Abstract
Atypical rearing has deleterious effects on chimpanzee behavior during development, some of which can be ameliorated with a responsive care intervention (RCI). Here, we obtained in vivo magnetic resonance images of adult brains of 27 chimpanzees given institutional care, with and without RCI, and compared them with those of 16 chimpanzees mother-reared from birth. We found significant long-term rearing effects on structural covariation and gray matter volume, specifically in the basal forebrain (i.e., caudate, putamen, nucleus accumbens, rectus gyrus, and orbital prefrontal cortex), indicating that RCI prevented brain changes due to atypical rearing. A significant correlation between covariation in these brain areas and caregiver nurturing, experienced in the first month of life, suggests a possible developmental mechanism for the effect of early experience on brain networks. We identified an early intervention that prevents changes in the basal forebrain that otherwise emerge as a consequence of institutionalized rearing without species-typical socioemotional experiences.
Keywords: great apes, brain, institutionalization, nurturing experiences, source-based morphometry
Although the chimpanzee brain is more developed at birth than the human brain (40% vs. ~28% of its adult size, respectively), there is still considerable plasticity in great ape brains during development, as shown by the influence of environmental and experiential factors (Bogart, Bennett, Schapiro, Reamer, & Hopkins, 2014; DeSilva & Lesnik, 2008; Gómez-Robles, Hopkins, Schapiro, & Sherwood, 2015). Interactions with caregivers who nurture species-typical development are critically important, especially when they occur early in life. In fact, socioemotional experiences contribute to good social relationships (e.g., engendering positive emotions; Bard, Bakeman, Boysen, & Leavens, 2014; and appropriate communicative skills; Bard, Dunbar, et al., 2014).
Studies in a variety of mammalian species demonstrate that the early development of positive socioemotional relationships is important for species-typical adult patterns of behavior. Acute or chronic disruption in the formation of reliable social relationships can have deleterious effects on the development of social, emotional, and cognitive functions in human and nonhuman primates (French & Carp, 2016). For instance, many children who experienced very poor quality caregiving early in life, such as Romanian orphans, have deficits or delays in cognition, attention regulation, and language, some of which are associated with differences in brain structure and function (Bick & Nelson, 2016; Loman, Wiik, Frenn, Pollak, & Gunnar, 2009; McLaughlin et al., 2014; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012). These findings in human children are consistent with reports in other primate species, including monkeys (Sackett, Ruppenthal, & Elias, 2005; Sanchez, Hearn, Do, Rilling, & Herndon, 1998; Spinelli et al., 2009) and great apes, notably chimpanzees (Bogart et al., 2014; Davenport & Rogers, 1970; Davenport, Rogers, & Rumbaugh, 1973; Morimura & Mori, 2010).
In the current study, we sought to examine the long-term consequences of early atypical rearing experiences on cortical and subcortical organization in chimpanzees and to assess the extent to which atypical organization can be ameliorated by an early socioemotional intervention. Specifically, in the late 1980s, National Institute of Health (NIH)-sponsored chimpanzee breeding programs produced an abundance of infants for potential use in biomedical research. Many infants were raised by their biological mothers, whose care consisted of continuous physical contact 24 hr per day, 7 days a week in the first 3 months, on-demand nursing, and nurturing of species-typical motoric, social, emotional, and communicative skills (Bard, 1994). However, some infants had to be raised by humans in a nursery setting (i.e., atypical rearing) because their mothers did not exhibit adequate maternal behaviors (Bard, 1994). Standard (ST) nurseries consisted of small groups of same-age peers, with humans providing health care checks, scheduled feedings, and diaper changes but spending a total of only 60 min per day with the chimpanzees. ST practices were associated with relatively poor infant outcomes, including disorganized attachment (consisting of a poorly functioning attachment system without mechanisms that provide infants with security under times of distress; van IJzendoorn, Bard, Bakermans-Kranenberg, & Ivan, 2009) and deficits in joint attention and cooperation (Bard, Bakeman, et al., 2014). Disorganized attachment in infancy had long-term deleterious consequences on adult chimpanzees, including increased abnormal behaviors, such as stereotypic rocking (Clay, Bloomsmith, Bard, Maple, & Marr, 2015). Such disruptions in species-typical development in the ST chimpanzees are consistent with a large body of research on the effects of early adverse rearing on social and behavioral development in various primate species.
In 1990, a responsive care intervention (RCI) was designed to optimize the development of species-typical behaviors in nursery-reared chimpanzees and potentially to ameliorate the effects of atypical rearing (Bard, 1996). RCI, given from birth to chimpanzees that would have otherwise experienced only ST nursery rearing, consisted of an additional 240 min per weekday of contact with human researchers, who provided constant cradling contact in the early months and nurtured chimpanzee motoric, communicative, social, and emotional species-typical behaviors during the first year of life. Chimpanzee infants given RCI, compared with ST care, had a well-functioning (i.e., organized) attachment system (van IJzendoorn et al., 2009) and significantly improved social cognition, emotional responses, and communication (Bard, Bakeman, et al., 2014; Bard, Dunbar, et al., 2014).
Here, we examined the long-term consequences of RCI and ST practices compared with species-typical mother rearing (MR) on cerebral gray matter organization. The RCI program is particularly important in the context of orphans and other human populations exposed to adverse rearing experiences, because the intervention started within hours after birth, and confounding factors known to influence brain development, such as nutrition and access to prenatal and postnatal health care, were systematically controlled. Indeed, both the duration of institutionalization and the age at intervention are critical factors for human orphans (Nelson, Bos, Gunnar, & Sonuga-Burke, 2011), but few studies have been able to characterize long-term changes in the brains of children adopted before the age of 6 months. Adoption into families, the primary intervention in humans, facilitates partial recovery of function but some effects of institutionalization on brain organization persist (e.g., decreased total gray matter volume; Sheridan et al., 2012).
We assessed changes in brain structure using source-based morphometry (SBM) to quantify structural gray matter covariation (Xu, Groth, Pearlson, Schretlen, & Calhoun, 2009). SBM is one approach to the analysis of structural magnetic resonance imaging (MRI) scans that allows for the determination of consistent covariation in gray matter volume across cortical and subcortical regions throughout the brain. Moreover, SBM offers advantages compared with other whole-brain methods, such as voxel-based morphometry (VBM; Alexander-Bloch, Giedd, & Bullmore, 2013: see the Supplemental Material available online for further details). Notably, SBM compares groups of subjects on clusters or component scores that reflect networks of voxels of gray matter that covary, whereas VBM analyses are at the level of each individual voxel. Thus, in our view, SBM can be particularly useful in studies with smaller sample sizes. By computing component scores with SBM, grouping voxels that are highly correlated with each other across subjects, one can meaningfully reduce the number of tests, thereby retaining more optimal alpha levels and minimizing Type II errors. If RCI were successful in ameliorating atypical rearing effects in gray matter covariation in the adult brain, then we hypothesized that RCI chimpanzees would show species-typical patterns of gray matter organization similar to MR individuals and different from those raised with ST institutional practices.
Method
Subjects
All available chimpanzee subjects housed at the Yerkes National Primate Research Center (YNPRC) were used for this research: 23 females and 20 males. Ages at the time of their MRI scans ranged from 9 to 23 years (M = 15.98 years, SD = 3.31). This is a unique sample in that it comprises a relatively large number of adult chimpanzees that have had brain scans and for whom rearing history is known explicitly. Sixteen chimpanzees were raised by their biological mothers (MR group) in “nuclear” family groups, with group sizes ranging from 4 to 20 individuals. MR chimpanzee infants remained with their conspecific mother for at least their first 2.5 years of life.
ST care was given to 17 chimpanzees (most placed in the nursery on the first day of life, n = 9; with others placed later because of injury or illness). The newborn chimpanzees were placed in incubators until their temperature regulated (typically 30 days) and were fed standard human infant formula (on a schedule), with health checks and diaper changes given by humans on a predetermined schedule. By 1 month, when other infants of the same age were available, infants were placed in peer groups. They remained with their peer group 24 hr per day, 7 days per week until they were approximately 5 years old, when they moved out of the nursery to join mixed-age, mixed-sex groups of chimpanzees residing at the YNPRC (Bard, 1996).
The RCI was given to 10 chimpanzees placed in the nursery within hours after birth because of inadequate maternal care. RCI was given each weekday afternoon for 4 hr and consisted of enhancing and nurturing species-typical patterns of motoric development, emotional development, and communicative development (Bard, 1996) for the first year of life. During the intervention, infants were fed on demand, given constant physical contact with an adult caregiver, groomed, and attended to much like infant chimpanzees are attended to by their biological mothers (Bard, Dunbar, et al., 2014). Except during this 4-hr period and after their first birthday, the RCI group experienced living conditions equivalent to those experienced by the ST group.
Chimpanzees were not assigned randomly to rearing condition. Every infant whose mother provided adequate maternal care was retained in the MR group; otherwise, infants were placed in the nursery. ST care was the standard regime from 1987 through 1995, whereas RCI was available only from 1991 to 1995 and was given only to infants who arrived in the nursery on the first day of life. Fifty-five percent of the RCI sample shared mothers and/or fathers with the ST sample.
Although every attempt was made to match the MR subjects in age with the other groups, the age at which MRI scans were taken differed across groups (ST: M = 18.2 ±3.1 years; RCI: M = 14.6 ±2.2 years; MR: M = 14.4 ±2.7 years). However, when analyses were run with age as a covariate, there were no significant main effects of age or any significant interactions with age on any of the SBM component scores. Since all the chimpanzees were young adults, minimally, when they were scanned, we concluded that their brain (gray matter) structures were equally mature.
Nurturing experiences
In a subset of chimpanzees, the nurturing of infant development by caregivers was directly observed and either coded from videotaped observations or recorded by caregivers on standardized reporting sheets. Although basic caregiving (e.g., feeding and health checks) ensured infant survival, nurturing enhanced the development of specific skills (e.g., motor development, social skills, independence; Bard, 1996; Bard, Dunbar, et al., 2014). In the first month of life, infant chimpanzees could experience “exercising” (e.g., mothers holding their hands while infants supported their partial weight with their legs) or “nurturing of social skills,” when mothers allowed or encouraged newborns to be groomed by others, providing them with extra social experiences (Bard, 1994). The amount of time that each infant experienced caregiver nurturing was calculated, and prorated (seconds per hour). For RCI chimpanzees, the average nurturing experienced was determined for 5 infants from 20 hr of caregiver interaction during the fourth week of age (recorded at the time of delivery by each infants’ caregiver). For ST chimpanzees, newborns (n = 13) had only about 60 min of contact with caregivers in 24 hr and did not experience any specific nurturing. There were nonzero values of nurturing for two infants who received ST care after being removed from their mother because of severe injury after inadequate maternal care (see Bard, Bakeman, et al., 2014, for details).
For the MR chimpanzee infants (n = 5), the average nurturing experienced was coded from 2-hr-long videotapes taken when infants were approximately 4 weeks old. Coders recorded maternal behavior during each minute, and 10% of the corpus was compared with ratings made by an expert third coder (K. A. Bard). There was good reliability between each (Coder 1 with expert: 72% agreement, Cohen’s κ = .67; Coder 2 with expert: 77% agreement, Cohen’s κ = .69), with a coding scheme of 12 potential maternal behaviors (Bakeman & Quera, 2011; Bard, Drescher, & Brindle, 2017).
MRI collection
Consistent with NIH guidelines, all the adult chimpanzees were scanned during one of their annual physical examinations. MRI scans followed standard procedures at the YNPRC and were designed to minimize stress. Thus, the animals were first sedated with ketamine (10 mg/kg) or telazol (3–5 mg/kg) and were subsequently anaesthetized with propofol (40–60 mg/(kg/h)). They were then transported to the MRI scanning facility and placed in a supine position in the scanner with their head in a human-head coil. On completion of the MRI scan, chimpanzees were briefly housed singly for 2 to 24 hr to permit close monitoring and safe recovery from the anesthesia, prior to returning to their home social group. All procedures were approved by the YNPRC Institutional Animal Care and Use Committees and also followed the guidelines of the Institute of Medicine on the use of chimpanzees in research. The chimpanzees were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions, Malvern, PA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2,300 ms, inversion time = 1,100 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320, slice thickness = 0.6 mm).
Source-based morphometry (SBM)
To characterize gray matter structural covariation, we used SBM (Xu et al., 2009). Consistent with previous studies with chimpanzees (Bianchi, Reyes, Hopkins, Taglialatela, & Sherwood, 2016), prior to the SBM analyses, the MRI scans were imported at 0.7 mm isotropic voxels, aligned in the anterior commissure-posterior commissure axis, skull-stripped, and processed though the FSL VBM pipeline procedures (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM/UserGuide). This included the initial segmentation of the T1-weighted scan into gray and white matter, registration to a chimpanzee standard template (Hopkins & Avants, 2013), and creation of a study-specific template brain. Each segmented gray matter volume was then linearly and subsequently nonlinearly registered to the study-specific template brain. To compensate for the expansion or enlargement due to the nonlinear component of the spatial transformation, we multiplied the nonlinear registered brains by the Jacobian warping field and appended to create a four-dimensional modulated gray matter volume. The images were then smoothed with an isotropic Gaussian kernel (σ = 2 mm, full width half maximum = ~ 4.7 mm). The modulated smoothed images were then subject to SBM analysis using the Group ICA of fMRI Toolbox (GIFT) software (Xu et al., 2009). The output from the SBM analysis produces a weighted score for each component and each individual subject that reflects their relative contribution to the strength of the covarying regions. The individual weighted scores were the dependent measures of interest. To visualize and identify those regions that were significant within each component, we used the source matrix maps. The source maps are scaled to standard-deviation units and z scores. Following previously used criteria (Xu et al., 2009), we thresholded the source maps at absolute z-score values ≥ 3.00. All clusters of voxels (i.e., components) that survived this threshold were considered significant.
Results
Source-based morphometry (SBM)
Because there are no previous reports of the use of SBM with chimpanzee MRI scans (or any nonhuman primates for that matter), we initially provide some descriptive data for the analyses. The SBM analysis revealed eight components within this sample of chimpanzees. The size of these components is shown in Table 1, and their anatomical locations are shown in Figures S1 to S8 in the Supplemental Material. Component 1 consisted of the anterior cingulate and medial dorsal prefrontal cortex. Components 2 and 5 were located within the cerebellum. Component 3 was composed of the basal forebrain, primarily including the caudate, putamen, ventral striatum, and nucleus accumbens. Regions comprising Component 4 were the left middle postcentral gyrus and the bilateral inferior temporal lobe, including portions of the amygdala. Components 6 and 8 were made up of the primary visual cortex and superior middle parietal lobe, respectively. Finally, Component 7 had the highest number of regions, including the left medial orbital prefrontal cortex, and bilaterally, the frontal pole, posterior superior temporal sulcus, visual cortex, and posterior parietal lobe.
Table 1.
Volumes (mm3) in the Brain Regions Associated With Components That Emerged From the Source-Based Morphometry Analysis (N = 43)
| Component and region | Bilateral | Left | Right |
|---|---|---|---|
| Component 1 | |||
| Anterior cingulate cortex | 202 | — | — |
| Medial prefrontal cortex | 1,928 | — | — |
| Component 2 | |||
| Cerebellar hemisphere | — | 3,902 | 2,991 |
| Component 3 | |||
| Basal forebrain | 2,759 | — | — |
| Component 4 | |||
| Middle left post central gyrus | — | 188 | — |
| Inferior temporal pole/amygdala | — | 1,726 | 1,914 |
| Component 5 | |||
| Cerebellum | 5,886 | — | — |
| Component 6 | |||
| Primary visual cortex | — | 1,406 | 1,498 |
| Component 7 | |||
| Medial orbital prefrontal cortex | 296 | — | — |
| Frontal pole | — | 179 | 209 |
| Posterior superior temporal sulcus | — | 78 | 185 |
| Visual cortex | — | 402 | 856 |
| Posterior parietal lobe | — | 368 | 607 |
| Component 8 | |||
| Superior middle parietal lobe | 6,331 | — | — |
Sex and rearing effects
We investigated the effects of rearing and sex on gray matter covariation with a repeated measures analysis of variance. The eight component SBM scores served as the repeated measures, and sex (male, female) and rearing group (MR, ST, RCI) were the between-groups factors. We found a significant main effect of rearing, F(2, 37) = 3.845, p = .030, ηp2 = .172, and a significant two-way interaction between rearing and SBM region, F(14, 259) = 2.199, p = .008, ηp2 = .106. The mean weighted SBM scores for each group and component are shown in Table 2. For the main effect of rearing, post hoc analysis indicated that RCI chimpanzees had higher scores than those in the ST group, but RCI chimpanzees did not differ from MR chimpanzees. Indeed, for every SBM region except Component 3, the ST chimpanzees had lower values than the RCI and MR chimpanzees. For the two-way interaction, we performed a secondary analysis using a multivariate analysis of variance with the SBM components serving as dependent measures and sex and rearing as between-groups factors. This analysis revealed a significant rearing effect only for Component 3, F(2, 37) = 8.300, p = .001, ηp2 = .310 (see Figs. 1 and 2a).
Table 2.
Weighted Gray Matter Covariation Scores for Each Source-Based Morphometry Component as a Function of Early Rearing Group
| Component | Standard (ST) nursery rearing |
Responsive care intervention (RCI) |
Mother rearing (MR) |
|---|---|---|---|
| M (SE) | M (SE) | M (SE) | |
| 1 | −.298 (.264) | −.145 (.321) | .539 (.287) |
| 2 | −.152 (.256) | .357 (.311) | .212 (.278) |
| 3 | .487 (.202) | −.696 (.245) | −.440 (.220) |
| 4 | −.292 (.266) | .069 (.322) | .355 (.288) |
| 5 | −.290 (.258) | .541 (.313) | .193 (.280) |
| 6 | −.378 (.258) | .643 (.313) | −.061 (.280) |
| 7 | .019 (.252) | .571 (.306) | −.001 (.273) |
| 8 | −.209 (.358) | .557 (.313) | .022 (.380) |
| Mean | −.139 (.090) | .237 (.109) | .102 (.097) |
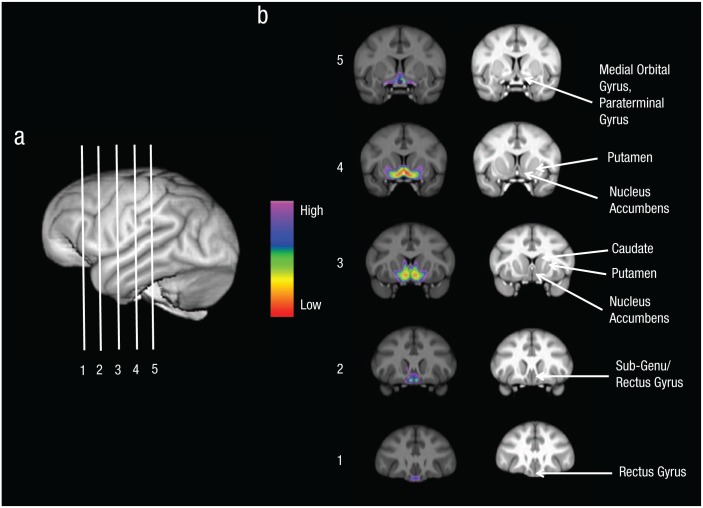
Fig. 1.
Brain regions of Component 3. A 3-D rendering of the chimpanzee brain (a) is shown with lines indicating the approximate location of the five slices illustrated in (b). The five coronal sections (b; labeled 1 to 5, anterior to posterior, respectively) illustrate the brain regions of Component 3 from the source-based morphometry (SBM) analysis. The left column shows the color-coded weighted voxel scores of Component 3 projected onto each coronal slice. The right column indicates the anatomical regions corresponding to the SBM weighted scores.
Fig. 2.

Results for Component 3. Mean weighted source-based morphometry score (a) and mean gray matter volume from the region-of-interest analysis (b) are shown for chimpanzees exposed to standard care, the responsive care intervention, and mother rearing. Error bars show ±1 SE.
Post hoc analysis indicated that the Component 3 weighted scores for the ST chimpanzees were significantly higher than those for the RCI group (p = .001), which did not differ from the MR group (p = .44; Fig. 2a). No other rearing effects were significant in the remaining seven components; however, there were nonsignificant trends for Component 1, F(2, 37) = 2.494, p = .096, ηp2 = .119, and Component 6, F(2, 37) = 3.207, p = .052, ηp2 = .148. For both of these components, the ST chimpanzees had much lower weighted scores than the RCI and MR groups.
We also quantified the gray matter volume of the cortical regions comprising Component 3, as a follow-up analysis, rather than solely using the weighted SBM component scores as the outcome measure. For this analysis, we simply converted the entire SBM Component 3 region to a binary mask by thresholding the volume at an absolute z score ≥ 3.00. We then placed that mask on the gray-matter-modulated volume for each chimpanzee and computed the gray matter volume within the mask. Not surprisingly, this analysis revealed an overall effect of rearing, F(2, 37) = 10.606, p = .001, ηp2 = .364. The mean gray matter volumes for ST, RCI, and MR chimpanzees are shown in Figure 2b. Post hoc analysis indicated that ST chimpanzees differed significantly from RCI and MR chimpanzees, but there was no difference between MR and RCI chimpanzees. As a means of validating the SBM results, we performed a more traditional VBM analysis, and the results largely confirmed the SBM findings (see the Supplemental Material).
Nurturing experience and SBM covariation
We selected the amount of time that an infant spent receiving nurturing experiences from an adult caregiver as one quantifiable aspect of the rearing experience that might relate to long-term brain outcomes. Although this information was not available for all the subjects for whom we had brain scans, we computed the rate of nurturing experienced by each of 25 chimpanzee infants at 1 month of age. Nurturing varied between 0 s per hr and 11.3 s per hr, with 1 infant experiencing several instances of neglect that were scored negatively (e.g., −0.5 s per hr of nurturing). We found after adjusting alpha for multiple comparisons (initial α =.05 for eight comparisons, adjusted α = .006), that nurturing experiences were significantly and meaningfully correlated with the Component 3 scores r(25) = −.632, p = .001. For the remaining seven SBM components, correlations between the nurturing values and the SBM weighted scores were nonsignificant, with rs between .350 and .008.
Discussion
Three significant findings emerged from this study on the influence of early rearing on long-term changes in cortical and subcortical organization in chimpanzees. First, we found that structural covariation in the gray matter of adult chimpanzees was significantly influenced by early rearing (Table 2). Chimpanzees raised with ST care (in an atypical nursery without species-typical socioemotional experiences) had the lowest degree of structural covariation, followed by MR chimpanzees (who had typical socioemotional experiences); the highest values were found in RCI chimpanzees (who received an early intervention that provided species-typical socioemotional experiences in the nursery setting). Second, consistently lower weighted scores for the ST chimpanzees were not found across all components. In Component 3 (brain areas typically associated with reward circuits; Fig. 1), ST chimpanzees had significantly higher structural covariation than RCI and MR chimpanzees (Fig. 2). Third, an RCI given in infancy had a significantly positive effect on long-term changes in structural covariation in gray matter in adulthood. Early experiences of species-typical nurturing appear to be a key variable in accounting for the lack of long-term institutionalization effects in RCI and MR chimpanzees. In both gray matter covariation in adulthood and rate of nurturing experiences in infancy, RCI chimpanzees more closely resembled MR than ST chimpanzees, suggesting that this intervention resulted in the development of a more species-typical pattern of brain organization.
We found a significant main effect of rearing on SBM component scores but also an interaction with components, indicating that the rearing effect was specific to Component 3. RCI chimpanzees were quantitatively similar to MR and significantly different from ST chimpanzees in both the structural covariation scores and gray matter volumes in Component 3, which included the striatum and dorsal forebrain. The evidence that early adverse rearing experiences impact the nucleus accumbens and related regions within the striatum is consistent with research in rodents and primates (Bennett & Pierre, 2010). Our findings suggest that disruptions in regulation of socioemotional behaviors found in ST chimpanzees might be attributable to atypical gray matter covariation within the areas typically associated with the reward system (e.g., caudate, putamen, nucleus accumbens, rectus gyrus, and orbital prefrontal cortex). Clinical and experimental research has clearly implicated these regions within the dorsal forebrain in motor, cognitive, socioemotional, and motivational systems in human and nonhuman primates (Cummings, 1993; Delgado, 2007; Glenn & Yang, 2012). For instance, individual differences in the neurobiology of the nucleus accumbens have been linked to addiction, poor delay of gratification, and a lack of impulsivity control (Casey et al., 2011; Franken, Booij, & van den Brink, 2005; Konova et al., 2012).
Constraints on generality
This study was conducted with chimpanzees raised under three different rearing conditions living in a laboratory setting. Although there are differences in everyday practice among great ape nurseries across laboratories (e.g., Bard, Brent, Lester, Worobey, & Suomi, 2011) and across other captive facilities (e.g., zoos), we expected that our results would generalize to chimpanzees residing in a variety of other captive facilities that included rearing protocols approximating our three conditions.
A variety of studies, by ourselves and others, illuminate the degree of similarity in psychological outcomes between chimpanzees and humans in the first year of life, including the effects of institutionalized care (and improvements due to RCI) on joint attention, cooperation, and quality of attachment (e.g., Bard, Bakeman, et al., 2014; van IJzendoorn et al., 2009). Additionally, a degree of similarity has been identified in some aspects of brain structure between adult chimpanzees and humans (Bianchi et al., 2016; Bogart et al., 2014; Cantalupo & Hopkins, 2001; Gómez-Robles et al., 2015) and congruence of function associated with some brain areas (e.g., gray matter asymmetries in the posterior superior temporal gyrus associated with better joint attention performance, Hopkins et al., 2014; morphometry and volume in the anterior cingulate cortex associated with engagement in joint attention, Hopkins & Taglialatela, 2013) even though there is a larger influence of language in the human brain. Therefore, we have theoretical and empirical reasons to predict that our core results would generalize to human participants, in the sense that an RCI would ameliorate brain changes caused by living in poor-quality institutionalized settings. Of course, responsive care for human participants would need to be species typical and culturally specific to approximate those nurturing experiences of human infants living outside the institutional setting (e.g., Keller & Bard, 2017).
Implications for future studies
Are there other behavioral, socioemotional, or cognitive outcomes that might also have been influenced positively by the RCI? RCI and ST chimpanzees differed, as predicted, in social cognition in infancy (Bard, Bakeman, et al., 2014), and “enculturated” adult apes perform significantly better than standard laboratory-raised individuals on problem-solving and cognitive tasks (e.g., Buttelmann, Carpenter, Call, & Tomasello, 2007; Furlong, Boose, & Boysen, 2008; Russell, Lyn, Schaeffer, & Hopkins, 2011). The extent to which RCI and ST chimpanzees might differ on other tasks linked with brain-structure differences warrants further investigation.
Standard institutional nursery care for chimpanzees appeared to cause changes in gray matter covariation that were prevented with an RCI. This finding is important for two reasons. First, there are significant implications for intervention programs for children raised in adverse social and physical environments. This intervention, given to young chimpanzees for only 4 hr per weekday, appeared to provide the experiences necessary to prevent the deleterious effects of atypical rearing on gray matter structural covariation, evident in the ST chimpanzees. In contrast, in children, differences in gray matter volume remain for those who experienced institutionalization, regardless of whether they had later interventions (Sheridan et al., 2012). This suggests that early interventions, even if only a few hours per day and in the first months of life, may ameliorate long-term neural changes due to institutionalization. Although caution is advised because of our small sample size and lack of random assignment, we conclude that RCI was effective in preventing the deleterious effects of atypical rearing on structural covariation in the chimpanzee brain and suggest that similar early interventions may be effective in humans.
Second, the extent to which chimpanzees may differ from humans in their ability to functionally and neurologically recover from adverse rearing when intervention is delayed in development is an interesting and timely issue. Specifically, there are ethical and scientific questions, based on our findings, as to how well ST chimpanzees can integrate into new social and physical environments and whether such changes would have positive effects (e.g., on brain organization or socioemotional behavior), given the recent decision by NIH to retire laboratory chimpanzees to sanctuaries. In many ways, NIH’s intervention program for chimpanzees is not very different from those initiated for institutionally raised children and offers an unprecedented opportunity to further study the role of early experiences on long-term outcomes in chimpanzees (Latzman & Hopkins, 2016). The identification of early rearing effects on specific areas of gray matter suggests that groups of adult chimpanzees may differ in sensitivity to primary rewards (e.g., food) or secondary rewards (e.g., establishing and maintaining social relationships). The assumption that the move to a sanctuary will have beneficial effects on well-being can be questioned if, in the ST group, for example, the reward value of close relationships is abnormally low, impacting the success of the intervention. NIH should continue to support research in this valuable yet dwindling population of apes, given the translational value of these studies to humans.
Acknowledgments
American Psychological Association and Institute of Medicine guidelines for the treatment of chimpanzees in research were followed during all aspects of this study.
Footnotes
Action Editor: Ralph Adolphs served as action editor for this article.
Author Contributions: K. A. Bard designed and conducted the studies of the effects of early rearing on chimpanzee development, and W. D. Hopkins designed and conducted the studies of source- and voxel-based morphometry in adult chimpanzees. K. A. Bard and W. D. Hopkins together analyzed these data, wrote the manuscript, and approved the final version of the manuscript for publication.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This research was supported by National Institute of Health Grants NS-42867, NS-73134, and HD-60563 to W. D. Hopkins; RR-06158 and HD-07105 to K. A. Bard; and RR-03951 to R. B. Swenson, and by Office of Research Infrastructure Programs Grant OD P51OD11132 (RR-00165) to Yerkes National Primate Research Center.
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617740685
References
- Alexander-Bloch A., Giedd J. N., Bullmore E. (2013). Imaging structural co-variance between human brain regions. Nature Neuroscience Reviews, 14, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R., Quera V. (2011). Sequential analysis and observational methods for the behavioral sciences. New York, NY: Cambridge University Press. [Google Scholar]
- Bard K. A. (1994). Evolutionary roots of intuitive parenting: Maternal competence in chimpanzees. Early Development and Parenting, 3, 19–28. doi: 10.1002/edp.2430030104 [DOI] [Google Scholar]
- Bard K. A. (1996). Responsive care: Behavioral intervention for nursery reared chimpanzees. Ridgefield, CT: Jane Goodall Institute. [Google Scholar]
- Bard K. A., Bakeman R., Boysen S. T., Leavens D. A. (2014). Emotional engagements predict and enhance social cognition in young chimpanzees. Developmental Science, 17, 682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard K. A., Brent L., Lester B., Worobey J., Suomi S. J. (2011). Neurobehavioral integrity of chimpanzee newborns: Comparisons across groups and across species reveal gene-environment interaction effects. Infant and Child Development, 20, 47–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard K. A., Drescher N., Brindle C. (2017). Can a responsive care program for nursery-reared chimpanzee infants simulate interactions between infants and their biological mothers? Unpublished manuscript, Psychology Department, University of Portsmouth, United Kingdom. [Google Scholar]
- Bard K. A., Dunbar S., Maguire-Herring V., Veira Y., Hayes K. G., McDonald K. (2014). Gestures and socio-emotional communicative development in chimpanzee infants. American Journal of Primatology, 76, 14–29. [DOI] [PubMed] [Google Scholar]
- Bennett A. J., Pierre P. J. (2010). Nonhuman primate research contributions to understanding genetic and environmental influences on phenotypic outcomes across development. In Hood K. E., Halpern C. T., Greenberg G., Lerner R. M. (Eds.), Handbook of developmental science, behavior, and genetics (pp. 353–359). Oxford, England: John Wiley & Sons. [Google Scholar]
- Bianchi S., Reyes L. D., Hopkins W. D., Taglialatela J. P., Sherwood C. C. (2016). Neocortical grey matter distribution underlying voluntary, flexible vocalizations in chimpanzees. Scientific Reports, 6, Article 34733. doi: 10.1038/srep34733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Nelson C. A. (2016). Early adverse experiences and the developing brain. Neuropsychophamacology, 41, 177–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart S. L., Bennett A. J., Schapiro S. J., Reamer L. A., Hopkins W. D. (2014). Different early rearing experiences have long-term effects on cortical organization in captive chimpanzees (Pan troglodytes). Developmental Science, 17, 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttelmann D., Carpenter M., Call J., Tomasello M. (2007). Enculturated chimpanzees imitate rationally. Developmental Science, 10(4), 31–33. [DOI] [PubMed] [Google Scholar]
- Cantalupo C., Hopkins W. D. (2001). Asymmetric Broca’s area in great apes. Nature, 414, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Somerville L. H., Gotlib I. H., Ayduk O., Franklin N. T., Askren M. K., . . . Shoda Y. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences, USA, 108, 14998–15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay A. W., Bloomsmith M. A., Bard K. A., Maple T. L., Marr M. J. (2015). Long-term effects of infant attachment organization on adult behavior and health in nursery-reared, captive chimpanzees ( Pan troglodytes). Journal of Comparative Psychology, 129, 145–159. [DOI] [PubMed] [Google Scholar]
- Cummings J. L. (1993). Frontal-subcortical circuits and human behavior. Archives of Neurology, 50, 873–880. [DOI] [PubMed] [Google Scholar]
- Davenport R. K., Rogers C. M. (1970). Differential rearing of the chimpanzee: A project survey. In Bourne G. H. (Ed.), The chimpanzee: Vol. 3. Immunology, infections, hormones, anatomy, and behavior of chimpanzees (Vol. 3, pp. 337–360). Baltimore, MD: University Park Press. [Google Scholar]
- Davenport R. K., Rogers C. M., Rumbaugh D. M. (1973). Long-term cognitive deficits in chimpanzees associated with early impoverished rearing. Developmental Psychology, 9, 343–347. doi: 10.1037/h0034877 [DOI] [Google Scholar]
- Delgado M. R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- DeSilva J., Lesnik J. J. (2008). Brain size at birth throughout human evolution: A new method for estimating neonatal brain size in hominins. Journal of Human Evolution, 55, 1064–1074. [DOI] [PubMed] [Google Scholar]
- Franken I. H. A., Booij J., van den Brink W. (2005). The role of dopamine in human addiction: From reward to motivated attention. European Journal of Pharmacology, 526, 199–206. [DOI] [PubMed] [Google Scholar]
- French J. A., Carp S. B. (2016). Early-life social adversity and developmental processes in nonhuman primates. Current Opinion in Behavioral Science, 7, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong E. E., Boose K. J., Boysen S. T. (2008). Raking it in: The impact of enculturation on chimpanzee tool use. Animal Cognition, 11, 83–97. [DOI] [PubMed] [Google Scholar]
- Glenn A. L., Yang Y. (2012). The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry, 72, 817–822. [DOI] [PubMed] [Google Scholar]
- Gómez-Robles A., Hopkins W. D., Schapiro S. J., Sherwood C. C. (2015). Relaxed genetic control of cortical organization in human brains compared with chimpanzees. Proceedings of the National Academy of Sciences, USA, 112, 14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. D., Avants B. B. (2013). Regional and hemispheric variation in cortical thickness in chimpanzees ( Pan troglodytes). The Journal of Neuroscience, 33, 5241–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. D., Misiura M., Reamer L. A., Schaeffer J. A., Mareno M. C., Schapiro S. J. (2014). Poor receptive joint attention skills are associated with atypical gray matter asymmetry in the posterior superior temporal gyrus of chimpanzees ( Pan troglodytes). Frontiers in Psychology, 5, Article 7. doi: 10.3389/fpsyg.2014.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. D., Taglialatela J. P. (2013). Initiation of joint attention is associated with morphometric variation in the anterior cingulate cortex of chimpanzees ( Pan troglodytes). American Journal of Primatology, 75, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H., Bard K. A. (2017). (Eds.). The cultural nature of attachment: Contextualizing relationships and development. London, England: MIT Press. [Google Scholar]
- Konova A. B., Moeller S. J., Tomasi D., Parvaz M. A., Alia-Klein N., Volkow N. D., Goldstein R. Z. (2012). Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. European Journal of Neuroscience, 36, 2979–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman R. D., Hopkins W. D. (2016). Letter to the editor: Avoiding a lost opportunity for psychological medicine: Importance of chimpanzee research to the National Institutes of Health portfolio. Psychological Medicine, 11, 2455–2457. [DOI] [PubMed] [Google Scholar]
- Loman M. M., Wiik K. L., Frenn K. A., Pollak S. D., Gunnar M. R. (2009). Postinstitutionalized children’s development: Growth, cognitive, and language outcomes. Journal of Developmental & Behavioral Pediatrics, 30, 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K. A., Sheridan M. A., Winter W., Fox N. A., Zeanah C. H., Nelson C. A. (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry, 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura N., Mori Y. (2010). Effects of early rearing conditions on problem-solving skill in captive male chimpanzees (Pan troglodytes). American Journal of Primatology, 72, 626–633. doi: 10.1002/ajp.20819 [DOI] [PubMed] [Google Scholar]
- Nelson C. A., III, Bos K., Gunnar M. R., Sonuga-Burke E. J. S. (2011). The neurobiological toll of early human deprivation. Monographs of the Society in Research in Child Development, 76, 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. L., Lyn H., Schaeffer J. A., Hopkins W. D. (2011). The role of socio-communicative rearing environments in the development of social and physical cognition in apes. Developmental Science, 14, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett G. P., Ruppenthal G. C., Elias K. (Eds.). (2005). Nursery rearing of nonhuman primates in the 21st century. New York, NY: Springer. [Google Scholar]
- Sanchez M. M., Hearn E. F., Do D., Rilling J. K., Herndon J. G. (1998). Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research, 812, 38–49. [DOI] [PubMed] [Google Scholar]
- Sheridan M. A., Fox N. A., Zeanah C. H., McLaughlin K. A., Nelson C. A. (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences, USA, 109, 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S., Chefer S., Suomi S. J., Higley J. D., Barr C. S., Stein E. (2009). Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry, 66, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn M. H., Bard K. A., Bakermans-Kranenberg M. J., Ivan K. (2009). Enhancement of attachment and cognitive development of young nursery-reared chimpanzees in responsive versus standard care. Developmental Psychobiology, 51, 173–185. [DOI] [PubMed] [Google Scholar]
- Xu L., Groth K. M., Pearlson G., Schretlen D. J., Calhoun V. D. (2009). Source-based morphometry: The use of independent component analysis to identify gray matter differences with application to schizophrenia. Human Brain Mapping, 30, 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]