Abstract
Youth with autism spectrum disorder (ASD) experience high rates of psychiatric symptoms, but the relation of verbal ability with psychiatric symptoms is unknown. This study utilized a large sample of clinically referred inpatient and outpatient youth with ASD to compare psychiatric comorbidity between verbal and minimally-verbal (MV) youth, adjusting for nonverbal IQ, age, and ASD symptom severity. Results indicated that verbal youth were more likely to present with and meet clinical cutoffs for depression and oppositional defiant disorder symptoms, with greater impairment associated with depression. Youth in inpatient settings had greater symptom severity and impairment across almost all psychiatric comorbidities. These results present the most direct estimate to date of the association between verbal ability and psychiatric comorbidity in ASD.
Keywords: Autism spectrum disorder (ASD), Psychiatric comorbidity, Verbal ability, Autism Inpatient Collection, minimally verbal, Child and Adolescent Symptom Inventory
Numerous studies indicate that autism spectrum disorder (ASD), a disorder characterized in DSM-5 by social communication deficits and stereotyped behaviors and interests (American Psychiatric Association, 2013), is commonly associated with increased rates of comorbid psychiatric symptoms and diagnoses. In more than 50% of cases, ASD co-occurs with a wide range of psychiatric symptoms including anxiety and mood disorders as well as attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD; Croen et al. 2015; Gadow, DeVincent, Pomeroy, and Azizian 2005; Kaat, Gadow, and Lecavalier 2013; Salazar et al. 2015; Simonoff et al. 2008). Given the wide range of intellectual and verbal abilities in ASD (Fombonne, 2005), most studies of psychiatric comorbidity have opted to focus on sub-groups of those either with or without intellectual disability (ID) (e.g. Gadow, DeVincent, and Schneider 2008; Joshi et al. 2013; Mattila et al. 2010; Tsakanikos et al. 2006), or primarily on those with spoken language (Leyfer et al. 2006). Thus, an understanding of how psychiatric symptom presentation differs based on the individual’s specific cognitive or verbal ability remains limited, restricting the ability of clinicians to recognize these important and common sources of impairment.
Historically, questions have been raised about whether individuals with very low measured intellectual ability had the capacity to experience psychiatric comorbidities such as affective disorders (Magnuson and Constantino 2011). However, there is now a wealth of research documenting high rates of psychiatric comorbidities in those with severe ID (Einfeld, Ellis, and Emerson 2011). Nonetheless, the relatively few studies of individuals with ASD that utilized samples with a broad range of intellectual abilities suggest that individuals without ID have higher rates of nearly all psychiatric disorders (e.g, Gotham et al. 2013; Salazar et al. 2015; Sukhodolsky et al. 2008; Witwer and Lecavalier 2010) except ADHD, which has been found to be more prevalent in individuals with ID (Olsson et al. 2016).
The historical focus on ID as a defining characteristic of ASD has overshadowed the potential role of verbal ability in psychiatric comorbidity, despite the fact that approximately 30% of individuals with ASD are minimally verbal (MV) and being MV does not necessarily indicate the presence of ID (Tager-Flusberg et al., 2016). Indeed, accurate characterization and identification of the unique clinical and neurobiological profile of MV individuals with ASD remains a high research priority (Kasari et al., 2013; Hus Bal et al., 2016; Jack & Pelphrey, 2017). The extant literature focuses primarily on the complexities inherent in identification and assessment when an individual cannot adequately verbalize internal emotional states, thoughts, or experiences (King, de Lacy, & Siegel, 2014). Although this problem could lead to reduced total symptom counts, there are multiple other ways to consider psychiatric illnesses, including symptom severity, symptom-induced impairment, and their combination (i.e., traditional clinical cut-off), each of which appears to provide unique information about clinical presentation (Gadow, Kaat, & Lecavalier, 2013). There is an untapped opportunity to explore how these various illness parameters vary between verbal and MV youth with ASD, which may reveal differences in psychiatric presentation related to verbal ability beyond the obvious assessment challenges.
There are multiple ways verbal ability could influence psychiatric symptom presentation. For example, verbally-mediated cognitive distortions have played a central role in models of depression and anxiety and related treatment-development efforts (e.g., March et al. 2004). Other disorders, such as ODD, are characterized in part by the inappropriate use of language, such as being verbally manipulative, swearing, or angry rants. Thus, limited verbal ability may be protective against meeting clinical criteria for specific disorders. Conversely, MV youth may experience greater psychiatric disorder-related impairment given their reduced ability to engage in verbally-mediated coping strategies and interventions, such as talking to others when upset or using joint problem-solving (Mazefsky & White 2014). Research outside of the ASD field has also found that impaired language abilities can interact with ADHD symptoms, above and beyond cognitive ability, to influence factors such as academic achievement and engagement with treatment (Cohen et al. 2000), which further supports the possibility of potentially greater impairment in MV youth.
The limited body of research that has considered the potential role of verbal ability in psychiatric symptom presentation in ASD has predominantly focused on anxiety, indicating that better functional communication is associated with higher levels of anxiety (Davis et al. 2011; Kerns et al. 2015; Sukhodolsky et al. 2008; Witwer and Lecavalier 2010). For the most part, the higher rates in more verbal youth were attributed to certain symptoms emphasizing verbal responses (e.g., Witwer & Lecavalier, 2010), though it is also possible that being more verbal exposes one to situations that might lead to anxiety (e.g., trying to start a conversation with an unfamiliar peer; Kerns et al., 2015). Witwer and Lecavalier (2010) also found that verbal children were significantly more likely to be diagnosed with ODD, whose diagnostic criteria include verbally-dependent symptoms. Only one study thus far indicates more psychiatric symptoms in those who are less verbal; specifically Hallett et al. (2013b) found that those with greater communication impairment had more social anxiety. Additional work has sought to taxonomize the behavioral symptoms that may serve as equivalent proxies for some psychiatric symptoms among individuals with ID (Fletcher, Loschen, Stavrakaki, 2007), though these have not tended to focus specifically on ASD, nor have they aimed to examine whether patterns of existing symptom sets (i.e., extant diagnostic entities) appear differentially across levels of verbal ability in this population. Indeed, no known prior studies have investigated the impact of verbal ability on psychiatric symptomatology in ASD adjusting for IQ and age.
The objective of this study was to investigate the association of verbal ability with psychiatric symptoms in ASD adjusting for nonverbal IQ, age and ASD symptom severity. Multi-site sampling from clinically-referred inpatient and outpatient ASD populations was utilized to increase the representativeness of the sample in terms of both psychiatric symptom severity and verbal ability. This also supported a subsidiary goal of ascertaining differences in psychiatric symptoms between inpatient and outpatient settings. Given conceptual and clinical distinctions between various illness parameters (Gadow et al. 2013), we considered symptom count, symptom severity, symptom-induced impairment, and clinical cut-offs, separately, for each targeted psychiatric disorder. We hypothesized that MV youth would have lower symptom counts than verbal youth owing to difficulties with expression and therefore challenges for parents in detecting some symptoms, but nevertheless show evidence of significant psychopathology. Compared to verbal youth, we expected MV youth to have greater symptom-induced impairment. Although the prior research is limited and mixed, we hypothesized that verbal participants would be more affected by anxiety and mood disorders and ODD, and ADHD would be more common among MV participants. Analyses for other disorders with extremely limited research in this area (e.g., schizophrenia) were considered exploratory. Because inpatient placement is generally associated with more severe ASD and psychiatric symptoms, we hypothesized that inpatients would have more severe depression and ODD (Mandell 2008; Siegel et al. 2012).
Method
Participants were from two sources, the Autism Inpatient Collection (AIC; n = 271) and a university developmental disabilities outpatient clinic (DDC) located on Long Island, NY (n = 162). The AIC is a six-site study of youth with ASD admitted to specialized psychiatric hospital units that treat ASD and other neurodevelopmental disorders. The full methods of the AIC have been published previously (Siegel et al. 2015). Briefly, patients between the ages of 4–20 years old with a score of ≥12 on the Social Communication Questionnaire (SCQ; Rutter et al. 2003), or high suspicion of ASD from the inpatient clinical treatment team, were eligible for enrollment. Enrollment was completed within ten days of admission to the hospital. Inclusion in the AIC dataset required confirmation of ASD diagnosis by research-reliable administration of the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al. 2012), administered during the inpatient stay when the patient was most stable. Diagnosis was further informed by historical information, SCQ, and observation over time. Exclusion criteria were not having a parent available who was proficient in English or the individual with ASD being a prisoner.
Case records for consecutive referrals to the DDC were screened for youth who were between 6 and 18 years old at time of evaluation and had the prerequisite assessment instruments. All youth met DSM-IV criteria for ASD (or, in DSM-IV terms, any of the pervasive developmental disorders), and represented the wide range of outpatient treatment-seeking youth. ASD diagnoses were confirmed by an expert diagnostician and based on the following sources of information: (a) comprehensive developmental history, (b) clinician interview with youth and caregiver(s), (c) direct observations of the youth, (d) review of validated ASD rating scales including the Child and Adolescent Symptom Inventory (CASI; Gadow, 2015) for current ASD symptoms and the SCQ for lifetime symptoms, (e) prior school and clinician ASD evaluations, and (f) the ADOS (Lord et al. 2000) administered by a certified, site-reliable examiner. In the present study, ADOS-2 algorithms were used to generate scores. The sample and procedures are described in greater detail in prior publications (Gadow, Perlman, Ramdhany, and de Ruiter 2016; Kaat et al. 2013). Both studies were approved by a university Institutional Review Board.
The demographic characteristics of participants are provided in Table 1. The combined sample comprised participants with a mean age of approximately 11 years who were mostly Caucasian males, with low-average to borderline nonverbal IQ, and moderate contemporary (but high historical) symptoms of ASD.
Table 1.
Sample Demographics Overall and in Comparison Between Minimally Verbal versus Verbal Youth with ASD
| Variable | Total sample (n=433) | MV (n=165) | V (n=268) | Analyses comparing MV & V samples | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| M | SD | M | SD | M | SD | t | p | Cohen’s d | |
| Age | 11.93 | 3.53 | 11.99 | 3.88 | 11.89 | 3.31 | 0.28 | .779 | 0.03 |
| ^ | 12.11 | 3.77 | 11.87 | 3.45 | |||||
| IQ, nonverbal | 79.20 | 28.95 | 53.48 | 19.72 | 94.67 | 21.71 | −15.59 | <.001 | −1.99 |
| ^ | 50.26 | 17.14 | 89.18 | 25.25 | |||||
| Income | 2.85 | 1.07 | 2.75 | 1.15 | 2.92 | 1.01 | −1.49 | .137 | −0.16 |
| Father Ed | 4.31 | 1.19 | 4.23 | 1.15 | 4.36 | 1.22 | −1.00 | .316 | −0.11 |
| Mother Ed | 4.16 | 1.18 | 4.08 | 1.09 | 4.20 | 1.23 | −1.00 | .316 | −0.10 |
| ADOS-2 CSS | 6.93 | 2.41 | 7.72 | 1.86 | 6.45 | 2.58 | 5.12 | <.001 | 0.57 |
| SCQ total | 20.19 | 8.21 | 24.82 | 7.04 | 17.29 | 7.54 | 10.04 | <.001 | 1.03 |
|
| |||||||||
| n | % | n | % | n | % | χ2 | p | OR | |
|
| |||||||||
| Gender (Male) | 347 | 80.1 | 128 | 77.6 | 219 | 81.7 | 1.10 | .294 | 0.77 |
| Informant (Mother) | 345 | 82.1 | 126 | 78.8 | 219 | 84.2 | 6.47 | .039 | 0.69 |
| Hispanic | 25 | 5.9 | 9 | 5.7 | 16 | 6.1 | 0.03 | .858 | 0.93 |
| African American | 39 | 9.0 | 22 | 13.3 | 17 | 6.4 | 6.03 | .014 | 2.26 |
| Asian | 16 | 3.7 | 5 | 3.0 | 11 | 4.1 | 0.34 | .560 | 0.73 |
| White | 359 | 83.1 | 127 | 77.0 | 232 | 86.9 | 7.15 | .008 | 0.50 |
Note. ASD = Autism Spectrum Disorder. MV = Minimally-verbal. V = Verbal. IQ = Intelligence quotient. Income: 1 = Less than $20,000; 2 = $20,000 to $70,000; 3 = $40,000 to $70,000; 4 = Over $70,000. Father/Mother Ed = Father/Mother’s highest level of education: 1 = Less than 8th grade; 2 = Some high school; 3 = Finished high school (or equivalent); 4 = Some college or AA degree; 5 = Bachelor’s degree (BA, BS); 6 = Post-graduate degree. ADOS-2 CSS = Autism Diagnostic Observation Schedule, Calibrated Severity Score. SCQ = Social Communication Questionnaire. Minimally-verbal participants exhibited lower nonverbal/performance IQ and higher ADOS CSS and SCQ scores than verbal participants. They were also less likely to have their mother be the informant on the extant measures, more likely to be African American, and less likely to be White than verbal participants.
values for the comparison in which MV is defined as requiring ADOS-2 Module 1 only.
Age range: 4 – 20 for MV and 5 – 18 for V groups; 4 – 20 for MV and 5 – 19 for V groups using the ADOS-2 Module 1 only cutoff.
Nonverbal IQ range: 30 – 123 for MV and 33 – 145 for V groups; 30 – 99 for MV and 31 – 145 for V groups using the ADOS-2 Module 1 only cutoff.
Participants were classified into verbal ability groups based on their required ADOS-2 module in accordance with ADOS-2 guidelines. Briefly, ADOS-2 Module 1 is intended for children and adolescents who do not consistently use phrase speech (e.g., with a language level of three-word phrases or less); Module 2 is for those who use phrase speech but are not fluent; and Modules 3 and 4 are for fluently verbal children/adolescents and adolescents/adults respectively. It has been advocated that the ADOS-2 is a useful direct assessment to aid identification of minimally-verbal subgroups (Kasari et al., 2013; Hus Bal et al., 2016; Jack & Pelphrey, 2017). Participants were considered MV if they required Module 1 or 2 (38%, n = 165) or verbal if they required Module 3 or 4 (62%, n = 268). There were significantly more MV participants in the AIC versus DDC sample (AIC: 48%, DDC: 21.6%; χ2 = 29.88, p < .001). As current practices for defining MV groups are not yet well-established, all analyses comparing MV and verbal participants were re-run in two ways: 1) by including only those requiring Module 1 as MV – a more stringent criterion that excludes those with appreciable phrase speech (Hus Bal et al., 2016), and 2) by excluding those individuals who were 4 or 5 years old (i.e., those for whom requirement of Module 2 could reasonably be argued would be close to a developmentally normative range of functioning). As the latter of these two approaches yielded results that were fully subsumed within the other two approaches (i.e., use of Module 1 and 2, or use of Module 1 only), they are not reported independently here.
The primary source of data was the CASI-4 and -5 (Gadow & Sprafkin 2005; 2013), which was completed by caregivers prior to initial outpatient evaluation (DDC) or during the inpatient admission (AIC). The symptom items in both versions of the CASI were identical for the disorders discussed in this paper: ADHD-inattentive (I), ADHD-hyperactive-impulsive (HI), ADHD-combined, ODD, generalized anxiety disorder (GAD), social anxiety disorder (SocAnx), separation anxiety disorder (SAD), major depressive episode (MDE), dysthymia, manic episode (ME) and schizophrenia. Individual items bear one-to-one correspondence with DSM symptoms, and are rated 0 (never), 1 (sometimes), 2 (often), and 3 (very often). On average participants in the DDC had significantly more missing CASI items than the AIC (M = 2.52, SD = 6.04 vs. M = 0.96, SD = 5.09; t = 2.88, p = .004). Seventy-eight percent of total participants had zero missing items. All analyses were conducted with listwise deletion, wherein participants were excluded from comparisons of a given disorder if insufficient items were present to calculate a score; the greatest number of cases excluded by this method was six for ME.
CASI scoring algorithms were applied to generate several illness parameters, four of which are reported in the present study: Symptom Severity is the sum of all item scores from a specific subscale. Symptom Count is the number of symptoms rated “often” or “very often”. To receive a Symptom Count Cutoff score, a youth must evidence the prerequisite number of symptoms specified in the DSM. For each subscale, informants are asked whether symptoms interfere with social or academic functioning (i.e., impairment), which is also rated from 0 (never) to 3 (very often). To meet the Impairment Cutoff, a youth must have a frequency rating of “often” or “very often” for the symptom-induced impairment item. Clinical Cutoff is a combination of Symptom Count Cutoff and Impairment Cutoff. Numerous studies indicate CASI subscales demonstrate satisfactory psychometric properties among typically developing and clinic-referred youth, including individuals with ASD and a wide range of verbal ability (reviewed by Gadow, 2015).
Nonverbal intellectual quotients (NVIQ) were based on the Leiter International Performance Scale – Third Edition (Leiter-3; Roid, Miller, Pomplun, & Koch, 2013) for the AIC sample (administered during the stay), or by the Wechsler (1999), Stanford-Binet (Thorndike, Hagen, and Sattler 1986), or Bayley (1993) tests for the DDC sample.
Data Analytic Plan
First, t- and χ2 tests were used to examine any demographic differences between MV and verbal participants. Next, ANOVA (for the continuous CASI variables of Symptom Severity and Symptom Count) and χ2 (for categorical CASI Impairment Cutoff and Clinical Cutoff) analyses were run to compare psychiatric comorbidity between MV and verbal participants. Finally, ANCOVA models and logistic regression models were used to examine whether relations found in the simple ANCOVA and χ2 models were robust to relevant covariates. First, given the use of different settings for data collection, site was utilized as a covariate (inpatient, which included the six inpatient facilities, versus outpatient). Second, given that verbal ability is often confounded with verbal IQ, NVIQ was used as a covariate to allow for a purer estimate of whether verbal ability per se – rather than broader cognitive functioning – contributes to comorbidity differences; thus, models were re-run with this covariate added. Third, given prior research supporting age effects for certain disorders (e.g., Gotham et al. 2013; Lecavalier 2006; White, Oswald, Ollendick, and Scahill 2009), age was added as a covariate. Fourth, ASD symptom severity, based on ADOS-2 Calibrated Severity Scores (ADOS CSS), was added as a covariate so that we could ensure that the effects were not an artifact of overall ASD severity. Finally, these procedures were repeated, but comparing the DDC and AIC samples rather than MV and verbal youth. Additionally, significant effects were probed by using the alternate MV thresholds mentioned above, as well as by covarying any unanticipated demographic differences.
Results
MV participants had lower NVIQ (t = −15.59, p < .001, d = −1.99) and higher SCQ (t = 10.04, p < .001, d = 1.03) and ADOS CSS (t = 5.12, p < .001, d = 0.57) compared to verbal participants. They were also less likely to have their mothers complete the CASI (χ2 = 6.47, p = .039, OR = 0.69), more likely to be African-American (χ2 = 6.03, p = .014, OR = 2.26), and less likely to be Caucasian (χ2 = 7.15, p = .008, OR = 0.50). Using the more stringent MV threshold, these sample differences were consistent for NVIQ (t = −11.89, p < .001, d = −1.80), SCQ (t = 9.79, p < .001, d = 1.16), ADOS CSS (t = 2.94, p = .003, d = 0.39), and African-American racial identification (χ2 = 4.73, p = .030, OR = 2.09). No significant differences were observed using the more stringent MV cutoff for informant and Caucasian racial identification (both p > .070). As such, after age, NVIQ, and ASD symptom severity as indexed by ADOS CSS were covaried per study objectives, African-American race was an added control in post-hoc analyses.
Table 2 summarizes the results comparing MV and verbal youth for each major disorder broken down by illness parameter. In general, the patterns were mostly similar across CASI Symptom Severity, Symptom Count, and Clinical Cut-off. Figure 1 summarizes the significant differences that remained after accounting for covariates across illness parameters.
Table 2.
Psychiatric Comorbidities in Minimally-Verbal versus Verbal Youth with ASD
| Disorder | Total (n=433) | MV (n=165) | V (n =268) | Analyses comparing MV & V samples | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Severity | M | SD | M | SD | M | SD | B | p | partial η2 (95% CI) |
| ADHD-I | 17.37 | 5.95 | 17.48 | 5.89 | 17.30 | 6.00 | 0.18 | .766 | .000 (−1.0–1.3) |
| ADHD-HI | 13.99 | 6.72 | 14.92 | 5.62 | 13.41 | 7.27 | 1.50 | .024 | .012 (0.2–2.8) |
| ADHD-Co | 31.36 | 11.13 | 32.39 | 9.89 | 30.72 | 11.81 | 1.68 | .128 | .005 (−0.5–3.8) |
| ODD | 12.02 | 6.64 | 9.68 | 5.22 | 13.48 | 7.01 | −3.80 | <.001a, b, c | .077 (−5.0–−2.6) |
| GAD | 10.15 | 5.02 | 9.54 | 4.26 | 10.52 | 5.40 | −0.99 | .047a, c | .009 (−2.0–−0.0) |
| SocAnx | 2.39 | 2.12 | 2.33 | 2.02 | 2.43 | 2.18 | −0.10 | .637 | .001 (−0.5–0.3) |
| SAD | 3.72 | 4.47 | 3.30 | 3.74 | 3.97 | 4.84 | −0.68 | .128 | .005 (−1.6–0.2) |
| MDE | 7.84 | 5.58 | 6.89 | 4.41 | 8.42 | 6.12 | −1.53 | .006a, c | .018 (−2.6–−0.5) |
| Dysthymia | 6.68 | 4.78 | 5.34 | 3.38 | 7.51 | 5.30 | −2.17 | <.001a, c | .049 (−3.1–−1.3) |
| ME | 7.23 | 6.09 | 7.34 | 5.42 | 7.17 | 6.47 | 0.17 | .785 | .000 (−1.0–1.4) |
| Schizophr | 3.60 | 3.35 | 3.58 | 3.02 | 3.61 | 3.55 | −0.04 | .916 | .000 (−0.7–0.6) |
|
| |||||||||
| Symptom count | M | SD | M | SD | M | SD | B | p | partial η2 (95% CI) |
|
| |||||||||
| ADHD-I | 5.91 | 2.80 | 5.90 | 2.75 | 5.91 | 2.84 | −0.01 | .980 | .000 (−0.5–0.5) |
| ADHD-HI | 4.56 | 2.82 | 5.00 | 2.47 | 4.30 | 3.00 | 0.70 | .012 | .015 (0.2 – 1.3) |
| ADHD-Co | 10.47 | 4.91 | 10.90 | 4.54 | 10.21 | 5.12 | 0.70 | .152 | .005 (−0.3–1.7) |
| ODD | 3.90 | 2.86 | 3.02 | 2.26 | 4.45 | 3.05 | −1.43 | <.001a, b, c | .059 (−2.0–−0.9) |
| GAD | 3.15 | 2.16 | 2.93 | 1.84 | 3.29 | 2.32 | −0.36 | .095 | .006 (−0.8–0.1) |
| SocAnx | 0.67 | 0.83 | 0.63 | 0.79 | 0.69 | 0.85 | −0.06 | .487 | .001 (−0.2–0.1) |
| SAD | 0.99 | 1.59 | 0.85 | 1.36 | 1.07 | 1.71 | −0.23 | .148 | .005 (−0.5–0.1) |
| MDE | 2.13 | 2.22 | 1.76 | 1.77 | 2.36 | 2.42 | −0.59 | .007a, c | .017 (−1.0–−0.2) |
| Dysthymia | 1.90 | 1.94 | 1.37 | 1.34 | 2.23 | 2.17 | −0.86 | <.001a, c | .046 (−1.2–−0.5) |
| ME | 2.05 | 2.37 | 2.01 | 2.24 | 2.07 | 2.45 | −0.07 | .783 | .000 (−0.5–0.4) |
| Schizophr | 1.00 | 1.30 | 1.09 | 1.26 | 0.95 | 1.32 | 0.14 | .263 | .003 (−0.1–0.4) |
|
| |||||||||
| Impairment | n | % | n | % | n | % | χ2 | p | OR (95% CI) |
|
| |||||||||
| ADHD | 311 | 77.8 | 127 | 81.4 | 184 | 75.4 | 1.98 | .159 | 1.43 (0.9–2.3) |
| ODD | 251 | 63.4 | 89 | 58.6 | 162 | 66.4 | 2.48 | .115 | 0.72 (0.5–1.1) |
| GAD | 208 | 52.8 | 84 | 54.9 | 124 | 51.5 | 0.45 | .504 | 1.15 (0.8–1.7) |
| SocAnx | 133 | 33.6 | 51 | 34.0 | 82 | 33.3 | 0.02 | .892 | 1.03 (0.7–1.6) |
| SAD | 60 | 15.0 | 23 | 14.9 | 37 | 15.0 | 0.00 | .990 | 0.99 (0.6–1.8) |
| MDE/Dys | 98 | 24.6 | 15 | 9.9 | 83 | 33.6 | 28.29 | <.001a, b, c | 0.22 (0.1–0.4) |
| ME | 149 | 37.6 | 55 | 36.7 | 94 | 38.2 | 0.10 | .758 | 0.94 (0.6–1.4) |
| Schizophr | 127 | 31.9 | 54 | 35.5 | 73 | 29.7 | 1.48 | .224 | 1.31 (0.8–2.0) |
|
| |||||||||
| Clinical cutoff | n | % | n | % | n | % | χ2 | p | OR (95% CI) |
|
| |||||||||
| ADHD-I | 239 | 59.8 | 94 | 60.3 | 145 | 59.4 | 0.03 | .869 | 1.03 (0.7–1.6) |
| ADHD-HI | 169 | 42.3 | 78 | 50.0 | 91 | 37.3 | 6.30 | .012 | 1.68 (1.1–2.5) |
| ADHD-Co | 146 | 36.5 | 66 | 42.3 | 80 | 32.8 | 3.72 | .054 | 1.50 (1.0–2.3) |
| ODD | 205 | 51.8 | 60 | 39.5 | 145 | 59.4 | 14.93 | <.001a, b, c | 0.45 (0.3–0.7) |
| GAD | 98 | 24.9 | 25 | 16.3 | 73 | 30.3 | 9.75 | .002a, c | 0.45 (0.3–0.7) |
| SocAnx | 56 | 14.1 | 18 | 12.0 | 38 | 15.4 | 0.91 | .340 | 0.75 (0.4–1.4) |
| SAD | 42 | 10.5 | 12 | 7.8 | 30 | 12.1 | 1.92 | .166 | 0.61 (0.3–1.2) |
| MDE | 19 | 4.8 | 0 | 0.0 | 19 | 7.7 | 12.20 | <.001 | 0.00 (N/A) |
| Dysthymia | 61 | 15.3 | 7 | 4.6 | 54 | 21.9 | 21.43 | <.001a, c | 0.17 (0.1–0.4) |
| ME | 71 | 17.9 | 25 | 16.7 | 46 | 18.7 | 0.26 | .609 | 0.87 (0.5–1.5) |
| Schizophr | 20 | 5.0 | 5 | 3.3 | 15 | 6.1 | 1.55 | .213 | 0.52 (0.2–1.5) |
Note. ASD = Autism Spectrum Disorder. MV = Minimally-verbal. V = Verbal. IQ = Intelligence quotient. ADOS-2 CSS = Autism Diagnostic Observation Schedule, Calibrated Severity Score. ADHD = Attention Deficit/Hyperactivity Disorder. ADHD-I = ADHD – Inattentive Type. ADHD-HI = ADHD-Hyperactive/Impulsive type. ADHD-Co = ADHD – Combined. ODD = Oppositional Defiant Disorder. GAD = Generalized Anxiety Disorder. SAD = Separation Anxiety Disorder. MDE = Major Depressive Disorder. ME = Manic Episode. Schizophr = Schizophrenia.
Significant at p < .05 when controlling for clinic (inpatient vs. outpatient) group, nonverbal IQ, age, and ADOS CSS.
Among those variables meeting criteria for a, also significant at p < .05 when using ADOS Module 1 only as the threshold for MV.
Among those variables meeting criteria for a, also significant at p < .05 after controlling for differences in African-American racial distribution across groups.
Figure 1. Summary of Group Differences by Illness Parameter.
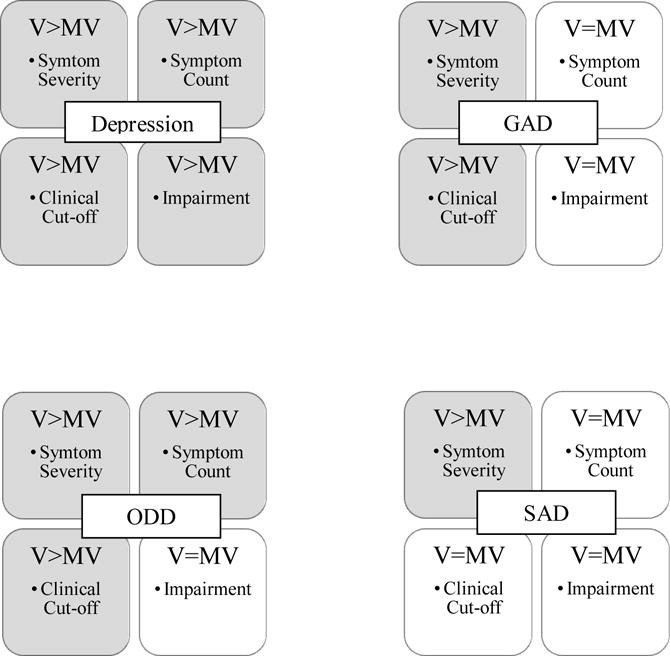
Note. MV = Minimally-verbal. V = Verbal. ODD = Oppositional Defiant Disorder. GAD = Generalized Anxiety Disorder. SAD = Separation Anxiety Disorder. Depression represents consistent effects for Major Depressive Episode and Dysthymia. Summarized effects are only those with raw score significant differences that remained significant after controlling for clinic group, nonverbal IQ, age, and ADOS calibrated severity score. Disorders without significant differences after controls are not included.
In terms of CASI symptom severity, MV participants exhibited more severe symptoms of ADHD-HI (B = 1.50, p = .024, partial η2 = .012), but less severe symptoms of ODD (B = −3.80, p < .001, partial η2 = .077), GAD (B = −0.99, p = .047, partial η2 = .009), MDE (B = −1.53, p = .006, partial η2 = .018), and Dysthymia (B = −2.17, p <.001, partial η2 = .049). Of these, the effects sizes for ODD, GAD, MDE, and Dysthymia were small (Cohen 1992), but robust to all covariates (age, NVIQ, ADOS CSS, race; ODD: B = −6.64, p < .001, partial η2 = .161; GAD: B = −2.23, p = .017, partial η2 = .026; MDE: B = −2.48, p = .012, partial η2 = .028; Dysthymia: B = −2.47, p = .004, partial η2 = .038). However, with the more stringent MV threshold, differences in symptom severity were non-significant for GAD (p = .812) and MDE (p = .313). While ANOVAs with this lower MV cutoff consistently revealed more severe ADHD-HI (B = 1.75, p = .017, partial η2 = .013) and less severe Dysthymia (B = −1.31, p = .012, partial η2 = .015) symptoms, these effects were not robust to covariates. Finally, ODD symptom severity remained significantly lower for MV participants using the more stringent ADOS-2 cutoff for MV (B = −1.31, p = .012, partial η2 = .015), and was robust to covariates (B = −4.96, p < .001, partial η2 = .089).
With regard to symptom count, MV participants exhibited more symptoms of ADHD-HI (B = 0.70, p = .012, partial η2 = .015), but fewer symptoms of ODD (B = −1.43, p < .001, partial η2 = .059), MDE (B = −0.59, p = .007, partial η2 = .017), and Dysthymia (B = −0.86, p < .001, partial η2 = .046) relative to verbal participants. Of these, the effects sizes for ODD, MDE, and Dysthymia were small, but robust to all covariates (age, NVIQ, ADOS CSS, race) (ODD: B = −2.67, p < .001, partial η2 = .139; MDE: B = −0.86, p = .041, partial η2 = .019; Dysthymia: B = −1.08, p = .003, partial η2 = .039). Using the more stringent MV threshold, similar differences were observed in the ANOVAs for ADHD-HI (B = 0.82, p = .008, partial η2 = .016), ODD (B = −1.18, p < .001, partial η2 = .033), and Dysthymia (B = −0.49, p = .021, partial η2 = .012), but not MDE (p = .353). Finally, only ODD symptom differences were robust to covariates with the lower MV cutoff (B = −2.05, p < .001, partial η2 = .081).
In contrast, for nearly all disorders, the groups did not differ for CASI Impairment Cutoff. The exception was that the MV participants were less likely to be rated as impaired by symptoms of MDE/Dysthymia (χ2 = 28.29, p < .001, OR = 0.22), and this effect was medium (for ORs <1, effect size conventions are calculated as 1/the threshold guidelines indicated by Chen, Cohen, & Chen, 2010), robust to covariates (p = .002, OR = 0.18), and remained the case using the more stringent ADOS-2 cutoff for MV (p = .026, OR = 0.25), as well as after controlling for race (p = .002, OR = 0.16).
Finally, the MV participants were more likely to reach CASI Clinical Cutoff for a diagnosis of comorbid ADHD-HI (χ2 = 6.30, p = .012, OR = 1.68), but less likely to reach Clinical Cutoffs for ODD (χ2 = 14.93, p < .001, OR = 0.45), GAD (χ2 = 9.75, p = .002, OR = 0.45), MDE (χ2 = 12.20, p < .001, OR = 0.04), and Dysthymia (χ2 = 21.43, p < .001, OR = 0.17). These differences were robust to all covariates (age, NVIQ, ADOS CSS, race) for ODD, GAD, and Dysthymia (ODD: p < .001, OR = 0.15; GAD: p = .024, OR = 0.36; Dysthymia: p = .025, OR = 0.17), and effect sizes were small, small, and medium, respectively. Using the more stringent MV cutoff, chi-squared tests were significant for ADHD-HI (χ2 = 7.85, p = .005, OR = 1.89), ODD (χ2 = 6.69, p = .010, OR = 0.55), MDE (χ2 = 6.97, p = .008, OR = 0.00), and Dysthymia (χ2 = 9.67, p = .002, OR = 0.27), but not GAD (p = .053). However, only ODD using the lower ADOS-2 cutoff for MV was robust to covariates (p = .002, OR = 0.27; all other p > .083).
Inpatient versus Outpatient
Table 3 summarizes differences between the inpatient and outpatient samples for each illness parameter. AIC participants showed significantly higher symptom severity than DDC participants for every domain (all p < .001) except social anxiety, and all comparisons were robust to controls (as well as use of the more stringent ADOS-2 cutoff for MV and added race control) and had small to medium effect sizes (partial η2: .027–.139). AIC participants showed significantly higher symptom counts (all p < .001) in these domains as well (also with small to medium effects; partial η2: .027–.165), though the effects for MDE, dysthymia, and schizophrenia did not remain after all controls. AIC participants also showed higher rates of impairment in terms of ADHD, ODD, GAD, separation anxiety, mania, and schizophrenia symptoms (all p ≤ .001), with all comparisons except schizophrenia robust to controls, evincing small to medium effects (OR: 0.15–0.37). AIC participants were also more likely to exceed clinical cutoffs in terms of ADHD (all subtypes; p ≤ .002), ODD (p < .001), GAD (p = .001), separation anxiety (p = .040), and manic episodes (p < .001), evincing small to medium effects (OR: 0.23–0.52); only comparisons for ADHD and ODD were robust to all controls.
Table 3.
Psychiatric Comorbidities in Outpatient (DDCLI) versus Inpatient (AIC) Youth with ASD
| Disorder | Total (n=433) | Outpatient (n=162) | Inpatient (n =271) | Analyses comparing clinic groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Severity | M | SD | M | SD | M | SD | B | p | partial η2 (95% CI) | |
| ADHD-I | 17.37 | 5.95 | 16.11 | 6.51 | 18.12 | 5.47 | −2.02 | .001a, b, c | .027 (−3.2–−0.9) | |
| ADHD-HI | 13.99 | 6.72 | 11.55 | 7.01 | 15.44 | 6.11 | −3.89 | <.001a, b, c | .078 (−5.2–−2.6) | |
| ADHD-Co | 31.36 | 11.13 | 27.65 | 11.92 | 33.56 | 10.03 | −5.91 | <.001 a, b, c | .066 (−8.0–−3.8) | |
| ODD | 12.02 | 6.64 | 8.69 | 6.05 | 14.05 | 6.16 | −5.36 | <.001 a, b, c | .154 (−6.6–−4.2) | |
| GAD | 10.15 | 5.02 | 8.24 | 5.27 | 11.29 | 4.50 | −3.04 | <.001 a, b, c | .086 (−4.0–−2.1) | |
| SocAnx | 2.39 | 2.12 | 2.56 | 2.24 | 2.30 | 2.04 | 0.27 | .211 | .004 (−0.2–0.7) | |
| SAD | 3.72 | 4.47 | 2.60 | 3.87 | 4.37 | 4.66 | −1.77 | <.001 a, b, c | .037 (−2.6–−0.9) | |
| MDE | 7.84 | 5.58 | 5.26 | 5.53 | 9.40 | 5.00 | −4.14 | <.001 a, b, c | .130 (−5.2–−3.1) | |
| Dysthymia | 6.68 | 4.78 | 4.94 | 4.99 | 7.74 | 4.32 | −2.79 | <.001 a, b, c | .080 (−3.7–−1.9) | |
| ME | 7.23 | 6.09 | 4.31 | 5.09 | 8.99 | 5.98 | −4.68 | <.001 a, b, c | .139 (−5.8–−3.6) | |
| Schizophr | 3.60 | 3.35 | 2.59 | 2.87 | 4.19 | 3.48 | −1.60 | <.001a, b, c | .053 (−2.2–−1.0) | |
|
| ||||||||||
| Symptom count | M | SD | M | SD | M | SD | B | p | partial η2 (95% CI) | |
|
| ||||||||||
| ADHD-I | 5.91 | 2.80 | 5.24 | 3.08 | 6.31 | 2.55 | −1.07 | <.001a, b, c | .034 (−1.6–−0.5) | |
| ADHD-HI | 4.56 | 2.82 | 3.63 | 2.84 | 5.12 | 2.67 | −1.49 | <.001a, b, c | .066 (−2.0–−1.0) | |
| ADHD-Co | 10.47 | 4.91 | 8.86 | 5.19 | 11.43 | 4.49 | −2.57 | <.001a, b, c | .064 (−3.5–−1.6) | |
| ODD | 3.90 | 2.86 | 2.41 | 2.58 | 4.81 | 2.64 | −2.39 | <.001 a, b, c | .165 (−2.9–−1.9) | |
| GAD | 3.15 | 2.16 | 2.36 | 2.15 | 3.63 | 2.02 | −1.27 | <.001 a, b, c | .081 (−1.7–−0.9) | |
| SocAnx | 0.67 | 0.83 | 0.72 | 0.88 | 0.63 | 0.80 | 0.09 | .284 | .003 (−0.1–0.3) | |
| SAD | 0.99 | 1.59 | 0.65 | 1.34 | 1.19 | 1.69 | −0.54 | .001 a, b, c | .027 (−0.9–−0.2) | |
| MDE | 2.13 | 2.22 | 1.41 | 2.00 | 2.57 | 2.23 | −1.16 | <.001a | .064 (−1.6–−0.7) | |
| Dysthymia | 1.90 | 1.94 | 1.40 | 1.92 | 2.21 | 1.90 | −0.81 | <.001a | .041 (−1.2–−0.4) | |
| ME | 2.05 | 2.37 | 1.04 | 1.82 | 2.65 | 2.46 | −1.61 | <.001 a, b, c | .109 (−2.1–−1.2) | |
| Schizophr | 1.00 | 1.30 | 0.72 | 1.08 | 1.17 | 1.38 | −0.45 | <.001 | .028 (−0.7–−0.2) | |
|
| ||||||||||
| Impairment | n | % | n | % | n | % | χ2 | p | OR (95% CI) | |
|
| ||||||||||
| ADHD | 311 | 77.8 | 88 | 65.7 | 223 | 83.8 | 16.99 | <.001 a, b, c | 0.37 (0.2–0.6) | |
| ODD | 251 | 63.4 | 47 | 35.1 | 204 | 77.9 | 69.94 | <.001 a, b, c | 0.15 (0.1–0.2) | |
| GAD | 208 | 52.8 | 38 | 28.4 | 170 | 65.4 | 48.64 | <.001 a, b, c | 0.21 (0.1–0.3) | |
| SocAnx | 133 | 33.6 | 41 | 31.1 | 92 | 34.8 | 0.57 | .452 | 0.84 (0.5–1.3) | |
| SAD | 60 | 15.0 | 12 | 9.0 | 48 | 17.9 | 5.52 | .019 | 0.45 (0.2–0.9) | |
| MDE/Dys | 98 | 24.6 | 30 | 22.6 | 68 | 25.7 | 0.46 | .498 | 0.84 (0.5–1.4) | |
| ME | 149 | 37.6 | 30 | 22.4 | 119 | 45.4 | 20.04 | <.001 a, b, c | 0.35 (0.2–0.6) | |
| Schizophr | 127 | 31.9 | 27 | 20.5 | 100 | 37.6 | 11.93 | .001 | 0.43 (0.3–0.7) | |
|
| ||||||||||
| Clinical cutoff | n | % | n | % | n | % | χ2 | p | OR (95% CI) | |
|
| ||||||||||
| ADHD-I | 239 | 59.8 | 66 | 49.3 | 173 | 65.0 | 9.23 | .002 a, b, c | 0.52 (0.3–0.8) | |
| ADHD-HI | 169 | 42.3 | 37 | 27.6 | 132 | 49.6 | 17.70 | <.001 a, b, c | 0.39 (0.2–0.6) | |
| ADHD-Co | 146 | 36.5 | 33 | 24.6 | 113 | 42.5 | 12.26 | <.001 a, b, c | 0.44 (0.3–0.7) | |
| ODD | 205 | 51.8 | 39 | 29.1 | 166 | 63.4 | 41.66 | <.001 a, b, c | 0.24 (0.2–0.4) | |
| GAD | 98 | 24.9 | 20 | 14.9 | 78 | 30.0 | 10.75 | .001a | 0.41 (0.2–0.7) | |
| SocAnx | 56 | 14.1 | 19 | 14.4 | 37 | 14.0 | 0.01 | .919 | 1.03 (0.6–1.9) | |
| SAD | 42 | 10.5 | 8 | 6.0 | 34 | 12.7 | 4.22 | .040a | 0.44 (0.2–1.0) | |
| MDE | 19 | 4.8 | 4 | 3.0 | 15 | 5.7 | 1.37 | .242 | 0.52 (0.2–1.6) | |
| Dysthymia | 61 | 15.3 | 19 | 14.3 | 42 | 15.8 | 0.17 | .683 | 0.88 (0.5–1.6) | |
| ME | 71 | 17.9 | 9 | 6.7 | 62 | 23.7 | 17.31 | <.001a | 0.23 (0.1–0.5) | |
| Schizophr | 20 | 5.0 | 3 | 2.3 | 17 | 6.4 | 3.14 | .077 | 0.34 (0.1–1.2) | |
Note. ASD = Autism Spectrum Disorder. IQ = Intelligence quotient. ADOS-2 CSS = Autism Diagnostic Observation Schedule, Calibrated Severity Score. ADHD = Attention Deficit/Hyperactivity Disorder. ADHD-I = ADHD – Inattentive Type. ADHD-HI = ADHD-Hyperactive/Impulsive type. ADHD-Co = ADHD – Combined. ODD = Oppositional Defiant Disorder. GAD = Generalized Anxiety Disorder. SAD = Separation Anxiety Disorder. MDE = Major Depressive Disorder. ME = Manic Episode. Schizophr = Schizophrenia.
Significant at p < .05 when controlling verbal ability (Minimally-verbal vs. Verbal), nonverbal IQ, age, and ADOS-2 CSS.
Among those variables meeting criteria for a, also significant at p < .05 when using ADOS Module 1 only as the threshold for MV.
Among those variables meeting criteria for a, also significant at p < .05 after controlling for differences in African-American racial distribution across groups.
Discussion
This study aimed to provide the first estimate of differences in comorbid psychiatric symptom profiles between MV versus verbal youth with ASD. A large multisite sample, representing the full range of functioning across inpatient and outpatient clinic settings, was utilized, and analyses were adjusted for age, NVIQ, and ASD Symptom Severity. As expected, both MV and verbal youth were clearly impacted by psychiatric comorbidity as assessed by symptom severity, symptom counts, and clinical cut-offs. In particular, CASI Clinical Cut-offs for ADHD, GAD, and dysthymia were exceeded across both inpatient and outpatient samples at high rates (>35%, 20%, and 15%, respectively).
This study also provided an opportunity for preliminary direct comparison of comorbidity between inpatient and outpatient samples. Participants in the inpatient units showed greater severity and symptom-induced impairment, and were more likely to exceed clinical cutoffs, than those in outpatient settings across almost all comorbidities. This extends prior work indicating that youth seen in psychiatric outpatient clinics present with greater psychiatric comorbidity than youth seen in ASD clinics (Joshi et al. 2010), and suggests that referral source may have an impact on estimates of psychiatric comorbidity prevalence.
Overall, there were few group differences based on verbal ability. The verbal group had higher symptom severity and a higher percentage exceeding clinical cut-offs for depression, GAD, and ODD. Symptom counts were also higher for depression and ODD in verbal as compared to MV youth. It is noteworthy that despite small effect sizes, these differences persisted after adjusting for other salient variables, including setting, NVIQ, age, and ASD symptom severity. The strongest difference, with a medium effect size, was more depression-related symptom-induced impairment in verbal youth. This is particularly interesting in light of the absence of a difference in depression-related impairment between inpatient and outpatient samples.
The two symptom domains that showed the greatest degree of difference between MV and verbal groups (ODD and depression) also had the greatest agreement across illness parameters. This suggests that these group differences are unlikely to be attributable solely to informant bias in rating some aspects of observed behavior (e.g., a parent who sees aggressive behavior as highly impairing, but not frequent). The relatively few symptom count differences between groups argues against a general problem with symptom detection. However, given the lack of any substantial differences in the direction of greater comorbidity in the MV youth, it is possible that their symptoms are less easily identified, leading to under-reporting. Alternatively, it has been suggested that disruptive behaviors in MV youth are more strongly related to communication than to an intent to be oppositional and defiant (Neidert, Rooker, Bayles, & Miller, 2013), which could be consistent with a lower incidence of ODD in MV youth. In addition, prior reports suggest markedly higher rates of depression in higher-functioning ASD youth, citing some contextual factors such as fewer structured daytime activities, which would also likely apply to those with MV ASD (Magnuson & Constantino, 2011).
When a more stringent definition of MV status was applied (e.g., requiring an ADOS-2 Module 1, which is for children with a language level of three-word phrases or less; Hus Bal et al., 2016), the primary group differences were still related to depression and ODD, but with some slight modifications. Interestingly, the most robust depression-related difference was higher symptom-induced impairment scores among the verbal group. It may be that discerning impairment due to depression is more difficult in youth with very limited verbal ability given that their overall level of impairment is often also measured to be greater (Kasari et al., 2013; Tager-Flusberg et al., 2016). The most robust group differences with the more stringent MV criteria were related to ODD, with the verbal group having significantly higher symptom count, symptom severity, and clinical cutoff scores. This finding is in line with arguments regarding ODD in non-ASD populations that although greater verbal ability is usually associated with more social-emotional competence, “children with oppositional defiant disorder who are also verbally gifted are skillful in their argumentativeness and verbal defiance” (Cole, Armstrong, & Pemberton, 2010, pp. 59). These results, though preliminary, extend prior work that has considered how psychiatric symptomatology differs based on intellectual ability or general high- or low-functioning categorizations. The finding of more depression and ODD in verbal versus MV youth is consistent with the notion that higher-functioning youth, who are generally verbal, may be more susceptible to these disorders. Specifically, prior work has argued that higher-functioning individuals may be more likely to experience depression given greater self-awareness of deficits, better theory of mind, and a stronger desire to fit in with peers (Magnuson & Constantino, 2011; Mendelson, Gates, & Lerner, 2016). Similarly, because some ODD symptoms require an ability to detect another’s intentions and attempts to manipulate people, it has been argued that ODD would be more likely to be diagnosed in more cognitively able individuals (Burke, Loeber, and Birmaher 2002; Oosterlaan, Scheres, and Sergeant 2005). These arguments for higher susceptibility to depression and ODD in higher-functioning youth are focused on cognitive processes more than verbal ability. The finding that verbal youth were more likely to experience depression and ODD than MV youth in our study is intriguing given that these differences persisted after controlling for NVIQ, which points to a specific role for verbal ability beyond the cognitive factors that have received the most attention as potential mechanisms leading to more comorbidity in high-functioning youth with ASD. Verbal youth may be more likely have experiences that could produce depressive or oppositional reactions, such as initiating social attempts with peers of differing levels of social ability, and experiencing rejection from these peers (Rowley, Chandler, Baird, Simonoff, Pickles, Loucas, & Charman, 2012). If verbal ability is a pathogenic factor in ODD and depression, then better understanding of the mechanism of action could improve treatment options. The importance of attempting to isolate the potential impact of verbal ability versus NVIQ is made further apparent by disorders that significantly differed between groups before controlling for NVIQ but not after (e.g., ADHD).
Despite this preliminary evidence for an association between psychiatric presentation and verbal ability, the latter was not associated with all illness parameters or all disorders, and many of the effects were small – once again highlighting that, despite these differences, the MV group is not “protected” from the risk for comorbid psychopathology. Specifically, verbal and MV youth were equivalent in their reported presentation of social anxiety and schizophrenia symptoms. The lack of association between verbal ability and social anxiety was unanticipated given prior research indicating that higher-functioning youth are more likely to experience social anxiety than more severely affected youth (Hallett et al., 2013a; Kerns et al., 2015; Sukhodolsky et al., 2008). This difference may be explained by an emphasis on observable aspects of social anxiety in CASI items, as opposed to the concept of fear of embarrassment, which has been emphasized as the reason that more cognitively-able and self-aware youth are more prone to experience social anxiety (White & Schry, 2011).
Another unexpected finding was the relative lack of between-group differences in symptom-induced impairment for all disorders except for dysthymia, which suggests the possibility that verbal ability has only a minor influence if any on parents’ perceptions of impairment. In addition, our results also support the assertion that although symptom-induced impairment overlaps other illness parameters, it is also unique (Kaat et al., 2013) and may provide additional insight into variables that may contribute to causality or exacerbate clinical presentation (Gadow et al., 2016) and even how patients respond to treatment (Gadow et al., 2014).
This is the first large-scale study of a representative sample of clinically-referred youth with ASD (i.e., inpatient and outpatient) to focus explicitly on verbal ability adjusting for age, ASD symptom severity and NVIQ. It employed multiple thresholds for identifying MV status (Jack & Pelphrey, 2017), as well as a series of stringent controls, thereby increasing confidence that obtained results were not simply artifacts of a specific selection procedure. There are several promising avenues for research to build on this work and address this study’s limitations. First, information about psychiatric symptom presentation was gathered by caregiver report. Future research might benefit from including perspectives from other reporters, particularly teachers, as verbal ability has important implications for adaptive functioning in school. Relatedly, direct behavioral observation and psychophysiological assessment (e.g., heart rate and skin conductance) can further aid in assessing psychiatric symptom constructs that may otherwise be difficult for parents or teachers to observe (Moskowitz et al., 2017). A more fine-grained analysis of verbal and communication ability may provide insight into how verbal ability relates to psychiatric symptomatology. For instance, these results indicate that several internalizing symptoms (depression, GAD) yielded differences between MV and verbal groups in terms of severity and clinical cutoffs only when using the less stringent threshold for MV status. The fact that we found different patterns of associations based upon differing definitions is consistent with previous research (Hus Bal et al., 2016), and highlights the fact that how such groups are defined matters substantively. Future research should examine what factors associated with differences in verbal or communicative ability across these differently-defined subgroups may also relate to severity of internalizing symptoms. Similarly, whereas ADOS-2 module has been proposed to be an especially useful approach for identifying MV status (Hus Bal et al.), it remains an imperfect index of verbal ability. Relatedly, although there is compelling evidence that MV and ID status are indeed distinct in the ASD population (Tager-Flusberg et al., 2016), it is also true there is both conceptual and functional overlap in many cases; thus, it is hoped that the present study can provide a benchmark for future research aiming to disentangle such concerns. Although current findings suggest an association with verbal ability (for certain disorders), this cross-sectional study cannot address causality. Longitudinal studies that prospectively track the development of psychiatric symptoms while at the same time measuring verbal and communication ability could better delineate the direction of effects. Finally, future steps should include item comparisons between groups to identify particular symptoms that might be difficult to detect in MV youth (e.g., not a single MV youth met clinical cutoff for MDE), or particular item phrasings that might be problematic to answer about an individual with limited verbal capacity.
Acknowledgments
We gratefully acknowledge the contributions of the coordinating site advisory group: Donald L. St. Germain, MD and Girard Robinson, MD, and our scientific advisory group: Connie Kasari, PhD., Bryan King, MD, James McCracken, MD, Christopher McDougle, MD, Lawrence Scahill, MSN, PhD, Robert Schultz, PhD and Helen Tager-Flusberg, PhD, the input of the funding organizations and the families and children who participated.
The Autism Inpatient Collection (AIC) phenotypic database and biorepository is supported by a grant from the Simons Foundation Autism Research Initiative and the Nancy Lurie Marks Family Foundation (SFARI #296318 to M.S.). Dr. Mazefsky also received support from NICHD grant K23HD060601 during the course of this project and is currently supported by NICHD; R01HD079512. The DDCLI data were supported by the Matt and Debra Cody Center for Autism and Developmental Disabilities. Dr. Lerner received support from NIMH grant R01MH110585 and the Simons Foundation Autism Research Initiative (SFARI# 381283), during the course of this project.
Footnotes
Conflict of Interest: K.D.G. declares Shareholder, Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory. All other authors declare they have no conflicts of interest.
Ethical approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committees where the data was collected and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in this study.
Contributor Information
Matthew D. Lerner, Department of Psychology at Stony Brook University in Stony Brook, NY, USA.
Carla A. Mazefsky, Department of Psychiatry at the University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Rebecca J. Weber, Department of Psychology at Stony Brook University in Stony Brook, NY, USA
Emilie Transue, Department of Psychiatry at the University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Matthew Siegel, Maine Medical Center Research Institute and the Departments of Psychiatry and Pediatrics of Tufts University School of Medicine.
Kenneth D. Gadow, Department of Psychiatry at the Stony Brook University School of Medicine in Stony Brook, NY
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. Psychological Corporation; 1993. [Google Scholar]
- Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: A review of the past 10 years, part II. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(11):1275–1293. doi: 10.1097/00004583-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics—Simulation and Computation®. 2010;39(4):860–864. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Vallance DD, Barwick M, Im N, Menna R, Horodezky NB, Isaacson L. The interface between ADHD and language impairment: An examination of language, achievement, and cognitive processing. Journal of Child Psychology and Psychiatry. 2000;41(3):353–362. [PubMed] [Google Scholar]
- Cole PM, Armstrong LM, Pemberton CK. The role of language in the development of emotion regulation. In: Calkins S, Bell MA, editors. Child Development at the Intersection of Emotion and Cognition. Washington, DC, US: American Psychological Association; 2010. pp. 59–77. [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, Kripke C. The health status of adults on the autism spectrum. Autism: The International Journal of Research and Practice. 2015;19(7):814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- Davis TE, III, Moree BN, Dempsey T, Reuther ET, Fodstad JC, Hess JA, Matson JL. The relationship between autism spectrum disorders and anxiety: The moderating effect of communication. Research in Autism Spectrum Disorders. 2011;5(1):324–329. [Google Scholar]
- Einfeld SL, Ellis LA, Emerson E. Comorbidity of intellectual disability and mental disorder in children and adolescents: A systematic review. Journal of Intellectual and Developmental Disability. 2011;36(2):137–143. doi: 10.1080/13668250.2011.572548. [DOI] [PubMed] [Google Scholar]
- Fletcher RJ, Loschen E, Stavrakaki C, editors. DM-ID: diagnostic manual-intellectual disability: a textbook of diagnosis of mental disorders in persons with intellectual disability. National Assn for the Dually Diagnosed; 2007. [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. Journal of Clinical Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- Gadow KD. The Symptom Inventories: An annotated bibliography. Stony Brook, NY: Checkmate Plus; 2015. [Google Scholar]
- Gadow KD, Arnold LE, Molina BSG, Findling RL, Bukstein OG, Brown NV, Aman MG. Risperidone added to parent training + stimulant medication: Effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(9):948–959. doi: 10.1016/j.jaac.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-aged children with PDD versus clinic and community samples. Autism. 2005;9(4):392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Schneider J. Predictors of psychiatric symptoms in children with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38(9):1710–1720. doi: 10.1007/s10803-008-0556-8. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Kaat AJ, Lecavalier L. Relation of symptom-induced impairment with other illness parameters in clinic-referred youth. Journal of Child Psychology and Psychiatry. 2013;54(11):1198–1207. doi: 10.1111/jcpp.12077. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Perlman G, Ramdhany L, de Ruiter J. Clinical correlates of co-occurring psychiatric and autism spectrum disorder (ASD) symptom-induced impairment in children with ASD. Journal of Abnormal Child Psychology. 2016;44(1):129–139. doi: 10.1007/s10802-015-9979-9. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory. Stony Brook, NY: Checkmate Plus; 2005; 2013. [Google Scholar]
- Gotham K, Bishop S, Hus V, Huerta M, Lund S, Buja A, Lord C. Exploring the relationship between anxiety and insistence on sameness in autism spectrum disorders. Autism Research. 2013;6(1):33–41. doi: 10.1002/aur.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Lecavalier L, Sukhodolsky DG, Cipriano N, Aman MG, McCracken JT, Sikich L. Exploring the manifestations of anxiety in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013a;43(10):2341–2352. doi: 10.1007/s10803-013-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Colvert E, Ames C, Woodhouse E, Lietz S, Happe F. Exploring anxiety symptoms in a large-scale twin study of children with autism spectrum disorders, their co-twins and controls. Journal of Child Psychology and Psychiatry. 2013b;54(11):1176–1185. doi: 10.1111/jcpp.12068. [DOI] [PubMed] [Google Scholar]
- Hus Bal V, Katz T, Bishop SL, Krasileva K. Understanding definitions of minimally verbal across instruments: evidence for subgroups within minimally verbal children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2016;57(12):1424–1433. doi: 10.1111/jcpp.12609. [DOI] [PubMed] [Google Scholar]
- Jack A, A Pelphrey K. Annual Research Review: Understudied populations within the autism spectrum–current trends and future directions in neuroimaging research. Journal of Child Psychology and Psychiatry. 2017;58(4):411–435. doi: 10.1111/jcpp.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Biederman J. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders. 2010;40(11):1361–1370. doi: 10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Joshi G, Wozniak J, Petty C, Martelon MK, Fried R, Bolfek A, Caruso J. Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: a comparative study. Journal of Autism and Developmental Disorders. 2013;43(6):1314–1325. doi: 10.1007/s10803-012-1679-5. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Gadow KD, Lecavalier L. Psychiatric symptom impairment in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41(6):959–969. doi: 10.1007/s10802-013-9739-7. [DOI] [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, Tager-Flusberg H. Assessing the minimally verbal school-aged child with autism spectrum disorder. Autism Research. 2013;6(6):479–493. doi: 10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC, Zickgraf H, Franklin ME, Miller J, Herrington J. Not to be overshadowed or overlooked: Functional impairments associated with comorbid anxiety disorders in youth with ASD. Behavior Therapy. 2015;46(1):29–39. doi: 10.1016/j.beth.2014.03.005. [DOI] [PubMed] [Google Scholar]
- King BH, de Lacy N, Siegel M. Psychiatric assessment of severe presentations in autism spectrum disorders and intellectual disability. Child & Adolescent Psychiatry Clinics of North America. 2014;23(1):1–14. doi: 10.1016/j.chc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman SB, Davis NO, Dinh E, Morgan J, Lainhart JE. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Western Psychological Services; 2012. [Google Scholar]
- Magnuson KM, Constantino JN. Characterization of depression in children with autism spectrum disorders. Journal of Developmental & Behavioral Pediatrics. 2011;32(4):332–340. doi: 10.1097/DBP.0b013e318213f56c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS. Psychiatric hospitalization among children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(6):1059–1065. doi: 10.1007/s10803-007-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Foa E, Gammon P, Chrisman A, Curry J, Fitzgerald D, Zhao N. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, Pauls DL. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: A community-and clinic-based study. Journal of Autism and Developmental Disorders. 2010;40(9):1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, White SW. Emotion Regulation: concepts & practice in autism spectrum disorder. Child & Adolescent Psychiatry Clinics of North America. 2014;23(1):15–24. doi: 10.1016/j.chc.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JL, Gates JA, Lerner MD. Friendship in school-age boys with autism spectrum disorders: A meta-analytic summary and developmental, process-based model. Psychological Bulletin. 2016;142(6):601–622. doi: 10.1037/bul0000041. [DOI] [PubMed] [Google Scholar]
- Moskowitz LJ, Rosen T, Lerner MD, Levine K. Assessment of anxiety in youth with autism spectrum disorder. In: Kerns C, Storch E, Kendall P, Wood JJ, Renno P, editors. Evidence Based Assessment and Treatment of Anxiety in Children and Adolescents with Autism Spectrum Disorder. Elsevier; 2017. [Google Scholar]
- Neidert PL, Rooker GW, Bayles MW, Miller JR. Functional analysis of problem behavior. In: Reed DD, Reed FDDG, Luiselli JK, editors. Handbook of crisis intervention and developmental disabilities: Issues in clinical child psychology. New York: Springer; 2013. pp. 147–167. [Google Scholar]
- Olsson M, Lundstrom S, Westerlund J, Giacobini M, Gillberg C, Fernell E. Preschool to school in autism: Neuropsychiatric problems 8 years after diagnosis at 3 years of age. Journal of Autism and Developmental Disorders. 2016;46(8):2749–2755. doi: 10.1007/s10803-016-2819-0. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Scheres A, Sergeant JA. Which executive functioning deficits are associated with AD/HD, ODD/CD and comorbid AD/HD+ ODD/CD? Journal of Abnormal Child Psychology. 2005;33(1):69–85. doi: 10.1007/s10802-005-0935-y. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ, Pomplun M, Koch C. Leiter International Performance Scale, Third Edition (Leiter-3) Western Psychological Services; 2013. [Google Scholar]
- Rowley E, Chandler S, Baird G, Simonoff E, Pickles A, Loucas T, Charman T. The experience of friendship, victimization and bullying in children with an autism spectrum disorder: Associations with child characteristics and school placement. Research in Autism Spectrum Disorders. 2012;6(3):1126–1134. [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Western Psychological Services; 2003. [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O’Sullivan T, Howlin P, Simonoff E. Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45(8):2283–2294. doi: 10.1007/s10803-015-2361-5. [DOI] [PubMed] [Google Scholar]
- Siegel M, Doyle K, Chemelski B, Payne D, Ellsworth B, Harmon J, Lubetsky M. Specialized inpatient psychiatry units for children with autism and developmental disorders: A United States survey. Journal of Autism and Developmental Disorders. 2012;42(9):1863–1869. doi: 10.1007/s10803-011-1426-3. [DOI] [PubMed] [Google Scholar]
- Siegel M, Smith KA, Mazefsky C, Gabriels RL, Erickson C, Kaplan D, Santangelo SL. The autism inpatient collection: methods and preliminary sample description. Molecular Autism. 2015;6(61) doi: 10.1186/s13229-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, Vitiello B. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology. 2008;36(1):117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Skwerer DP, Joseph RM, Brukilacchio B, Decker J, Eggleston B, Yoder A. Conducting research with minimally verbal participants with autism spectrum disorder. Autism. 2016 doi: 10.1177/1362361316654605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. Riverside Publishing Company; 1986. [Google Scholar]
- Tsakanikos E, Costello H, Holt G, Bouras N, Sturmey P, Newton T. Psychopathology in adults with autism and intellectual disability. Journal of Autism and Developmental Disorders. 2006;36(8):1123–1129. doi: 10.1007/s10803-006-0149-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Witwer AN, Lecavalier L. Validity of comorbid psychiatric disorders in youngsters with autism spectrum disorders. Journal of Developmental and Physical Disabilities. 2010;22(4):367–380. [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review. 2009;29(3):216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Schry AR. Social anxiety in adolescents on the autism spectrum. In: Alfano CA, Beidel DC, editors. Social anxiety in adolescents and young adults: Translating developmental science into practice. Washington, DC: American Psychological Association; 2011. pp. 183–201. [Google Scholar]


