standard deviations (95% expected) in normal weight, overweight, and obese women, respectively. Predicted week-specific values were within 1.2 kg of crude weight measurements for all weeks between 6 and 39 weeks, with the majority of predicted values within 0.5 kg.
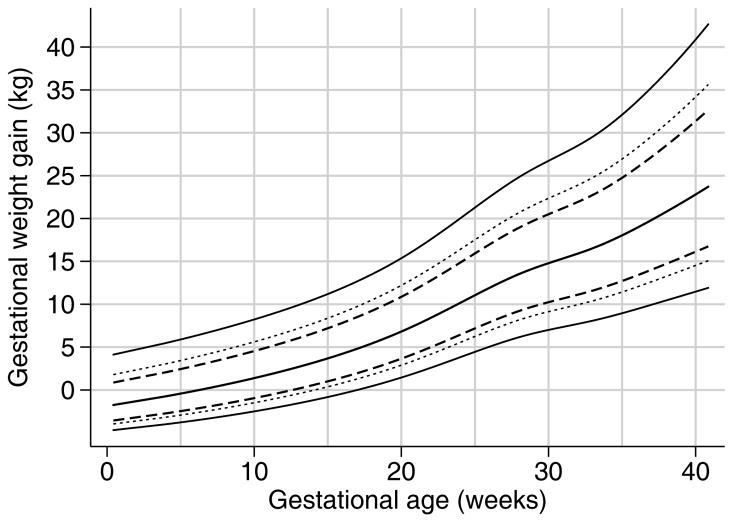
Table 2 and Figure 2 show the final weight-gain-for-gestational age charts for normal weight women (Table S1 and Table S2 for overweight and obese, respectively, and Figure S1). The week-specific log mean and log standard deviations can be used to calculate a given woman’s weight gain z-score as follows: first, add the BMI-category specific constant (provided in Appendix S1) to the woman’s weight gain measurement (i.e., weight gain measurement + constant c). Second, log transform this adjusted weight measurement (i.e., log[weight gain measurement + constant c]). Third, identify the log mean and log SD values for that week of gestation from Table 2, and insert into the formula: (log[weight gain measurement + constant c] − logmean)/logSD. For example, the z-score for a woman who had gained 12 kg at 24 weeks would be calculated as: (log[12kg + constant of 7.59kg] – 2.876)/0.235 = 0.42, where 2.876 and 0.235 are obtained from the log mean and log SD columns for 24 weeks’ gestation of Table 2. The z-score of 0.42 indicates that this woman was above the population average of weight gain for gestational age (where a z-score 0 corresponds to the population median weight), confirmed by comparing her 12 kg gain to the 50th percentile estimate for 24 weeks of 10.2 kg. A z-score below 0 would indicate a weight gain below the population average. In Appendix S2, we provide Stata code to convert weight gain measurements into age-standardized z-scores using day-specific estimates of gestational age (rather than weeks).
Table 2.
Pregnancy weight gain for gestational age among normal weight women with twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.
| Normal weight women (Pre-pregnancy BMI 18.5–24.9) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gestational age (week) | Weight observations (N) | Weight gain (kg) | ||||||
|
| ||||||||
| p3 | p10 | p50 | p90 | p97 | Log mean | Log SD | ||
| 5 | 20 | −3.8 | −2.9 | −0.4 | 3.4 | 5.9 | 1.97 | 0.336 |
| 6 | 34 | −3.5 | −2.7 | −0.1 | 3.8 | 6.3 | 2.015 | 0.329 |
| 7 | 77 | −3.3 | −2.4 | 0.3 | 4.3 | 6.8 | 2.06 | 0.321 |
| 8 | 139 | −3 | −2.1 | 0.6 | 4.7 | 7.2 | 2.105 | 0.315 |
| 9 | 140 | −2.8 | −1.8 | 1 | 5.1 | 7.7 | 2.15 | 0.308 |
| 10 | 140 | −2.5 | −1.5 | 1.4 | 5.6 | 8.2 | 2.195 | 0.301 |
| 11 | 128 | −2.2 | −1.2 | 1.8 | 6.1 | 8.8 | 2.24 | 0.295 |
| 12 | 135 | −1.9 | −0.8 | 2.2 | 6.6 | 9.3 | 2.285 | 0.289 |
| 13 | 151 | −1.5 | −0.4 | 2.7 | 7.2 | 9.9 | 2.33 | 0.283 |
| 14 | 137 | −1.2 | 0 | 3.2 | 7.8 | 10.5 | 2.376 | 0.277 |
| 15 | 142 | −0.8 | 0.4 | 3.7 | 8.4 | 11.2 | 2.423 | 0.271 |
| 16 | 141 | −0.4 | 0.8 | 4.2 | 9 | 11.9 | 2.47 | 0.266 |
| 17 | 139 | 0 | 1.3 | 4.8 | 9.7 | 12.7 | 2.518 | 0.261 |
| 18 | 151 | 0.5 | 1.8 | 5.4 | 10.5 | 13.5 | 2.567 | 0.256 |
| 19 | 145 | 0.9 | 2.3 | 6.1 | 11.3 | 14.4 | 2.617 | 0.252 |
| 20 | 165 | 1.5 | 2.9 | 6.8 | 12.2 | 15.4 | 2.668 | 0.248 |
| 21 | 148 | 2 | 3.5 | 7.6 | 13.2 | 16.4 | 2.72 | 0.244 |
| 22 | 165 | 2.6 | 4.2 | 8.4 | 14.2 | 17.6 | 2.773 | 0.241 |
| 23 | 173 | 3.2 | 4.8 | 9.3 | 15.3 | 18.8 | 2.825 | 0.237 |
| 24 | 190 | 3.8 | 5.5 | 10.2 | 16.4 | 20 | 2.876 | 0.235 |
| 25 | 205 | 4.4 | 6.2 | 11.1 | 17.5 | 21.3 | 2.925 | 0.233 |
| 26 | 214 | 5.1 | 6.9 | 11.9 | 18.6 | 22.5 | 2.971 | 0.231 |
| 27 | 209 | 5.6 | 7.6 | 12.8 | 19.7 | 23.7 | 3.013 | 0.229 |
| 28 | 275 | 6.2 | 8.2 | 13.5 | 20.7 | 24.8 | 3.05 | 0.228 |
| 29 | 260 | 6.6 | 8.7 | 14.2 | 21.6 | 25.8 | 3.081 | 0.227 |
| 30 | 330 | 7 | 9.1 | 14.8 | 22.4 | 26.7 | 3.108 | 0.227 |
| 31 | 285 | 7.4 | 9.5 | 15.3 | 23.1 | 27.6 | 3.133 | 0.228 |
| 32 | 379 | 7.7 | 10 | 15.9 | 23.9 | 28.5 | 3.157 | 0.228 |
| 33 | 334 | 8.1 | 10.4 | 16.5 | 24.8 | 29.5 | 3.183 | 0.229 |
| 34 | 434 | 8.5 | 10.9 | 17.2 | 25.8 | 30.7 | 3.212 | 0.231 |
| 35 | 403 | 9 | 11.4 | 18 | 26.9 | 32.1 | 3.244 | 0.233 |
| 36 | 295 | 9.4 | 12 | 18.9 | 28.2 | 33.6 | 3.277 | 0.235 |
| 37 | 190 | 9.9 | 12.6 | 19.8 | 29.6 | 35.3 | 3.311 | 0.238 |
| 38 | 94 | 10.4 | 13.3 | 20.8 | 31 | 37 | 3.346 | 0.241 |
| 39 | 20 | 11 | 13.9 | 21.8 | 32.6 | 38.9 | 3.38 | 0.244 |
Figure 2.
Pregnancy weight gain for gestational age among normal weight women carrying twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013. Lines indicate 50th percentile (central solid line), 10th and 90th percentiles (dashed lines), +/− 1 standard deviation (dotted lines), and 3rd and 97th percentiles (outer solid lines).
Comment
Main Findings
This study shows the patterns of maternal weight gain across gestation in women delivering uncomplicated twin pregnancies in a large, contemporary US-based cohort. The week-specific means and standard deviations in our chart can be used to standardize a woman’s total pregnancy weight gain to account for her gestational duration, providing a tool to conduct analyses of the relation between weight gain in twin pregnancies and a variety of maternal and child health outcomes.
Interpretation
The total pregnancy weight gain in our cohort was very similar to the mean values in a cohort of 706 uncomplicated twin pregnancies from Baltimore, Miami, Charleston, and Ann Arbour used to derive the IOM’s current provisional guidelines.5,22 For example, the mean cumulative weight gains at 37–40 weeks in their cohort were 20.9, 18.9, and 15.7 kg in normal weight, overweight, and obese women, respectively, compared with 20.8, 19.1, and 15.4 kg at 38 weeks in our cohort. Data on patterns of weight gain throughout gestation in twin pregnancies are less common. Lantz and colleagues used serial prenatal weight gain measurements in 189 uncomplicated twin pregnancies to estimate average weekly weight gains before 20 weeks and between 20 weeks and delivery,23 while Luke and colleagues estimated the average weekly weight gain rates associated with good fetal growth (based on serial ultrasounds) from 0–20, 20–28, and ≥28 weeks in 2,324 pregnancies.22 Data from this cohort were used by the IOM committee to estimate the mean and interquartile range of weight gain rate (kg/week) in uncomplicated twin pregnancies between 2–13 weeks, 14–26 weeks, and 27 weeks to delivery.5 Our study extends this work by producing tables that directly estimate the means, standard deviations, and select percentiles for each week of pregnancy (and equations providing day-specific values), rather than values averaged across the period of a trimester or longer (which assumes a linear pattern of weight gain). Further, our charts were created using a multi-level longitudinal model, which correctly accounts for the correlation between a woman’s repeated weight measurements.24 This is important when creating weight-gain-for-gestational age charts, because ignoring the correlation will cause the standard deviations and the upper and lower percentiles to be artificially narrow.
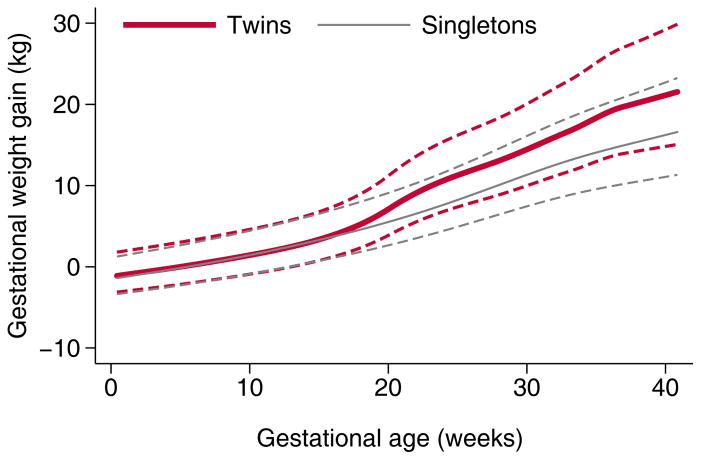
As expected, the total weight gain in twin pregnancies in our cohort was higher than that of singleton pregnancies at Magee-Womens Hospital.16 Figure 3, which plots the gestational age-specific weight gain patterns in twin pregnancies in our study with previously-published values for singletons,16 shows that weight gain patterns appeared similar until mid-pregnancy (approximately 17–19 weeks), but sharply diverged shortly thereafter. Of interest, the timing of divergence in twin and singleton gestational weight gain in this study was earlier than that observed in fetal growth, which was shown in the National Institute of Child Health and Human Development fetal growth study to diverge at approximately 32 weeks.25
Figure 3.
Pregnancy weight gain patterns of twin vs. singletons pregnancies in women delivering at Magee-Womens Hospital, Pittsburgh PA, based on previously published singleton values (16). Solid lines indicate the mean, dashed lines indicate one standard deviation.
Limitations
Despite having a sample size over 50% larger than that used to produce the current IOM provisional values, our sample size was smaller than anticipated (e.g., 573 normal weight women vs. 687 expected). As a result, our more extreme percentiles are less precise than planned. We did not have a sufficient number of underweight mothers to produce charts for this group of women, for whom data are extremely scarce. Our study population was drawn from a single academic medical center in Pittsburgh, Pennsylvania, with predominantly Non-Hispanic White women and the weight gain patterns may not be generalizable to all US women. Nevertheless, the total weight gains at 38 weeks in our cohort were very similar to those seen in the cohort of women from 4 different US cities used by the IOM committee. We have previously demonstrated that weight gain in women delivering singleton births at Magee-Womens Hospital is comparable to those of other contemporary cohorts from across the United States.16 Finally, while our pre-pregnancy BMI measurements likely have some measurement error introduced by self-report of weight,26 they reflect the information available to clinicians seeking to evaluate weight gain in the real-world setting.
Conclusions
The charts presented herein describe the week-by-week pattern of weight gain in women with twin gestations in a contemporary United States cohort. Linking the z-scores or percentiles of these charts with a broad range of adverse short and longer-term maternal and child health outcomes is a critical next step for establishing the optimal range of pregnancy weight gain for twin gestations. With higher weight gains among twin pregnancies, it is particularly important to relate gestational weight gain with longer-term outcomes such as excess post-partum weight retention, obesity, and associated metabolic complications. After studies have determined the range of weight gain z-scores associated with lowest risks of adverse outcomes, the chart could potentially be used as a tool to guide weight gain in prenatal care.
Supplementary Material
Regression equations to calculate z-scores for exact gestational age
Stata code to calculate pregnancy weight gain z-scores for twin pregnancies using exact gestational age
Figure S1. Pregnancy weight gain values in overweight and obese women delivering twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013. In panels A and B, grey dots indicate observed weight gain measurements, solid black line indicates predicted mean, dashed lines indicate predicted +/− 1 standard deviation, and dotted lines indicate predicted +/− 2 standard deviations. In panels C and D, lines indicate 50th percentile (central solid line), 10th and 90th percentiles (dashed lines), +/− 1 standard deviation (dotted lines), and 3rd and 97th percentiles (outer solid lines).
Table S1. Pregnancy weight gain for gestational age among overweight women with twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.
Table S2. Pregnancy weight gain for gestational age among obese women with twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.
Acknowledgments
We thank Melissa J. Papic and Sara M. Parisi, University of Pittsburgh, for leading the chart abstractions and data management of the study cohort. This research was supported by NIH grant R01 NR014245 (PI: LM Bodnar & JA Hutcheon).
Footnotes
Disclosures
All authors report no conflicts of interest. BA receives royalties as an UptoDate reviewer.
Details of Ethics Approval
This study was approved by the University of Pittsburgh Institutional Review Board on June 7, 2013 as a minimal risk study (IRB# PRO13060025).
References
- 1.Luke B, Brown MB. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertility and Sterility. 2007;88:283–293. doi: 10.1016/j.fertnstert.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwendemann WD, O’Brien JM, Barton JR, Milligan DA, Istwan N. Modifiable risk factors for growth restriction in twin pregnancies. American Journal of Obstetrics and Gynecology. 2005;192:1440–1442. doi: 10.1016/j.ajog.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 3.Fox NS, Saltzman DH, Kurtz H, Rebarber A. Excessive weight gain in term twin pregnancies: examining the 2009 Institute of Medicine definitions. Obstetrics & Gynecology. 2011;118:1000–1004. doi: 10.1097/AOG.0b013e318232125d. [DOI] [PubMed] [Google Scholar]
- 4.Fox NS, Rebarber A, Klauser CK, Roman AS, Saltzman DH. Intrauterine growth restriction in twin pregnancies: incidence and associated risk factors. American Journal of Perinatology. 2011;28:267–272. doi: 10.1055/s-0030-1270116. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: 2009. Report No.: 1473–656X. [PubMed] [Google Scholar]
- 6.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC) The American Journal of Clinical Nutrition. 2011;93:1285–1292. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker JD, Abrams B. Differences in postpartum weight retention between black and white mothers. Obstetrics & Gynecology. 1993;81:768–774. [PubMed] [Google Scholar]
- 8.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15:1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 9.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstetrics & Gynecology. 2002;100:245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar LM, Pugh SJ, Abrams B, Himes KP, Hutcheon JA. Gestational weight gain in twin pregnancies and maternal and child health: a systematic review. Journal of Perinatology. 2014;34:252–263. doi: 10.1038/jp.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Paediatric and Perinatal Epidemiology. 2012;26:109–116. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 13.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 14.Cheikh Ismail L, Bishop DC, Pang R, et al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ. 2016;352:i555. doi: 10.1136/bmj.i555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson K, Hutcheon JA, Stephansson O, Cnattingius S. Pregnancy weight gain by gestational age and BMI in Sweden: a population-based cohort study. The American Journal of Clinical Nutrition. 2016;103:1278–1284. doi: 10.3945/ajcn.115.110197. [DOI] [PubMed] [Google Scholar]
- 16.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. The American Journal of Clinical Nutrition. 2013;97:1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) 2015;23:532–535. doi: 10.1002/oby.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodnar LM, Abrams B, Bertolet M, Gernand AD, Parisi SM, Himes KP, et al. Validity of birth certificate-derived maternal weight data. Paediatric and Perinatal Epidemiology. 2014;28:203–212. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of O, Gynecologists. ACOG Practice Bulletin No. 101: Ultrasonography in pregnancy. Obstetrics & Gynecology. 2009;113:451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York, USA: Springer-Verlag; 2001. [Google Scholar]
- 21.Altman DG, Chitty LS. Charts of fetal size: 1. Methodology. British Journal of Obstetrics and Gynaecology. 1994;101:29–34. doi: 10.1111/j.1471-0528.1994.tb13006.x. [DOI] [PubMed] [Google Scholar]
- 22.Luke B, Hediger ML, Nugent C, Newman RB, Mauldin JG, Witter FR, et al. Body mass index--specific weight gains associated with optimal birth weights in twin pregnancies. Journal of Reprodudctive Medicine. 2003;48:217–224. [PubMed] [Google Scholar]
- 23.Lantz ME, Chez RA, Rodriguez A, Porter KB. Maternal weight gain patterns and birth weight outcome in twin gestation. Obstetrics & Gynecology. 1996;87:551–556. doi: 10.1016/0029-7844(95)00485-8. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Ohuma EO, International F Newborn Growth Consortium for the 21st C. Statistical considerations for the development of prescriptive fetal and newborn growth standards in the INTERGROWTH-21st Project. British Journal of Obstetrics and Gynaecology. 2013;120(Suppl 2):71–76. doi: 10.1111/1471-0528.12031. [DOI] [PubMed] [Google Scholar]
- 25.Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. American Journal of Obstetrics and Gynecology. 2015;213:449, e1–e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Journal of the American Dietic Association. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regression equations to calculate z-scores for exact gestational age
Stata code to calculate pregnancy weight gain z-scores for twin pregnancies using exact gestational age
Figure S1. Pregnancy weight gain values in overweight and obese women delivering twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013. In panels A and B, grey dots indicate observed weight gain measurements, solid black line indicates predicted mean, dashed lines indicate predicted +/− 1 standard deviation, and dotted lines indicate predicted +/− 2 standard deviations. In panels C and D, lines indicate 50th percentile (central solid line), 10th and 90th percentiles (dashed lines), +/− 1 standard deviation (dotted lines), and 3rd and 97th percentiles (outer solid lines).
Table S1. Pregnancy weight gain for gestational age among overweight women with twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.
Table S2. Pregnancy weight gain for gestational age among obese women with twin pregnancies at Magee-Womens Hospital, Pittsburgh PA, 1998–2013.