Abstract
Background
Premature birth occurs when nephrogenesis is incomplete and has been linked to increased renal pathologies in the adult. Metabolic factors complicating preterm birth may have additional consequences for kidney development. Here, we evaluated the effects of prematurity and hyperglycemia on nephrogenesis in premature baboons when compared to term animals.
Methods
Baboons were delivered prematurely (67% gestation; n=9) or at term (n=7) and survived 2–4 weeks. Preterm animals were classified by glucose control during the first five days of life (DOL): normoglycemic (PtN; serum glucose 50–100mg/dL, n=6) and hyperglycemic (PtH; serum glucose 150–250mg/dL, n=3). Kidneys were assessed histologically for glomeruli relative area, maturity, size, and overall morphology. Kidney lysates were evaluated for oxidative damage with 4-hydroxynonenal (4-HNE) antibody.
Results
Histological examination revealed decreased glomeruli relative area (p<0.05), fewer glomerular generations (p<0.01), and increased renal corpuscle area (p<0.001) in preterm compared to term animals. Numbers of apoptotic glomeruli were similar between groups. PtH kidneys exhibited reduced nephrogenic zone width (p<0.0001), increased numbers of mature glomeruli (p<0.05), and increased 4-HNE staining compared to PtN kidneys.
Conclusion
Prematurity interrupts normal kidney development, independent of glomerular cell apoptosis. When prematurity is complicated by hyperglycemia; kidney development shifts towards accelerated maturation and increased oxidative stress.
INTRODUCTION
Premature birth is increasing worldwide and in 2010 was estimated at 11.1% of all live births (1). Although preterm birth is the largest contributor to neonatal death, advances in perinatal practices have improved preterm infant survival (2). However, prematurity remains associated with many long and short term morbidities, including chronic kidney disease. The factors contributing to the development of chronic kidney disease in preterm infants are not well understood, but are believed to be related to interruption of normal kidney development resulting in reduced nephron endowment and abnormal vascularization (3). Nephrogenesis begins at week 5 of gestation and is ongoing until 36 weeks. Therefore, premature birth occurs at a time when the kidney is actively developing and the abrupt transition to an ex utero environment interrupts this process.
Numerous studies have investigated the relationship between prematurity and abnormal kidney development. A study examining human autopsy samples found that preterm infants had a reduced number of glomerular generations compared to term infants, suggesting preterm infants do exhibit nephron deficit (4). However, these findings may be confounded by the population of intrauterine growth restricted infants within the sample group, which has also been shown to adversely affect nephron numbers (5). A subsequent study in baboons concluded that preterm birth was not associated with diminished nephron endowment when compared to gestational controls, but did find a strong correlation between renal size and glomerular number. In addition, the proportion of abnormal glomeruli was quite high in some, but not all preterm animals, suggesting the possibility that some preterm infants could be more susceptible to glomerular injury and the development of adult diseases (6). Similar findings with respect to the prevalence of abnormal glomeruli were seen in a corresponding human study including evidence of increased glomerular size and accelerated nephron maturation in preterm infants when compared to stillborn gestational controls (7).
Hyperglycemia is a common comorbidity in premature infants and may exacerbate this disruption in kidney development. Although glucose levels in the infant are routinely monitored, the definition continues to be accepted at >150 mg/dL or even >216 mg/dL in some neonatal intensive care units (8). Therefore, iatrogenic hyperglycemia from total parenteral nutrition continues to be a problem (9–12). In adult diabetics, poor glucose control and the resulting hyperglycemia are associated with a variety of complications, including retinopathy, neuropathy, and nephropathy (13–15). Mitochondrial overproduction of reactive oxygen species (ROS) is believed to contribute to the microvascular changes underlying these pathologies (13,14). Interestingly, hyperglycemia in preterm infants is associated with many morbidities that are known to be, or may be mediated by, microvascular complications, including retinopathy and intraventricular hemorrhage. Additionally, ROS have been suspected of contributing to many other complications associated with prematurity and hyperglycemia including necrotizing enterocolitis and bronchopulmonary dysplasia (16). However, the relationships between hyperglycemia, oxidative stress, and disease remain relatively understudied in preterm infants. Whether a relationship exists between prematurity, environmental factors such as hyperglycemia, and the development of chronic kidney disease has not been described. The purpose of this study was first to evaluate the effects of prematurity on kidney development, specifically nephrogenesis, and also to investigate whether hyperglycemia is associated with increased oxidative stress and abnormal kidney development in preterm infants using a non-human primate model of prematurity.
MATERIALS AND METHODS
Animal Care
A total of 16 animals were studied. Surgeries and procedures were performed at the University of Texas Health Science Center (UTHSCSA, San Antonio, TX 78229) or the Texas Biomedical Research Institute (TBRI, San Antonio, TX 78227). All animal experiments were approved by the respective institutional animal use and care committees and were conducted in accordance with accepted standards of humane animal use. Animals were delivered from healthy, nondiabetic mothers.
Care of term animals
Term infants were delivered at approximately 185 days gestation via spontaneous vaginal delivery and survived for 2–4 weeks (14–28 days). Animals were cared for by their mothers for up to 72 hours before being transferred to UTHSCSA. After transfer, animals received 24 hour nursery care and were monitored by veterinary staff daily. Animals were housed in a temperature controlled environment and were fed Similac (Abbott, Abbott Park, Illinois) formula orally every 3–4 hours. At approximately DOL 14 (n=3) or 28 (n=4), animals were euthanized with phenobarbital followed by exsanguination and immediately necropsied. Kidney samples were snap frozen in liquid nitrogen or placed in 10% neutral buffered formalin for fixation. After 24 hours of fixation, kidney samples were transferred to 70% ETOH. Term animals were considered healthy controls, therefore, blood chemistries and other parameters were not monitored unless clinically indicated. The blood chemistry values reported for term animals in this manuscript have been previously published in animals from the same non-human primate colony (17).
Care of preterm animals
Care of preterm animals was performed as previously described (18). Briefly, animals were delivered by caesarian section at 125±2 days of gestation (67% gestation) and survived for two weeks. Immediately after delivery, animals were intubated and placed on mechanical ventilation. Parenteral nutrition, containing a mixture of pediatric amino acids, was initiated at 24 hours of life. IV lipids were not administered. Preterm animals were subdivided by glucose target for the first 5 days of life into hyperglycemic (PtH, target glucose 150–250 mg/dL, n=3) and normoglycemic (PtN, target glucose 50–100 mg/dL, n=6). IV dextrose was administered shortly after birth and glucose infusion rate (GIR) was titrated by a glucose sliding protocol to maintain respective target glucoses. After 5 days, all animals were maintained normoglycemic until necropsy. Serum glucose was measured after birth and at minimum every 4 hours thereafter and urine glucose was measured every 12 hours. Blood chemistries (including blood urea nitrogen, BUN, and creatinine) were analyzed every 72 hours. Additional measurements were performed as needed. Other clinical parameters were also evaluated at set intervals: vitals including blood pressure (BP) every hour, arterial blood gas (ABG) measurements every two hours, and blood electrolytes every six hours. Values collected over the course of 24 hours were combined and compared between PtH and PtN animals. At 14±2 DOL, animals were euthanized with phenobarbital and exsanguination. Necropsy was performed and kidneys were snap frozen or fixed in 10% formalin as previously described.
Histological Analysis
Fixed kidney samples were sent to the UTHSCSA Pathology Core for processing. Samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological examination. Analysis was performed with the Computer Assisted Stereology Toolbox (CAST) 2.0 system (Visiopharm, Ballerup, Denmark) coupled with an Olympus BX61 microscope (Olympus Corporation of the Americas, Center Valley, PA). Two slides per animal were assessed with three animals per group. The relative area of mature glomeruli was determined by selecting at least 30 random fields within the cortex (~25% of total cortical tissue). Counting was performed at 20× magnification on a field of 225 points with at least 6750 total points within the tissue. The relative area of mature glomeruli was then calculated by dividing the number of points intersecting mature glomeruli by the total number of points in the tissue. The result was then multiplied by 100 to be reported as a percentage of total tissue area (19,20). The glomerular generation number was assessed as described by Rodriguez and colleagues (4). Briefly, glomeruli along the medullary ray were counted in 10 areas per kidney, averaged, and compared amongst the groups. Nephrogenic zone width (NZW) in preterm kidneys was determined by averaging the width of renal cortex containing immature glomeruli in five separate areas per kidney. The renal corpuscle cross-sectional area was calculated from the length and width (A = π × 1/2L × 1/2W) of 100 random glomeruli per kidney. Glomerulus morphology was assessed in 300 random fields per kidney at 40× magnification. Only mature glomeruli were considered in the morphological assessment. Kidneys were then categorized as “normal” or “abnormal.” Abnormal was characterized by a dilated Bowman’s space and shrunken glomerular tuft (7). Glomerulus maturity was determined according to presence of characteristic features as described in the literature (7,21,22). Because these animals were shared with other protocols, samples were not obtained with the intention of running stereological analysis. Therefore, the results obtained from the CAST system were further verified by 2D analysis. Briefly, slides were scanned at 20X magnification on an Aperio CS2 (Leica Biosystems, Buffalo Grove, IL) slide imaging system. Images were analyzed using Aperio ImageScope (Leica Biosystems) and ImageJ (National Institute of Health, Bethesda, Maryland) as described for the CAST system. The results obtained by this analysis supported the results obtained using the CAST system, therefore, only the CAST system (which allowed a more systematic and random approach to analysis) results are reported here.
Immunohistochemistry
Unstained kidney sections were dehydrated using serial xylene and alcohol dilutions. Staining was then performed with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) according to manufacturer’s instructions. Cleaved caspase-3, for apoptosis quantification, was detected with cleaved caspase-3 primary antibody (1:200 dilution; Cell Signaling Technology, Danvers, MA). Detection of lipid peroxidation, a marker of oxidative damage, was achieved with 4-HNE primary antibody (1:200 dilution; Abcam, Cambridge, MA). Primary staining was followed by diluted biotinylated secondary antibody solution and incubation in peroxidase substrate solution (Vector Laboratories). Nuclei were counterstained with hematoxylin. Sections were then rehydrated in serial xylene and alcohol dilutions and mounted. Staining was visualized with a Nikon DXM1200C camera mounted on a Nikon 80i microscope (Nikon, Melville, NY). Apoptosis quantification was accomplished using the CAST system to obtain random kidney cortex fields at 10× magnification. A total of ~400 mature glomeruli per kidney were visualized and labeled as apoptotic (positive cleaved caspase-3 staining) or non-apoptotic (negative staining).
Immunoblots
Kidney tissue was lysed in homogenizing (Kei) buffer (0.02M Tris pH 7.5, 5mM EDTA, 10mM Na4P2O7, 0.1M NaF, 2mM Na3VO4, 1% NP-40, 0.01 mg/ml Apoprotinin, 0.01 mg/mL Leupeptine, 3mM Benzamidine, 1mM PMSF) and 1.0mm Zirconia beads (Biospec Products, Bartlesville, OK) using a Mini-Bead Beater-16 (Biospec Products). Protein concentration was estimated using an Epoch Microplate Reader (BioTek, Winooski, VT) with Gen5 (BioTek) software. 50 μg of protein was loaded per lane to 10% Mini-Protean TGX precast gels (Bio-Rad, Hercules, CA). Following transfer, nitrocellulose membranes were blocked with 5% nonfat dry milk or 5% BSA and incubated with primary antibody overnight at 4°C. Primary antibodies were used to detect 4-HNE (Abcam) and GAPDH (Santa Cruz Biotechnology, Dallas, TX). Secondary anti-rabbit IRDye® 800CW antibody compatible with the LI-COR infrared imaging system (LI-COR, Lincoln, NE) was used. Immunoblots were performed in triplicate. Blots were imaged on a LI-COR Odyssey system and processed using Image Studio Lite V3.1 (LI-COR) software.
Statistics
Statistical analysis was performed using GraphPad Prism 5 (GraphPad, San Diego, CA) with data expressed as mean ± standard error of the mean (SEM). Statistical analyses included the student’s unpaired t-test to compare two groups, one-way ANOVA with the Newman-Keul posttest for analysis of multiple groups, and Kruskall-Wallis test with the Dunn’s Multiple Comparison Test for analysis of multiple group non-parametric data. Correlation of nonparametric to parametric data was assessed with Spearman’s correlation, rs. Asterisks or other identifying mark indicate the degree of significant differences compared with the controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
A sample size calculation was performed to determine the number of animals needed to detect a difference in kidney size and glomerular number derived from our preliminary data, with an alpha of 0.05, beta of 80%, a n=6 animals was calculated per group (preterm and term).
RESULTS
Animal Characteristics
Animal characteristics including gender and birth weight are summarized in Table 1.
Table 1. Details of animals used in this study.
Average birth weights, the male to female ratio, and the number of animals per group are indicated. Asterisks indicate significant difference versus Term results.
P< 0.001
Clinical Parameters
Preterm vs. Term animals
The serum blood chemistry values acquired up to DOL 7 were combined to yield a week 1 average and values after DOL 7 were combined into a week 2 average. Preterm animals had significantly higher serum BUN and creatinine compared to term animals (mean ± S.E.: 30.10 ± 2.71 vs 12.14 ±0.60 and 0.93 ± 0.055 vs 0.67 ± 0.013, respectively). Blood sodium levels were significantly lower in preterm animals vs term animals (mean ± S.E.: 138.33 ± 2.71 vs 144.08 ± 0.60, respectively), whereas blood potassium levels were significantly higher in preterm vs term animals (mean ± S.E.: 4.92 ± 0.055 vs 4.32 ± 0.013, respectively).
Fasting serum glucose was similar between term and PtN animals at similar times of extrauterine life (DOL 5±2 mean 55.57 vs 68.00, p=0.153, and DOL 14±2 mean 51.33 vs 67.03, p=0.06). Serum glucose was not checked as frequently in term animals as they were on enteral feeds, and were considered to be the normal controls.
Urine was collected and assessed from both preterm groups as well as term control animals during the first week of life (Table 2). The conversion between the scale used here and the corresponding clinical values are summarized in Table 3. There were no significant differences in pH, glucose, blood or protein content between PtN and term animals. PtH baboons tended to have more blood in their urine than PtN animals, but this trend was not statistically significant (p=0.07). Finally, in preterm animals, over 127 samples (average number of samples per animal=19.67±1.19) were evaluated for urine glucose and no correlations were found between serum glucose and presence/amount of glucosuria (Figure 1a–d).
Table 2. Comparison of Urinalysis Results.
Median values were calculated from urinalysis results for pH, glucose, blood, and protein taken during the first week of life. Medians were compared amongst the groups using the Kruskall-Wallis test with the Dunn’s Multiple Comparison Test as a post-test. Significance was assessed in comparison to term animals.
| pH | Glucose | Blood | Protein | ||
|---|---|---|---|---|---|
| PtN | Median (Range) |
6.25 (6.00–8.00) |
0 (NA) |
1.5 (0–3.5) |
0 (0–1.5) |
| ptH | Median (Range) |
6.50 (6.00–6.50) |
0 (0–1.0) |
3.0* (3.0–4.0) |
1 (0–2.0) |
| Term | Median (Range) |
7.0 (5.00–7.50) |
0 (NA) |
0 (NA) |
0 (0–1.0) |
P<0.05
Table 3. Urinalysis Reporting Scale.
Scale used for data analysis is shown along with the corresponding clinical value for parameters included in urinalysis.
| Scale for data | 0 | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| Clinical Value | Protein (mg/dL) | Negative | Trace | 30 | 100 | 300 | 2000+ |
| Blood, Non-hemolyzed | Negative | Trace | Moderate, 80+ | N/A | N/A | N/A | |
| Blood, Hemolyzed | Negative | Trace | Small, 25+ | Moderate, 80+ | Large, 200+ | N/A | |
| Glucose (mg/dL) | Negative | 100 | 250 | 500 | 1000 | 2000+ | |
| Bilirubin | Negative | Small, + | Moderate, ++ | Large, +++ | N/A | N/A | |
| Ketones (mg/dL) | Negative | 5 | 15 | 40 | 80 | 160 |
Figure 1. Correlation between urine and serum glucose measurements.
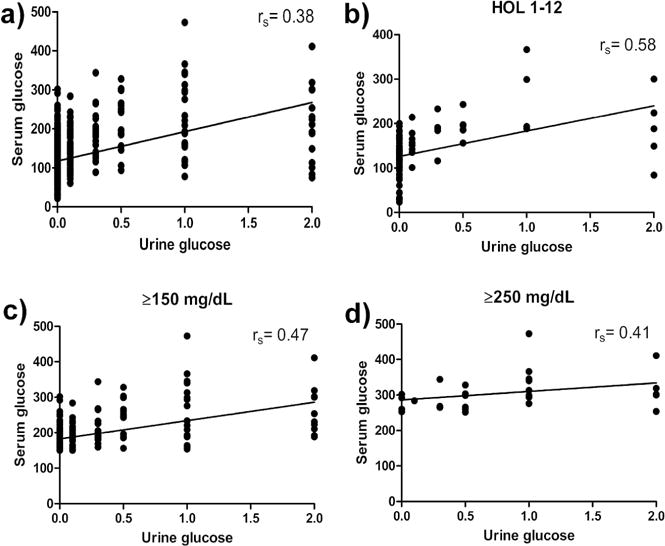
(a) All values, (b) those obtained within the first 12 hours of life, and (c) values with serum glucose ≥150 mg/dL or (d) ≥200 mg/dL were plotted and a line of best fit was added. The spearman r was calculated in order to judge the strength of correlation between serum and urine glucose in the above conditions (nA=1086, nB= 100, nC=249, nD= 28)
Preterm Normoglycemic vs Preterm Hyperglycemic
Renal function was measured frequently in preterm animals for monitoring of well-being (blood urea nitrogen (BUN) and creatinine were measured several times per week, whereas sodium and potassium were measured twice daily). Electrolyte details between PtH and PtN animals are shown in Figure 2a–b and no differences were found. Serum calcium and phosphorus were also measured several times per week, but did not yield significant differences (data not shown). Serum BUN was significantly lower in PtH animals compared to PtN animals during week 1, but this trend normalized by week 2 (Figure 2c). There were no differences in serum creatinine measurements between PtH or PtN animals (Figure 2d).
Figure 2. Serum Markers of Kidney Stress.
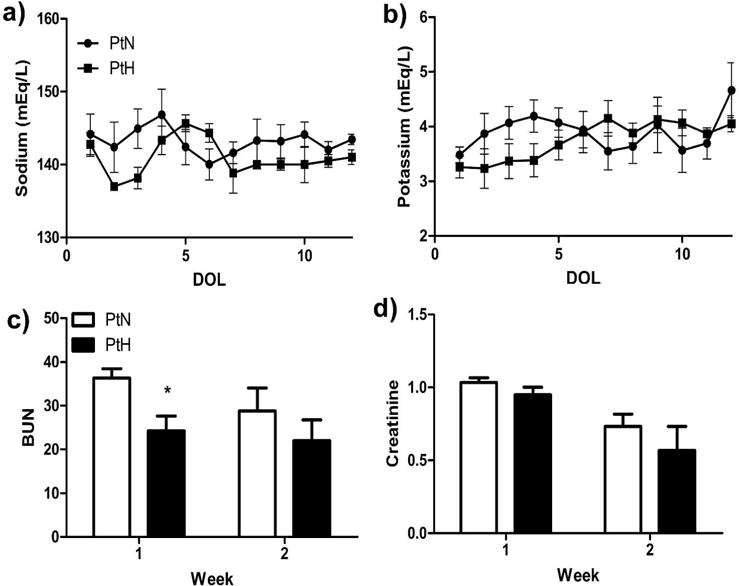
Serum chemistry including (a) sodium, (b) potassium, (c) BUN and (d) Creatinine were assessed; values were combined to yield a daily or weekly average. Results were compared between the groups daily or weekly. (n=6 PtN; n=3 PtH; *, P<0.05)
Compared to PtN, PtH baboons had significantly higher mean arterial pressure (MAP) at DOL 1 and at DOL 10 (Figure 3a). The difference in BP measurements could not be attributed to the administration of pressor medications including dopamine or epinephrine as an equal number of preterm animals (n=1) per group required pressor support. There were no significant differences in other vital signs including heart rate or oxygen saturation (data not shown). Similar to BP, urine output (UOP) was also significantly higher in PtH animals compared to PtN at DOL 1 and DOL 8 (Figure 3b). The differences in UOP for the PtH group could not be explained by an excess in fluid intake as recorded fluids were similar between groups at all DOL (Figure 3c).
Figure 3. Summary of Clinical Parameters.

(a) mean arterial pressure, (b) urine output (UOP), and (c) fluid intake were assessed several times per day in each animal and combined to yield a daily average value that was compared between groups. (d) GIR was calculated for each DOL and compared daily until necropsy. (e) Glucose levels from serum were assessed several times per day and averaged for each DOL; The PtH group included n=2 for days 5 and 7–11 due to equipment issues. (n=6 PtN; n=2–3 PtH***, P<0.001; **, P<0.01; *, P<0.05)
PtH animals were maintained hyperglycemic and had significantly higher glucose infusion rates for the first 5 DOL (Figure 3d). During the hyperglycemic period, PtH animals had between 70–104% higher serum glucose levels compared to PtN animals (Figure 3e). Serum glucose remained 42–71% higher in PtH animals for DOL 6–7, despite decreased GIR, likely due to the time it takes for insulin sensitive tissues to adapt, and was taken as a transition period to normoglycemia. As planned, from DOL 8 to necropsy, serum glucose was within normoglycemic parameters in all preterm animals.
Kidney development and oxidative stress
Preterm vs. Term animals
Figure 4a and b summarizes data obtained from the histological analysis including nephrogenic zone width and area of the renal corpuscle. Representative images of the outer boundaries of H&E stained kidney cortices are shown in figure 4c. There was no apparent nephrogenic zone in term animals, whereas both preterm groups exhibited a nephrogenic zone. The cross-sectional area of the renal corpuscle was significantly increased in both preterm groups compared to term animals. Data on glomerular relative area, generation number, proportion with abnormal features, apoptosis and maturity are shown in figure 5a–e. Both preterm baboon groups exhibited significantly reduced relative area of mature glomeruli when compared to term animals. Similarly, glomerular generation number was significantly reduced in preterm animals compared to term animals. In addition, to ensure the additional survival days did not contribute to the differences found in term animals, we compared term animals that survived 14 days versus term animals that survived 28 days, and found no differences in their histological analyses (glomerular generation number: p=0.25, cross-sectional area of renal corpuscle: p=0.48, relative area of mature glomeruli: p=0.78, percent of abnormal glomeruli: p=1.0).
Figure 4. Parameters Assessed In Evaluation of Kidney Histology.
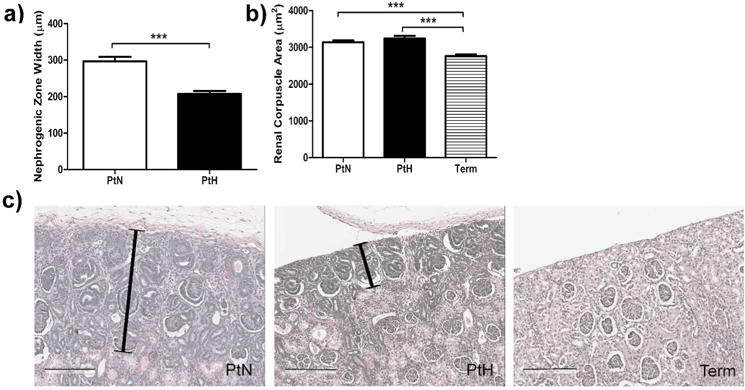
Paraffin embedded kidney sections stained with H&E were assessed to calculate (a) thickness of cortex containing immature glomeruli undergoing nephrogenesis (nephrogenic zone, there was no apparent nephrogenic zone in kidneys from term animals), and (b) renal corpuscle area. Error bars represent standard deviation (***, P<0.001; **, P<0.01; *, P<0.05). (c) Representative images of the outer boundaries of the kidney cortices in histological samples stained with H&E. In both preterm animal groups (PtN= D1, PtH= D2) there is evidence of darker hematoxylin staining with immature glomeruli. This area, the nephrogenic zone, is decreased in width in PtH animals. Nephrogenic zone is indicated by capped black line. Black scale bar is 200 μm.
Figure 5. Glomeruli Assessment.
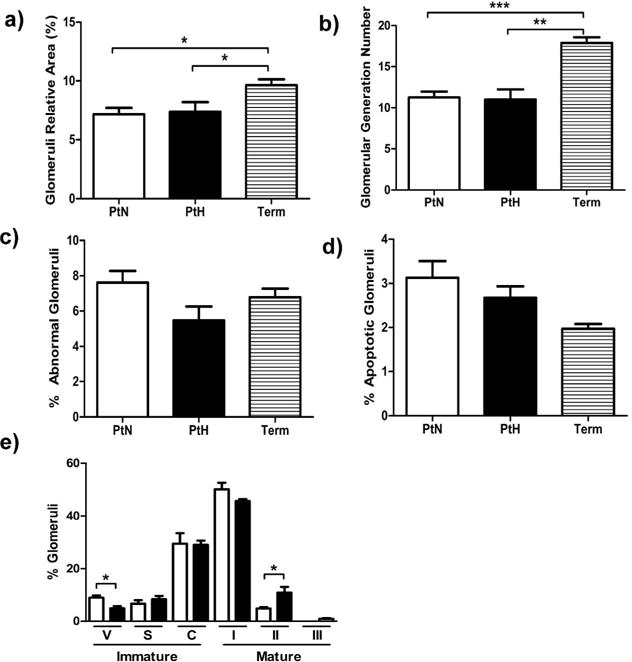
(a) Relative area of glomeruli, (b) estimated glomerular generation number, (c) percentage of mature glomeruli with an abnormal appearance, (d) and percentage of apoptotic glomeruli are shown. (e) Stages of glomerular development in PtH (black bar) and PtN (white bar) kidneys are shown [Immature stages: Vesicular Stage (V), S-Shaped or Comma-Shaped (S), Capillary loop stage (C); Mature Stages: Stage I (I), Stage II (II), Stage III (III)]. Error bars represent standard deviation (***, P<0.001; **, P<0.01; *, P<0.05).
Although it was evident that prematurity resulted in alterations in glomerular number and size, there were concerns that prematurity would also induce higher oxidative stress. Therefore, kidney protein lysates were analyzed via immunoblots for oxidative stress using the lipid peroxidation marker, 4-HNE (Figure 6a). No differences were found between preterm and term animals (p=0.63). Furthermore, in order to evaluate the localization of increased oxidative stress within the kidney, immunohistochemistry for 4-HNE in histological samples was performed (Figure 6b). Kidney samples from all groups demonstrated some positive staining for 4-HNE and similar staining was found between PtN and term animals whereas PtH had significantly more staining.
Figure 6. Oxidative Stress in Preterm and Term Kidneys.
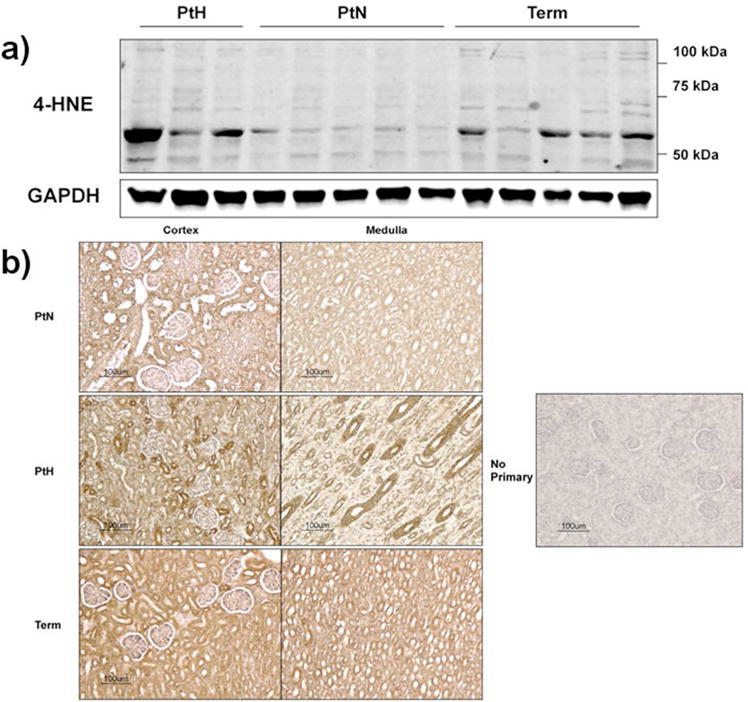
(a) Representative immunoblots for 4-HNE, a marker of lipid peroxidation, and corresponding GAPDH control in kidney protein lysates are shown (n=3 PtH; n=5 PtN; n=5 Term). (b) Representative images from PtN, PtH, and Term animal kidneys stained with 4-HNE are shown. Images on the left represent the renal cortex whereas images on the right represent the medulla. The far right image is the staining control, which did not include primary antibody.
Preterm Normoglycemic vs Preterm Hyperglycemic
There was no difference between the PtH and PtN groups in glomerular relative area, generation number, abnormal glomeruli or apoptotic glomeruli (Figure 5a–d). When compared to PtN animals, kidneys of PtH animals exhibited accelerated maturation. For example, the nephrogenic zone was significantly decreased in PtH animals compared to PtN animals (Figure 4a). Additionally, kidneys from PtH animals demonstrated a 59.6% reduction in the most immature Vesicular Stage (Stage V) glomeruli when compared to PtN animals (Figure 5e). Simultaneously, PtH kidneys exhibited a 77.3% increase in more mature Stage II glomeruli. Finally, PtH kidneys contained glomeruli in the most mature stage of glomerular development, Stage III, whereas these were not seen in PtN kidneys. Therefore, when Stage II and III numbers were combined and compared amongst the groups, PtH kidneys had an 83.7% increase in the most mature glomeruli in comparison to PtN kidneys.
To assess if transient hyperglycemia resulted in oxidative stress, 4-HNE protein content was examined by immunoblot as before. PtH animals had significantly higher protein content of 4-HNE compared to ptN animals (p<0.05; Figure 6a). Additionally, on immunohistochemical analysis, kidneys from the PtH group had significantly more staining than PtN (Figure 6b). Furthermore, the images from PtH kidneys revealed increased 4-HNE staining was predominantly within the renal tubules, and was particularly evident in the loops of Henle and collecting ducts in the medulla.
DISCUSSION
Prematurity continues to be a major contributor to neonatal morbidities and mortality. Furthermore, prematurity has been linked to an increased incidence of a number of adult pathologies, including obesity, type I and type II diabetes, hypertension, and chronic kidney disease (23,24). An increasing body of work supports the hypothesis that environmental exposures at critical periods of development, including metabolic disturbances such as hyperglycemia early in life, may pre-dispose an individual towards adult disease (25,26). Premature surviving infants frequently develop hypertension by the time they reach term gestation; hypertension is frequently attributed to the development of chronic lung disease which can potentially alter the angiotensin-renin-aldosterone axis (27). Few have investigated if intrinsic renal causes during late gestation or after preterm birth play an important role in the development of hypertension in surviving premature infants. To further complicate their outcomes, the preterm neonate may also be exposed to a wide variety of clinical interventions and/or pathological conditions during the first several weeks of life. In particular, transient hyperglycemia can affect up to 80% of extremely immature infants and may adversely affect renal function, as has been shown in diabetes. Herein, we induced transient hyperglycemia in preterm baboons to evaluate its effects on the already disrupted development of preterm baboons.
In this study, we demonstrated that prematurity alone interrupts normal kidney development. Furthermore, if prematurity is coupled with hyperglycemia, there is an increase in oxidative stress in the premature baboon kidney. The kidney is at particular risk for disrupted development in the preterm neonate as nephrogenesis is still ongoing after birth (24). As anticipated, premature baboon kidneys were less mature (fewer mature glomeruli and fewer glomerular generations) when compared to the kidneys of term animals. Although these results differ from those found by Gubhaju et al. (6), our study examined preterm baboons that were chronically ventilated and survived for 2 weeks and were compared to term counterparts rather than gestational controls, which were sacrificed immediately after birth. Animals killed right after birth have not gone through the stressors of being born prematurely and likely demonstrate fetal kidney development rather than postnatal development, and this, in itself, could account for these contrasting findings. A potential pitfall is the difference in postnatal developmental days within the term baboon group. In a sub-analysis comparing term animals that survived 14 days versus those that survived 28 days, we found no differences in their histological analyses. Although the numbers are small, the additional postnatal days are unlikely to contribute to the differences in glomerular generation number. Interestingly, we did not find evidence for increased apoptosis in preterm kidney glomeruli when compared to their term counterparts. This suggests that glomerular cell death is not a major contributor to early alterations in kidney structure and function seen in preterm infants. Preterm animals also exhibited greater kidney stress (higher serum BUN and creatinine) when compared to term animals. This result is consistent with the hypothesis that preterm birth is detrimental to kidney development. It remains to be determined if these changes persist and translate into altered kidney function later in life.
In addition to the effects of prematurity on kidney development, hyperglycemia alone induced significant changes in kidney morphology. When compared to the kidneys of preterm normoglycemic animals, hyperglycemic kidneys exhibited a significantly smaller nephrogenic zone. Hyperglycemic kidneys also had a greater number of mature glomeruli and correspondingly fewer immature glomeruli than their normoglycemic counterparts. Collectively, these results suggest hyperglycemia causes a shift in kidney development towards accelerated maturation. This is consistent with in vitro induced hyperglycemia in human derived dendritic cells, where it was found that hyperglycemia promotes their maturation and ability to induce T-cell proliferation (28). Although very few studies have investigated the effects of postnatal hyperglycemia on kidney development, several studies have explored the effects of maternal diabetes and the resulting hyperglycemic environment on fetal kidney development. Studies in mouse (29) and rat (30) models of impaired maternal glucose tolerance found that offspring had decreased nephron endowment and increased glomerular volume. However, these fetuses were exposed to prolonged hyperglycemia, which is likely why we did not find such differences in the PtH compared to PtN preterm baboons. A limitation of the study is that we could not investigate long term effects in this premature baboon model, due to the severity of illness related to extreme prematurity in these animals. An additional limitation is the inclusion of only three animals in the hyperglycemic preterm group due to the prohibitive cost and animal availability. Although more studies with additional animals need to be done in order to fully delineate the effects of hyperglycemia during prematurity on nephrogenesis, this work provides an important first step.
Prematurity alone did not have an increase in oxidative damage, whereas prematurity paired with hyperglycemia resulted in significantly increased levels of oxidative damage in the kidneys of PtH baboons. 4-HNE, a marker of lipid peroxidation induced by oxidative damage, was significantly increased in the kidneys of hyperglycemic animals. Using immunohistochemistry, we found that the oxidative damage was localized predominantly within the loops of Henle and the collecting ducts of the renal tubules. Tubule damage due to hyperglycemia is a major contributor to the development and progression of diabetic nephropathy (31).
Despite significant alterations in kidney development and increased oxidative stress, there were no significant differences in serum BUN or creatinine between normoglycemic and hyperglycemic animals. These findings are extremely important as they suggest these parameters may not be as sensitive for assessing short term effects of prematurity and hyperglycemia and should not be considered as surrogates for kidney injury in clinical practice. Furthermore, there were no correlations between serum glucose and the amount of glucosuria in the neonatal period. This is often utilized as a marker for treatment of hyperglycemia in the neonatal period and the hypothetical reasoning for utilizing a cutoff value of 150 mg/dL for treating hyperglycemia, as this is the level where glucosuria developed in older infant studies (32). These findings further support the conclusion that glucosuria is not a reliable indicator of hyperglycemia in preterm infants, as has been suggested by other studies (33,34).
This study further demonstrates that prematurity alone has the potential to significantly alter kidney development and, if paired with hyperglycemia, it increases oxidative stress in the developing kidney. Oxidative damage has been demonstrated to contribute significantly to hyperglycemic tissue damage in adults and its significance in the developing neonate should not be overlooked. Further research is needed to better understand the long-term impacts of these hyperglycemia induced developmental changes.
In conclusion, premature baboon kidneys exhibited nephrogenic zones containing immature glomeruli, whereas term baboon kidneys did not. Furthermore, the cross-sectional area of the renal corpuscle was significantly increased in both preterm groups compared to term animals. In addition, preterm baboons have significantly reduced relative area of mature glomeruli and, if exposed to transient hyperglycemia, it results in accelerated glomerular maturation. Finally, hyperglycemia also increases oxidative stress in the premature kidney. Disruption of normal kidney development by premature birth and/or environmental exposures could contribute to the development of adult renal pathologies commonly seen in surviving preterm infants.
Acknowledgments
Special appreciation to Drs. Jean Jiang and Sumin Gu for their assistance with the immunohistochemistry portion of this project, and to Dr. Jay Peters for the use of the Aperio system. We thank Dr. Michelle Leland for her dedication to Non-Human Primate research and her invaluable help with this project.
FINANCIAL SUPPORT: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118 (L.M.), the American Diabetes Association (C.B., R.A.D., and N.M.), The Robert Wood Johnson Foundation (C.B.), UTHSCSA CTSA (UL1RR025767) (C.B.), and University Health System Research Fund (C.B.). This investigation used resources that were supported by the Southwest National Primate Research Center grant P51 OD011133 from the Office of Research Infrastructure Programs, National Institutes of Health and conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number C06 RR 014578 and 1 C06 RR015456.
Footnotes
DISCLOSURE STATEMENT: The authors have no conflict of interest relevant to the subject matter of this manuscript.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol SN. 2000;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 3.Luyckx VA, Brenner BM. The Clinical Importance of Nephron Mass. J A. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 5.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 6.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol. 2009;297:F1668–1677. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol JASN. 2011;22:1365–74. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stensvold HJ, Strommen K, Lang AM, et al. Early enhanced parenteral nutrition, hyperglycemia, and death among extremely low-birth-weight infants. JAMA Pediatr. 2015;169:1003–10. doi: 10.1001/jamapediatrics.2015.1667. [DOI] [PubMed] [Google Scholar]
- 9.Slidsborg C, Jensen LB, Rasmussen SC, Fledelius HC, Greisen G, De Cour M. Early postnatal hyperglycaemia is a risk factor for treatment-demanding retinopathy of prematurity. 2017:1–5. doi: 10.1136/bjophthalmol-2016-309187. [DOI] [PubMed] [Google Scholar]
- 10.Auerbach A, Eventov-Friedman S, Arad I, et al. Long duration of hyperglycemia in the first 96 hours of life is associated with severe intraventricular hemorrhage in preterm infants. J Pediatr. 2013;163:388–93. doi: 10.1016/j.jpeds.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Beardsall K, Vanhaesebrouck S, Ogilvy-stuart AL, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE Study. J Pediatr. 2010;157:715–719.e3. doi: 10.1016/j.jpeds.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Harris DL, Hons M, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161:787–91. doi: 10.1016/j.jpeds.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137–42. doi: 10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa T, Edelstein D, Brownlee M. The missing link: A single unifying mechanism for diabetic complications. Kidney Int. 2000;58:26–30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic compliations. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Donovan DJO, Fernandes CJ. Free radicals and diseases in premature infants. Antioxid Redox Signal. 2004;6 doi: 10.1089/152308604771978471. [DOI] [PubMed] [Google Scholar]
- 17.Havill LM, Snider CL, Leland MM, Hubbard GB, Theriot SR, Mahaney MC. Hematology and blood biochemistry in infant baboons (Papio hamadryas) J Med Primatol. 2003;32:131–8. doi: 10.1034/j.1600-0684.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 18.Blanco CL, McGill-Vargas LL, Gastaldelli A, et al. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology. 2015;156:813–23. doi: 10.1210/en.2014-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn AR, Blanco CL, Perego C, et al. The ontogeny of the endocrine pancreas in the fetal/newborn baboon. J Endocrinol. 2012;214:289–99. doi: 10.1530/JOE-12-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guardado-Mendoza R, Davalli AM, Chavez AO, et al. Pancreatic islet amyloidosis, betacell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–7. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naruse K, Fujieda M, Miyazaki E, et al. An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int. 2000;57:1836–46. doi: 10.1046/j.1523-1755.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 22.Thony HC, Luethy CM, Zimmermann A, Laux-End R, Oetliker OH, Bianchetti MG. Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur J Pediatr. 1995;154:S65–68. doi: 10.1007/BF02191509. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: Systematic review and meta-analysis. Obes Rev. 2014;15:804–11. doi: 10.1111/obr.12214. [DOI] [PubMed] [Google Scholar]
- 24.Chong E, Yosypiv IV. Developmental programming of hypertension and kidney disease. Int J Nephrol. 2012;2012 doi: 10.1155/2012/760580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canani RB, Di Costanzo M, Leone L, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24:198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 26.Jang H, Serra C. Nutrition, epigenetics, and diseases. Clin Nutr Res. 2014;3:1–8. doi: 10.7762/cnr.2014.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stritzke A, Thomas S, Amin H, Fusch C, Lodha A. Renal consequences of preterm birth. Mol Cell Pediatr. 2017 doi: 10.1186/s40348-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao K, Ge J, Sun A, et al. Effects and mechanism of hyperglycemia on development and maturation and immune function of human monocyte derived dendritic cells. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:60–4. [PubMed] [Google Scholar]
- 29.Hokke S, Arias N, Armitage JA, et al. Maternal glucose intolerance reduces offspring nephron endowment and increases glomerular volume in adult offspring. Diabetes Metab Res Rev. 2016;32:816–26. doi: 10.1002/dmrr.2805. [DOI] [PubMed] [Google Scholar]
- 30.Amri K, Freund N. Adverse effects of hyperglycemia on kidney development in rats in vivo and in vitro studies. Di. 1999;48:2240–5. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- 31.Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20:551–5. doi: 10.1016/j.ejim.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Kairamkonda VR, Khashu M. Controversies in the management of hyperglycemia in the ELBW infant. Indian Pediatr. 2008;45 [PubMed] [Google Scholar]
- 33.Wilkins BH. Renal function in sick very low birthweight infants : 4. Glucose excretion. Arch Dis Child - Fetal Neonatal Ed. 1992;67:1162–5. doi: 10.1136/adc.67.10_spec_no.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983;71:467–75. doi: 10.1172/JCI110791. [DOI] [PMC free article] [PubMed] [Google Scholar]


