Abstract
Preventing morbidity and mortality from infectious disease through the development and use of effective vaccines is one of medicine’s greatest achievements and greatest frustrations. We are struggling with improving vaccine efficacy for some of the most globally widespread diseases, such as malaria and tuberculosis. In an effort to gain an edge, systems biology approaches have begun to be employed to more broadly investigate the pathways leading to protective vaccine responses. As such, we are now at a critical juncture, needing to evaluate how fruitful these approaches have been. Herein we discuss the level of success achieved as compared to the original promise of systems methodologies, and conclude that while we have indeed begun to make clear inroads into understanding the immune response to vaccines, we still have much to learn and gain from the more comprehensive approach of systems-level analysis.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
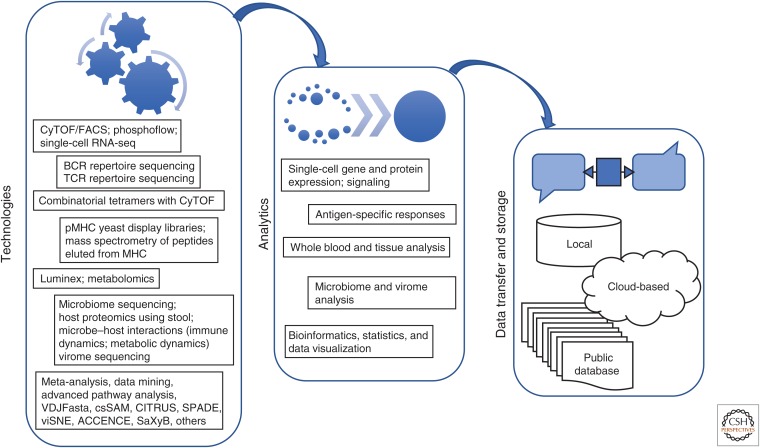
As frequently noted, vaccination is the most successful medical intervention of all time in terms of lives saved and serious illness prevented. But for decades we have been in an era in which vaccines against complex diseases such as HIV, adult tuberculosis (TB), malaria, and Dengue have failed, even though they have largely employed the same approach that was so successful for Pasteur and the generations of vaccinologists that followed him for many other infectious diseases. Even more modern innovations such as adjuvants and DNA vaccines have not proved decisive. Animal models, even primates such as macaques, have also proved unreliable in predicting the success of a candidate HIV vaccine in at-risk human populations. The only recourse then are large, multiyear efficacy studies that are hugely expensive and generally can only test one formulation at a time. Thus, since the first trial of an HIV vaccine 20+ years ago, only four different vaccines have been tested on human subjects, each at a cost of millions of dollars (Gray et al. 2016). Furthermore, such studies often leave vaccinologists without an understanding of why a vaccine has failed. It is to address this pressing need for an alternative approach to vaccine development that has given rise to systems methodologies to characterize vaccine responses, sometimes called “systems vaccinology” (Fig. 1) (Pulendran 2014; Hagan et al. 2015). Work in this area has sought to use the knowledge of modern immunology together with relatively inexpensive high-throughput assays to gain a deeper understanding of how established vaccines work by comparing some of the most successful (such as yellow fever virus vaccine) with some of the less successful such as influenza (Gaucher et al. 2008; Querec et al. 2009; Obermoser et al. 2013; Li et al. 2014). These studies have started to produce a wealth of data and insights into important aspects of vaccine responses, but have not yet produced a “silver bullet” that will be enabling for the most difficult diseases. They are, however, our best hope for the future of vaccine development, as well as generally giving us important new insights into human immunology (Davis 2008).
Figure 1.
The systems way. Systems vaccinology has taken advantage of a number of different high-throughput technologies that enable multiparameter interrogation of blood, tissue, and fecal specimens. Many of these technologies are already used regularly, whereas others, such as microbiome sequencing and receptor repertoire sequencing, are just beginning to provide additional dimensions to data collection. New algorithms are also bringing better analytic, visualization, and interpretive power to these data sets. These computational tools can be implemented on a wide variety of platforms both locally and through cloud-based computing, and a number of new and improved databases are being built to help provide more standardization and global accessibility to raw data. CyTOF, Cytometry by time-of-flight; FACS, fluorescence-activated cell sorting; BCR, B-cell receptor; TCR, T-cell receptor; pMHC, peptide–major histocompatibility complex.
TECHNOLOGIES
The immune system is composed of specialized cell types that communicate with each other and the tissues they inhabit with cytokines. Also important are signaling sequences that move normally quiescent lymphocytes to rapid proliferation, functional routines (killing, stimulating B cells to secrete antibodies, etc.), and then to a memory phase, which arms the system for a rapid and robust response upon reexposure. The goal of most vaccines is to generate these memory cells, or reinvigorate existing memory.4 In an attempt to conceptualize the complexity of multiple and simultaneous cellular interactions occurring at various time points after vaccination, it has become the purpose of “systems technologies” to provide a wide-angle lens capturing greater breadth of information in each snapshot. The technologies that are most commonly used in systems vaccine studies are shown in Table 1.
Table 1.
Influenza studies
| References | Cohort (n) | Vaccine | Technology | Concept |
|---|---|---|---|---|
| Bucasas et al. 2011 | Adult males (119), age 18–40 years, 2008 flu season | TIV | Microarray analysis on PBMCs D0, D1, D3, D14 | Response to vaccine is associated with common transcriptional signatures; early gene up-regulation of IFN-related genes and antigen processing and presentation Late gene up-regulation of proliferation and biosynthesis-related genes Gene signature correlates with magnitude of antibody response |
| Nakaya et al. 2011 | Adults (56) over 3 years, 2007–2009 | TIV versus LAIV | FACS analysis; microarray analysis on PBMCs D0, D3, D7 | Early TIV transcriptional response correlates with increase in antibody titer; B-cell signature LAIV produced a T-cell and monocyte signature; IFN signature; link between host innate immunity and antibody response Identified a kinase that may be involved with regulation of antibody responses |
| Furman et al. 2013 | Older adults (59); younger adults (30) | TIV | Microarry; CyTOF; Luminex on PBMCs from D0, D28 | Preexisting antibody repertoire against HA epitopes can predict response to vaccination Biomarkers for predicting antibody response include genes related to apoptosis |
| Bentebibel et al. 2013 | Adults (12), 2009; children (19), 2010; adults (37), 2011 | TIV, same B strain both years; different H3N2 and H1N1 | FACS analysis D0, D7 | Increased blood TFH 7 days after vaccination (ICOS+) provide help to memory B cells, and correlate with increased antibody titers in subjects with preexisting antibody titers Plasmablast number 7 days postvaccination does not correlate with antibody response in children |
| Furman et al. 2014 | Adult males (34); females (53) | TIV | Microarray, FACS on PBMCs from D0, D28 | Testosterone levels affect response to vaccination |
| Tsang et al. 2014 | Adults (63), 2009 | TIV and pH1N1 | Microarray, FACS analysis on PBMCs on D-7, D0, D1, D7, D70 | Prevaccination parameters can predict antibody response Used baseline cell subpopulation frequencies to predict response |
| Furman et al. 2015 | Y1: Young (30), older (61); Y2: young (25), older (52) | TIV | Micorarray, on PBMCs from D0, D28 | Chronic viral infection (CMV) enhances immune response after vaccination in young, but not older individuals |
| Nakaya et al. 2015 | Elderly (54), young (141), diabetic (17) over 5 years, 2007–2011; previous cohort (218) | TIV | Microarray, FACS analysis (2010 cohort); microRNA (miRNA) profiling (2010 cohort) on PBMCs on D0, D1, D3, D7, D14 | Prevaccination signatures correlate with antibody response to vaccination Antibody response correlates with age Early IFN signature Later ASC and cell-cycle signatures Decreased IFN response and enhanced NK and monocyte response in elderly miRNAs were up-regulated in elderly and correlated with antibody response |
| Sobolev et al. 2016 | Adults (178) 18–63 years old | Adjuvanted H1N1 | Microarray, FACS analysis, Luminex on PBMCs on D-7, D0, D1, D7 | Increased adverse effects after vaccination is associated with increased number of transitional B cells at baseline No common signature for nonresponders |
| Nakaya et al. 2016 | Children (90) 14–24 months old, 2012–2013 | TIV ATIV (MF59) | Microarray, FACS analysis on PBMCs from D0, D1, D3, D7, D28 | Adjuvanted vaccine provides more uniform and robust response across the entire cohort Adjuvant enhances kinetics of serum antibody titers and induction of multi-cytokine-producing CD4 T cells |
TIV, Trivalent-inactivated influenza vaccine; PBMC, peripheral blood mononuclear cell; IFN, interferon; LAIV, live attenuated influenza vaccine; FACS, fluorescence-activated cell sorting; CyTOF, cytometry by time-of-flight; HA, hemagglutinin; ASC, antibody secreting cell; NK, natural killer; ATIV, adjuvanted trivalent influenza vaccine.
TRANSCRIPTIONS
Using whole genome arrays or, in some cases, subset arrays, has been popular because they use well-established technologies and produce expression data on most of the estimated 23,000 genes in the human genome. Another advantage is that standard blood-collection tubes for this purpose can be used interchangeably with specialized containers, which immediately fix blood cells, leaving almost no time for changes that are likely occur in vitro, and which are later used for RNA extraction for array analysis or RNAseq. The problem with this technology is that it is quite noisy, and much effort needs to be spent deconvoluting the data with respect to the large variations in cell-subset frequency that are seen among individuals.
CYTOKINES
Cytokines related to immune function are generally measured in two distinct ways—by intracellular staining, where up to 14 different ones can be measured by mass cytometry or fluorescence-activated cell sorting (FACS), or by multiplex assays of serum cytokines using Luminex beads or other systems. The advantage of intracellular cytokine analysis is that one can tie particular functions to particular cells, as most subsets are defined by their cytokine expression. But the number that can be used together with other necessary markers is quite limited, five to six, typically. This is where mass cytometry is particularly useful. In particular, Newell et al. (2012) used stains for nine intracellular markers (eight cytokines and one secretory granule marker) to show that hundreds of different contributions could be expressed in human CD8+ T cells and in specific viral responses (Newell et al. 2012). Highly multiplexed assays for serum cytokines lack the cell specificity of intracellular staining, but provide coverage of a broader range of molecules and allow the products of epithelial cells to be accounted for. At Stanford, we recently surveyed 62 different cytokines at one time, using the Luminex system. But even more powerful systems are starting to be used—MSD Technology has an immunoassay platform that currently surveys ∼300 serum molecules, and SomaLogic claims to assay over 1300 proteins in their SOMAscan system. Whether these expansions represent too much of a good thing remains to be seen, but at least in the case of mass cytometry, the additional labels led to important insights and revealed complexities that were not known after decades of FACS analysis.
FUNCTIONAL ASSAYS
In vitro assays have been a mainstay of immunology for many years, but most are not high throughput. Peptide–major histocompatibility complex (MHC) tetramers and other multimer formats are now much more sensitive (to <0001% or so), compared to traditional ELISPOT assays (∼0.02%), which is in the low range of the naïve repertoire, and recent advances in combinatorial tetramer staining allow >100 specificities to be used simultaneously (Newell et al. 2013). Tetramers can also be combined with intracellular staining to link both specificity and function using FACS. Another approach to physiology was developed by Nolan and colleagues, which is known as Phosphoflow, where antibodies against phosphorylated forms of signaling molecules are used to interrogate the responses of immune cells (Krutzik et al. 2008). This has recently been used to measure chronic inflammation in elderly subjects (Shen-Orr et al. 2016) and in predicting which subjects would recover quickly or slowly following surgery.
CELL PHENOTYPING
Here, the classic technology is FACS, which has been the workhorse of immunology since it was developed by the Herzenbergs in the 1970s. Because of the overlap in emissions spectra in most cases, 12 or so colors is an operational limit, although some laboratories have achieved 16 or 18. Recent advances in the instrument and dyes by BD Biosciences report a 28-color capability, and it seems possible that this number could go higher. In 2009, a small Canadian company, DVS Sciences, developed an instrument that uses mass spectroscopy as a readout instead of fluorescence, and because this technology produces line spectra for the different metal ions used to make specific labels, many more labels can be used. Bendall et al. (2011) took advantage of this capability to demonstrate that ∼25 labels could be used simultaneously to label single cells, and this number has risen to 45–50 at present. A number of assays, including our own, have used this significantly broader array of labels to reexamine the diversity of particular lymphocyte populations; thus, human CD8+ T cells and NK cells have been discovered to subdivide into a variety of unique phenotypic subsets (Horowitz et al. 2013; Newell et al. 2013).
B- AND T-CELL REPERTOIRE ANALYSIS
The dramatic increases in nucleic acid sequencing technology throughput, and decreases in cost, have made it possible to sequence millions of immunoglobulins (Igs) or T-cell receptors (TCRs) that relate to a given response. Single-cell methods are complementary and allow antigen receptor pairs to be characterized from single T or B cells and then reconstructed in various ways to find the antigen (Birnbaum et al. 2014; Han et al. 2014; Tan et al. 2014). Although the data analysis methodology is somewhat limited at this time, we expect that in the near future “systems” approaches will incorporate TCR and Ig data into the overall picture of how individuals or cohorts respond to a vaccine and whether that is protective or not.
THE TEST CASE: FLU VACCINATION
The most intense efforts to use systems approaches have centered around influenza vaccinations, as this is the most common form of adult vaccination. New strains are a constant threat, especially to the very young or very old, and even greater threats lay in the probability of particularly deadly strains such as H5N1 (bird flu) becoming transmissible and causing severe illness or death. We also know very little about why the standard split virus vaccine is not very effective in elderly individuals, and much less than optimal even in healthy adults. The flu vaccine analysis thus far relies on transcriptional data, cytokine, cell phenotyping, and peptide-specific antibodies and has focused on two main objectives:
Characterizing the progression of the immune response in blood samples.
Trying to predict from prevaccination blood draws who will or who won’t respond effectively to the vaccine that is about to be administered.
To this end, we have learned a number of broad-stroke lessons from these initial study undertakings (see Table 1) with regard to vaccine responses being affected by age, sex, and prior exposure history. Transcriptional signatures early after vaccination have been found to correlate with antibody production at later time points, and different vaccine formulations have been observed to promote shifted antibody profiles. We have not identified the key pathway to a successful response, but we now have a greater appreciation for the dynamic range of the human immune system and the variability in the progression of an immune response for a given challenge. We have not yet achieved the promise of finding biomarkers that can serve as immune correlates or predictors of efficacy, but we now know that an individual’s prevaccination immune state can impact their responses postvaccination, and the potential for adverse effects. So we are scratching the surface and gaining some insight, but still have much to learn.
OTHER LESSONS
Vaccines for other diseases are also under intense study, such as yellow fever, Herpes Zoster, and meningitis, to learn whether there are common signatures of success among them. Studying the effects of these efficacious vaccines has helped us understand better how the innate immune response informs the adaptive response, such as through the triggering of “master switch” transcriptional programs that lead to neutralizing antibody responses after yellow fever vaccination (Gaucher et al. 2008; Querec et al. 2009). We have learned that inflammasome activation may be one of the key success factors for this live, attenuated vaccine and this is also a pathway important to the function of at least some adjuvants. Whereas there may be common signaling networks that promote protection, it is clear that there are likely multiple paths to success. Comparison of responses to vaccines with different formulations, such as polysaccharide-containing or conjugated meningococcal vaccines, shows they have different transcriptional signatures in the blood and exhibit antibody responses of diverse magnitudes and duration, yet both are effective at providing protection (Li et al. 2014). The response to the multivalent pneumococcal vaccine exhibits both shared and distinct molecular pathways when compared to the response to the trivalent flu vaccine, and which of these pathways is most important for vaccine potency will be the focus of future study (Obermoser et al. 2013). Ultimately, however, and likely the most complicating factor in understanding what it takes to invoke an effective vaccine response, is how the variability in response from host to host, may make these interventions more or less successful. For example, a longitudinal study of infants receiving bacille Calmette–Guérin (BCG) vaccination revealed a parceling of the cohort into two groups that exhibited distinct constellations of immune response genes postvaccination, and which correlated with susceptibility to TB disease within 2 years (Fletcher et al. 2016). Studies such as these suggest that we may need to implement different vaccine strategies for individuals with disparate immune response profiles, making vaccination another focus for personalized medicine.
ON THE HORIZON
Novel study design paired with evolving technologies will continue to provide fertile ground from which new insights may be reaped. Beyond the traditional clinical trial for a new vaccine, coming into the picture is a renewed interest in challenge studies. These studies employ controlled human infection models, where volunteers are deliberately infected with live pathogens, such as Plasmodium falciparum, Salmonella typhi, and Vibrio cholerae, that can be arrested with drugs before too much damage is done, or influenza where healthy nonvulnerable subjects can be studied (Killingley et al. 2011; Shirley and McArthur 2011; Seder et al. 2013; Darton et al. 2014). These types of studies are especially important for pathogens that are difficult to study because of a lack of analogous animal models for either infection or vaccination. Additionally, along with increasing the number of parameters measured using systems approaches, has come access to more sourcing points from which biological data can sampled and collected in real time. Wearables or implantables are bringing into view the prospect that devices that can continuously monitor aspects of a person’s immune physiology could be worn, with such things as glasses monitoring fluid in the eye or implantable detectors of cytokines that could collect valuable data uniquely. Devices like these will be important tools for understanding the kinetics of immune responses, the effect of host environment, and the impact of individual response variability.
CONCLUSIONS
With respect to the main question posed—has systems analysis delivered on its promise to improve vaccines that are currently not optimal or point the way to a new generation of vaccines that could be used against the most refractory diseases today? The answer must be “no.” But this is not because it is hopeless, but more that when this effort started, we were massively ignorant about what actually happens when even a common vaccine like flu are administered to people of different ages. But we are learning and data is coming in that is revealing important things regarding both what biomarkers predict a successful response and how that response is elaborated with time in different individuals. It was also necessary to develop (and continue developing) the most useful technologies to measure major aspects of the immune response and the analytical tools that are needed to parse the data. We are much better able to do this now than in 2008 and we will be much better in the next 5 years than we are now. A case in point is that we are just now applying these methods to parse the effects of adjuvants on flu responses and to derive meaning from our newly acquired ability to sequence massive numbers of TCRs and Igs. So progress is slow, but steady, and one could turn the question around and ask, because we know the immune system is complex and consists of many functional components, how could we not want to know how the whole system is responding to a given vaccine with or without an adjuvant?
Recently, it was found that adults already have a small population of specific T cells for pathogens (such as HIV) that they have never encountered although the precise role of these memory phenotype T cells is not known (Su et al. 2013).
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, et al. 2011. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. 2013. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 5: 176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW, Garcia KC. 2014. Deconstructing the peptide–MHC specificity of T cell recognition. Cell 157: 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Niño D, Arden N, Quarles JM, Couch RB, Belmont JW. 2011. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 203: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton TC, Blohmke CJ, Pollard AJ. 2014. Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol 30: 7–17. [DOI] [PubMed] [Google Scholar]
- Davis MM. 2008. A prescription for human immunology. Immunity 29: 835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, Matsumiya M, Tanner R, O’Shea MK, Dheenadhayalan V, et al. 2016. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 7: 11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H, Lund P, Maecker HT, et al. 2013. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 9: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci 111: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, et al. 2015. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 7: 281ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR III, Castro E, et al. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205: 3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GE, Laher F, Lazarus E, Ensoli B, Corey L. 2016. Approaches to preventative and therapeutic HIV vaccines. Curr Opin Virol 17: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan T, Nakaya HI, Subramaniam S, Pulendran B. 2015. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine 33: 5294–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Glanville J, Hansmann L, Davis MM. 2014. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol 32: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. 2013. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 5: 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingley B, Enstone J, Booy R, Hayward A, Oxford J, Ferguson N, Nguyen Van-Tam J. 2011. Potential role of human challenge studies for investigation of influenza transmission. Lancet Infect Dis 11: 879–886. [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Crane JM, Clutter MR, Nolan GP. 2008. High-content single-cell drug screening with phosphospecific flow cytometry. Nat Chem Biol 4: 132–142. [DOI] [PubMed] [Google Scholar]
- Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, et al. 2014. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 15: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. 2011. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 12: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, et al. 2015. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity 43: 1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, Patel NB, Zak DE, Aderem A, Dong T, et al. 2016. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci 113: 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Nair N, Kidd BA, Greenberg HB, Davis MM. 2013. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat Biotechnol 31: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, Thompson-Snipes L, Ranganathan R, Zeitner B, Bjork A, et al. 2013. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity 38: 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. 2014. Systems vaccinology: Probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci 111: 12300–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. [DOI] [PubMed] [Google Scholar]
- Shirley DA, McArthur MA. 2011. The utility of human challenge studies in vaccine development: Lessons learned from cholera. Vaccine (Auckl) 2011: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolev O, Binda E, O'Farrell S, Lorenc A, Pradines J, Huang Y, Duffner J, Schulz R, Cason J, Zambon M, et al. 2016. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat Immunol 17: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. 2013. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity 38: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YC, Blum LK, Kongpachith S, Ju CH, Cai X, Lindstrom TM, Sokolove J, Robinson WH. 2014. High-throughput sequencing of natively paired antibody chains provides evidence for original antigenic sin shaping the antibody response to influenza vaccination. Clin Immunol 151: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, et al. 2014. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell 157: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]