Abstract
The advent of high-throughput “omics” technologies, combined with the computational and statistical methods necessary to analyze such data, have revolutionized biology, enabling a global view of the complex molecular processes and interactions that occur within a biological system. Such systems-based approaches have begun to be used in the evaluation of immune responses to vaccination, with the promise of identifying predictive biomarkers capable of rapidly evaluating vaccine efficacy, transforming our understanding of the immune mechanisms responsible for protective responses to vaccination and contributing to a new generation of rationally designed vaccines. Here we present our opinion that systems biology does indeed have a critical role in the future of vaccinology. Such approaches have shown potential in identifying transcriptional and cellular signatures of responsiveness to vaccination using diverse vaccines, adjuvants, and human populations. These findings, coupled with further mechanistic evaluation in animal models, will guide development of targeted vaccine and adjuvant formulations designed to optimally induce protective responses in populations of differing immune status.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
SYSTEMS VACCINOLOGY INTRODUCTION
Although vaccines have historically been extremely effective public health tools (Plotkin 2003), vaccinologists have encountered difficulties in developing vaccines against many present-day global pandemics, such as human immunodeficiency virus (HIV) (Johnston and Fauci 2008), malaria (Vaughan and Kappe 2012), tuberculosis (Orme 2013), and dengue fever (McArthur et al. 2013). A recurring theme for many of these diseases is that the immune components required for protection from infection have not been well established and there remains a very limited understanding of the mechanisms by which vaccines elicit protective immune responses. Additionally, the prohibitive cost of large and lengthy clinical trials required for licensure greatly limits the number of vaccine formulations that are tested in phase III trials.
One of the major obstacles to identifying correlates and mechanisms of protection is that traditional immunological assays (enzyme-linked immunospot [ELISpot], flow cytometry, hemagglutination inhibition [HAI], etc.) typically measure a small number of immune parameters at a time, making it difficult to capture a global view of the immune system as it responds to infection or vaccination and interacts with other biological systems in the body. In recent decades, advances in high-throughput “omics” technologies have enabled researchers to quantitatively examine entire classes of molecules in a given tissue, even down to the single-cell level. The application of these technologies, along with the computational algorithms developed to analyze and interpret such data, to study immune responses to vaccination has generated a new field of “systems vaccinology.” The aim of this approach is to capture the responses and interaction between individual immune components in the context of vaccination to develop a holistic understanding of the immune system (Pulendran 2009, 2014; Pulendran et al. 2010; Nakaya et al. 2012; Hagan et al. 2015).
DELINEATING SIGNATURES OF VACCINE EFFICACY
The first studies to use high-throughput data to evaluate vaccine responses focused on the live attenuated yellow fever vaccine 17D (YF-17D). By integrating gene-expression data generated through microarray analysis of peripheral blood mononuclear cells (PBMCs) with flow cytometry measurements of innate immune cells and neutralizing antibody assays, our laboratory was able to identify gene signatures capable of accurately predicting CD8+ T-cell and antibody responses to vaccination (Querec et al. 2009). This study provided proof-of-concept evidence of the capacity of systems approaches to delineate “molecular signatures” or biomarkers of vaccine immunity. At the same time, Gaucher et al. (2008), through analysis of an independent cohort, identified similar transcriptional responses to YF-17D, including interferon and complement pathways driven by signal transducers and activators of transcription (STAT1) and interferon regulatory factor (IRF7) transcription factors.
Following this initial work, the field has expanded to include studies of vaccines against a multitude of pathogens, including live attenuated and inactivated seasonal influenza (Bucasas et al. 2011; Nakaya et al. 2011, 2016; Furman et al. 2013), meningococcal vaccines (Li et al. 2014), shingles (Qi et al. 2016; Li et al. 2017), malaria (Vahey et al. 2010; Kazmin et al. 2017; van den Berg et al. 2017), smallpox (Reif et al. 2009), and HIV (Zak et al. 2012). Recent work has used systems approaches to identify molecular signatures capable of predicting the efficacy of vaccine-induced immunity in controlled human malaria infection (CHMI) models (Kazmin et al. 2017). These trials mostly focused on examining the transcriptional and cellular responses immediately after vaccination (days 1–7) in healthy adults, including identification of transcriptional signatures capable of discriminating between successful and diminished immune responses to vaccination. Recently, we and others have extended this approach to evaluate potential baseline markers of vaccine responsiveness (Tsang et al. 2014; Nakaya et al. 2015). In our analysis of responses to inactivated influenza vaccine in young and elderly adults across five influenza seasons, we identified several transcriptional modules whose expression prevaccination was associated with an increased or diminished day 28 antibody response. Included in these modules was a monocyte-related module associated with reduced antibody titers. We also observed increased baseline frequencies and expression of CCR5, a receptor for inflammatory chemokines, among monocytes in elderly subjects (≥65 yr old), suggesting a possible connection between age-related changes in the innate immune system and reduced responsiveness to vaccination (Nakaya et al. 2015). Such analyses show that it is possible to identify baseline markers of immune status associated with vaccine efficacy in different populations. Further studies in other groups at risk for infections, such as infants, HIV-positive individuals, and transplant patients on immunosuppressant medications could help identify dysregulated transcriptional pathways and in turn guide development of vaccines optimized for these at-risk groups.
BEYOND SIGNATURES: DELINEATING MECHANISMS OF VACCINE EFFICACY
Although such biomarkers are useful for profiling and evaluating vaccine effectiveness, they fall short of providing the kind of mechanistic information required to further vaccine design on their own. However, these predictive signatures can become a source for hypotheses about potential immune mechanisms and serve as a basis for follow-up experiments in vitro or in animal models. As an example, our group has used this approach to elucidate an unappreciated role of the integrated stress response involved in sensing cellular stress such as amino acid starvation and endoplasmic reticulum stress in controlling innate immune responses and inflammation. In our initial systems biology analysis of responses to YF-17D, general control nonderepressible 2 kinase (GCN2), also known as eukaryotic initiation factor 2α-kinase 4 (EIF2AK4), expression in PBMCs on day 7 postvaccination was identified in several gene signatures as capable of accurately predicting the CD8+ T-cell response to vaccination (Querec et al. 2009). A sensor of amino acid starvation, GCN2 regulates protein synthesis through phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), initiating translational arrest and production of stress granules in response to accumulation of uncharged transfer RNA (tRNA) (Anderson and Kedersha 2002). Despite its well-known role in regulating the integrated stress response, any potential function of GCN2 within the immune system was not understood. The capacity of GCN2 expression to predict CD8+ T-cell responses suggested a potential for GCN2 in priming the adaptive response to YF-17D. Follow-up experiments using in vitro cell culture and GCN2 knockout mice revealed that GCN2 generated increased autophagy and antigen presentation in dendritic cells in response to YF-17D-induced amino acid starvation (Ravindran et al. 2014). In addition, further work with knockout mice showed that GCN2-induced autophagy inhibits inflammation and the development of T helper (TH)17 responses during intestinal colitis through a reduction in inflammasome activation and interleukin (IL)-1β signaling, and that this protective function can be enhanced through administration of an amino acid–restricted diet (Ravindran et al. 2016).
Systems-based findings have also guided investigation into the potential influence that intestinal microbiota have in shaping immune responses to vaccination. Analysis of transcriptional responses to inactivated seasonal influenza vaccine identified a strong correlation between expression of TLR5, a Toll-like receptor (TLR) specific for bacterial flagellin, on day 3 postvaccination with antibody titers measured 28 days postvaccination (Nakaya et al. 2011). The association between a bacterial-sensing TLR with adaptive responses to a viral vaccine was unexpected and prompted our investigation into the impact of gut microbiota on vaccine responses. This work revealed that flagellin activates lymph node macrophages to produce B-cell growth factors IL6 and APRIL, as well as acting directly on activated B cells through TLR5. Consequently, antibiotic-treated and TLR5 knockout mice have reduced plasma cell and antibody responses to inactivated influenza vaccination. This effect was not extended to adjuvanted or live viral vaccines, suggesting that gut bacteria may serve as an endogenous adjuvant in the absence of external sources (Oh et al. 2014). We are currently examining the potential for analogous mechanisms in humans through a clinical trial of subjects given a broad-spectrum cocktail of antibiotics for 3 days before administration of the inactivated seasonal influenza vaccine.
A growing area in vaccinology that would benefit from improved mechanistic understanding is in the development of vaccine adjuvants. Modern vaccine formulations increasingly use purified recombinant or synthetic antigens that are less immunogenic than vaccines containing live or inactivated whole organisms. These purified vaccines often require coadministration with an adjuvant to achieve protective immune responses. As a result, there is a great need for the development of effective and safe adjuvants capable of augmenting targeted immune pathways and this is an area of intense research. Although a wide variety of natural and synthetic compounds are being investigated as potential adjuvants, many have been abandoned because of excessive toxicity/side effects, limited stability, and development cost (McElrath 2017). As a result, only a small number of adjuvants are currently licensed for use in humans (Rappuoli et al. 2011). In addition, there remain significant gaps in our understanding of the precise mechanisms by which adjuvants augment immune responses (Pulendran and Ahmed 2011). Even the mechanism of action of alum, which increases antigen presentation and TH2 responses and has been in widespread use for >70 yr, is still being debated within the field (Reed et al. 2013).
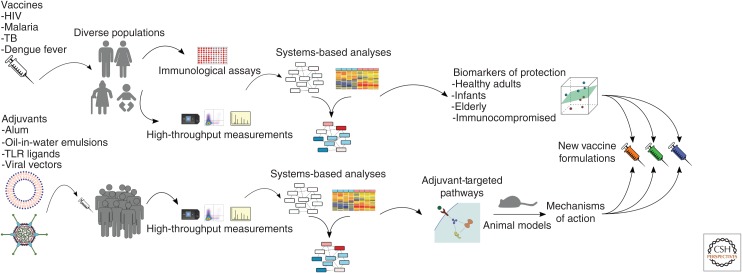
There have already been several preliminary systems-based analyses of adjuvants and adjuvanted vaccines, including studies of RTS,S malaria vaccine with AS01/AS02 monophosphoryl lipid (MPL) A–based adjuvants (Vahey et al. 2010; Kazmin et al. 2017; van den Berg et al. 2017) and of synthetic double-stranded RNA (poly-ICLC) (Caskey et al. 2011) in healthy adults as well as seasonal inactivated influenza vaccine using squalene-based oil-in-water emulsion MF59 in healthy infants (Nakaya et al. 2016). Interestingly, both analyses of oil-in-water emulsions (AS02 and MF59) revealed a strong up-regulation of immunoproteasome-related genes in response to the adjuvant, which agrees with work in mouse models suggesting these delivery systems up-regulate antigen presentation at the site of injection (Mosca et al. 2008). Hopefully, further studies will expand on these initial transcriptomic analyses and seek to include other data types such as metabolomic profiling, as oil-in-water emulsions have been shown to induce intracellular accumulation of lipids in vitro (Kalvodova 2010) and may induce innate responses partially through alterations in lipid metabolism. By enabling the high-resolution and global analysis of transcriptomic and metabolic changes that are induced during administration of various candidate adjuvants, systems biology approaches will be a useful tool as researchers seek to understand the unique immune response profile of each adjuvant. The resulting knowledge can be paired with insights about transcriptional and cellular correlates of protection identified through systems-based vaccine studies to inform development-optimized vaccine–adjuvant combinations that induce optimum protection from infection in a disease- and population-specific manner (Fig. 1).
Figure 1.
Systems biology–driven pipeline of vaccine development. In the context of clinical vaccine trials, systems-based analyses can be used to integrate immunological measurements of correlates of protection such as neutralizing antibody titers with high-throughput transcriptional, cellular, and metabolic data to establish biomarkers of protection in specific populations. Simultaneously, these approaches may also be used to identify the biological pathways activated by candidate adjuvants. This information can guide experimental validation in animal models to understand the unique mechanisms by which each adjuvant elicits protective immune responses. Together, this knowledge can be used to develop targeted vaccine–adjuvant combinations that are designed to enhance the most protective immune pathways unique to various at-risk populations. HIV, Human immunodeficiency virus; TB, tuberculosis; TLR, Toll-like receptor.
CHALLENGES
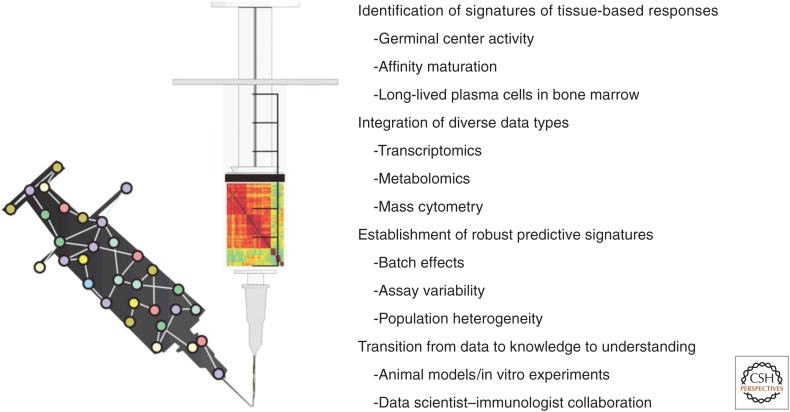
Although systems-level studies offer great promise toward high-resolution profiling of vaccine responses and identification of molecular and cellular signatures of protective immunity, challenges faced by the initial research in this area have also highlighted some of its limitations (Fig. 2).
Figure 2.
Challenges of systems vaccinology. Systems-based approaches to understanding vaccine-driven immune responses face several important challenges. First, constraints on the feasibility of biopsy collection during clinical trials has led to limited development of molecular signatures associated with tissue-based responses such as germinal center activity and affinity maturation. In addition, analysis of molecular responses to vaccination has thus far been largely focused on transcriptional data. The high-throughput technologies and computational algorithms necessary to integrate diverse data types and foster a true systems-level understanding of vaccine responses are just beginning to be developed. Another important obstacle that has emerged is the difficulty in identifying robust predictive signatures of protective immune responses caused by various sources of biological and technical variability, which must be overcome if clinically relevant diagnostic tools are to be established. Finally, the foremost challenge in systems vaccinology is the need to successfully convert knowledge extracted by high-throughput data analysis into a meaningful understanding of the biological mechanisms that generate protective immune responses through hypothesis-driven animal models, in vitro experiments, and intimate collaboration between data scientists and immunologists.
Identifying Predictors of Immune Responses in Tissues
Because of the difficulty in obtaining immune tissue samples during clinical trials, studies in the field thus far have focused on defining signatures of the magnitude of antigen-specific B- and T-cell responses in the blood. There has been little effort toward identifying early predictors of other variables of the immune response such as the persistence of antibody titers and the affinity of the antibody response. The immune response is a highly choreographed sequence that occurs at multiple sites in the body. Thus, for example, activated B cells differentiate into memory B cells within dynamic structures in lymph nodes called germinal centers (GCs) and some GC cells migrate to the bone marrow where they reside as long-lived plasma cells (Crotty 2011). Such plasma cells contribute toward the persistence of high-affinity antibody titers after vaccination. Therefore, a major challenge for the future is to identify signatures in the blood that can be used to predict the magnitude and longevity of the GC response or long-lived plasma cells as well as persistent high-affinity antibody responses. In this context, our recent work with a recombinant envelope (Env) protein against simian immunodeficiency virus (SIV), adjuvanted with nanoparticle encapsulated ligands for TLRs in nonhuman primates, revealed that protection against SIV infection was correlated with the prechallenge titers of Env-specific immunoglobulin G (IgG) antibodies in serum and vaginal secretions (Kasturi et al. 2017). Transcriptional profiling of PBMCs isolated within the first few hours to days after primary vaccination revealed that adjuvanted vaccines induced a molecular signature similar to that induced by YF-17D. This systems approach identified early blood transcriptional signatures that correlate with Env-specific antibody responses in vaginal secretions and protection against infection. These results show that transcriptional profiling can be used to identify molecular signatures that correlate with antibody titers in vaginal tissues.
Furthermore, recent work by Havenar-Daughton et al. (2016) found that plasma CXCL13 levels correlated with GC activity in draining lymph nodes of immunized mice, immunized macaques, and HIV-infected humans. Whereas the utility of this biomarker in predicting GC activity in vaccine-induced immunity remains to be seen, it highlights the feasibility of identifying such biomarkers in the blood of immune responses within lymph nodes. An important component of the GC response is follicular helper T (TFH) cells, which help to initiate formation of the GC and aid the affinity maturation process through expression of costimulatory molecules and cytokines such as CD40L, IL-21, and IL-4 (Crotty 2011). Recently, several subsets of circulating memory TFH cells have been identified in the blood (Schmitt et al. 2014), and these cells have been shown to correlate with development of broadly neutralizing antibodies against HIV (Locci et al. 2013) and with antibody responses to seasonal influenza (Bentebibel et al. 2013). Our laboratory is currently working to identify early transcriptional signatures within peripheral blood associated with these TFH cells and with changes in antibody affinity during responses to an AS03-adjuvanted avian H5N1 influenza vaccine. Such blood-based markers of affinity maturation could help us to understand the mechanisms by which vaccines and adjuvants induce this critical process.
Beyond Transcriptomics
In addition to sampling location, the immature state of high-throughput measurement technologies limits our ability to interrogate certain molecular components. The chief obstacle in this regard is in the measurement of proteins. Whereas proteins are the primary effector molecules within cells, measurement of cellular processes in clinical samples is frequently limited to transcriptional data through microarrays or RNA sequencing, despite the fact that messenger RNA (mRNA) levels only explain 40% of variability in protein levels (Schwanhausser et al. 2011). This is attributable to the challenges current proteomic technologies face in terms of complex sample preparation, reproducibility, limited dynamic range, and detection of posttranslational modifications (Chandramouli and Qian 2009). An alternative to more high-throughput proteomic approaches is multiplex bead assays, which can provide quantitative information for a small number (up to 20–30) of serum cytokines. However, these assays also suffer from sensitivity and reproducibility issues (Breen et al. 2011).
Although the study of the immune response to YF-17D involved an integrated analysis of transcriptomics, flow cytometry, and plasma cytokine analysis using Luminex (Querec et al. 2009), analysis of signatures of vaccine immunity was confined to transcriptomics data. Indeed, until recently, almost all studies in this field focused on identifying transcriptional signatures of vaccine immunity. Brodin et al. (2015) were able to generate an immune network consisting of cell populations, cytokines, and serum protein levels among >200 twins showing that the majority of immune parameters, including responses to seasonal influenza vaccination, are driven primarily by nonheritable factors. In our recent study with the shingles vaccine, we performed an integrated analysis of orthogonal datasets (transcriptomics, metabolomics of plasma metabolites, flow cytometry, and Luminex analysis of cytokines) to construct a multiscale multifactorial response network (MMRN) of immunity in healthy young and older adults immunized with the live attenuated shingles vaccine Zostavax (Li et al. 2017). This MMRN revealed robust associations of orthogonal datasets and helped delineate metabolic correlates of vaccine immunity. Interestingly, this analysis revealed sterol regulatory binding protein 1 (SREBP-1) and its targets as potentially key integrators of antibody and TFH responses. This study reveals the power of analyzing orthogonal datasets to identify predictors of vaccine immunity. Such work highlights the value in integration of diverse high-throughput data to uncover a more complete picture of the biological processes induced by vaccination.
Robustness of Predictors
A major goal of this field, which has emerged as a significant challenge, is the development of robust signatures of protective immune responses capable of predicting vaccine efficacy in a clinical setting. Systems-based analyses of vaccine responses must deal with significant amounts of variability, arising from both technological and biological sources. The sensitivity of many high-throughput technologies limits reliable detection of low-abundance molecules, such as transcription factors. Owing to the high cost of many high-throughput technologies, studies are often limited to small sample sizes, further reducing the signal-to-noise ratio and hindering discovery of robust molecular signatures of protection. Additionally, many of these measurements suffer from batch effects, making comparison of data across cohorts or datasets difficult (Leek et al. 2010). Even HAI and virus neutralization assays, two of the most common techniques for measuring antibodies, have been shown to be poorly reproducible (Stephenson et al. 2007). Biological variability is also particularly high in clinical studies because of the huge amount of heterogeneity across the human population. Immune responses are impacted by a variety of factors, including age (Duraisingham et al. 2013), genetics (Orru et al. 2013), exposure history to previous infections/vaccinations, gut microbial composition (Hooper et al. 2012), stress (Padgett and Glaser 2003), and physical activity (Woods et al. 2006). All of the above factors contribute to the enormous difficulty in finding broadly predictive biomarkers capable of successfully discriminating between protective and deficient responses to vaccination at an individual level.
Data to Knowledge to Understanding
Finally, the most important challenge facing this field is the need to translate the wealth of data generated in high-throughput studies into meaningful understanding about the mechanisms of immune responses to vaccines. As mentioned above, systems biology approaches are an essential tool for identifying signatures of vaccine response and extracting knowledge about the molecular pathways involved. These signatures, when combined with human knowledge and insight, can generate hypotheses that can be explored in animal models and in vitro experiments to further our understanding about the biological mechanisms responsible for protective immunity. As bridging the gap between data and understanding requires a combination of biological insight, rigorous data analysis, and creativity, close collaboration between systems biologists, bioinformaticians, and immunologists is critical to this process.
CONCLUSION
The human body, including the immune system, is incredibly complex and multifaceted. As technology develops, allowing us to interrogate immune responses at higher resolution and dimensionality, systems biology approaches will become an increasingly necessary step in the process of understanding the components and interactions within the immune system in the context of response to vaccination. Although systems biology is not a stand-alone method for vaccine development, discoveries thus far in the field suggest that the identification of molecular and cellular pathways induced in response to vaccination as well as signatures predictive of protective responses, combined with traditional methods of experimental validation, will help us to understand at greater depth the mechanisms by which vaccines and adjuvants elicit protective immune responses. This knowledge will improve our ability to design more effective vaccines and administer them in a more targeted manner, particularly for populations at risk of infection.
ACKNOWLEDGMENTS
This work is supported by grants from the U.S. National Institutes of Health (Grants R37 DK057665, R37 AI048638, U19 AI090023, and U19 AI057266) and from the Bill and Melinda Gates Foundation to B.P.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Anderson P, Kedersha N. 2002. Visibly stressed: The role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. 2013. Induction of ICOS+ CXCR3+ CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 5: 176ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, Dibben O, Margolick JB, Bream JH, Sambrano E, et al. 2011. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol 18: 1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, et al. 2015. Variation in the human immune system is largely driven by non-heritable influences. Cell 160: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, Arden N, Quarles JM, Couch RB, Belmont JW. 2011. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 203: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, Breton G, Trumpfheller C, Pollak S, Shimeliovich I, et al. 2011. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 208: 2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli K, Qian PY. 2009. Proteomics: Challenges, techniques and possibilities to overcome biological sample complexity. Hum Genomics Proteomics 10.4061/2009/239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29: 621–663. [DOI] [PubMed] [Google Scholar]
- Duraisingham SS, Rouphael N, Cavanagh MM, Nakaya HI, Goronzy JJ, Pulendran B. 2013. Systems biology of vaccination in the elderly. Curr Top Microbiol Immunol 363: 117–142. [DOI] [PubMed] [Google Scholar]
- Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H, Lund P, Maecker HT, et al. 2013. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 9: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR III, Castro E, et al. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205: 3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan T, Nakaya HI, Subramaniam S, Pulendran B. 2015. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine 33: 5294–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, et al. 2016. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci 113: 2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MI, Fauci AS. 2008. An HIV vaccine—Challenges and prospects. N Engl J Med 359: 888–890. [DOI] [PubMed] [Google Scholar]
- Kalvodova L. 2010. Squalene-based oil-in-water emulsion adjuvants perturb metabolism of neutral lipids and enhance lipid droplet formation. Biochem Biophys Res Commun 393: 350–355. [DOI] [PubMed] [Google Scholar]
- Kasturi SP, Kozlowski PA, Nakaya HI, Burger MC, Russo P, Pham M, Kovalenkov Y, Silveira EL, Havenar-Daughton C, Burton SL, et al. 2017. Adjuvanting a simian immunodeficiency virus vaccine with Toll-like receptor ligands encapsulated in nanoparticles induces persistent antibody responses and enhanced protection in TRIM5α restrictive macaques. J Virol 91: e01844–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, Ballou WR, Jongert E, Wille-Reece U, Ockenhouse C, et al. 2017. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci 114: 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Scharpf RB, Bravo HC, Simcha D, Langmead B, Johnson WE, Geman D, Baggerly K, Irizarry RA. 2010. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet 11: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, et al. 2014. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 15: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, McCausland M, Chiu C, Canniff J, Dubey S, et al. 2017. Metabolic phenotypes of response to vaccination in humans. Cell 169: 862–877; e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. 2013. Human circulating PD-1+ CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39: 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur MA, Sztein MB, Edelman R. 2013. Dengue vaccines: Recent developments, ongoing challenges and current candidates. Expert Rev Vaccines 12: 933–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ. 2017. Adjuvants: Tailoring humoral immune responses. Curr Opin HIV AIDS 12: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci 105: 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. 2011. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 12: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Li S, Pulendran B. 2012. Systems vaccinology: Learning to compute the behavior of vaccine induced immunity. Wiley Interdiscip Rev Syst Biol Med 4: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, et al. 2015. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity 43: 1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, Patel NB, Zak DE, Aderem A, Dong T, et al. 2016. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci 113: 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, et al. 2014. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM. 2013. Vaccine development for tuberculosis: Current progress. Drugs 73: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru V, Steri M, Sole G, Sidore C, Virdis F, Dei M, Lai S, Zoledziewska M, Busonero F, Mulas A, et al. 2013. Genetic variants regulating immune cell levels in health and disease. Cell 155: 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. 2003. How stress influences the immune response. Trends Immunol 24: 444–448. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. 2003. Vaccines, vaccination, and vaccinology. J Infect Dis 187: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Pulendran B. 2009. Learning immunology from the yellow fever vaccine: Innate immunity to systems vaccinology. Nat Rev Immunol 9: 741–747. [DOI] [PubMed] [Google Scholar]
- Pulendran B. 2014. Systems vaccinology: Probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci 111: 12300–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. 2011. Immunological mechanisms of vaccination. Nat Immunol 12: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Li S, Nakaya HI. 2010. Systems vaccinology. Immunity 33: 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Cavanagh MM, Le Saux S, Wagar LE, Mackey S, Hu J, Maecker H, Swan GE, Davis MM, Dekker CL, et al. 2016. Defective T memory cell differentiation after varicella zoster vaccination in older individuals. PLoS Pathog 12: e1005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R, Mandl CW, Black S, De Gregorio E. 2011. Vaccines for the twenty-first century society. Nat Rev Immunol 11: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, et al. 2014. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, Machiah DK, Lawson B, Hakimpour P, Wang YC, et al. 2016. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 531: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat Med 19: 1597–1608. [DOI] [PubMed] [Google Scholar]
- Reif DM, Motsinger-Reif AA, McKinney BA, Rock MT, Crowe JE Jr, Moore JH. 2009. Integrated analysis of genetic and proteomic data identifies biomarkers associated with adverse events following smallpox vaccination. Genes Immun 10: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Bentebibel SE, Ueno H. 2014. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 35: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. 2011. Global quantification of mammalian gene expression control. Nature 473: 337–342. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Das RG, Wood JM, Katz JM. 2007. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: An international collaborative study. Vaccine 25: 4056–4063. [DOI] [PubMed] [Google Scholar]
- Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, et al. 2014. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell 157: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey MT, Wang Z, Kester KE, Cummings J, Heppner DG Jr, Nau ME, Ofori-Anyinam O, Cohen J, Coche T, Ballou WR, et al. 2010. Expression of genes associated with immunoproteasome processing of major histocompatibility complex peptides is indicative of protection with adjuvanted RTS,S malaria vaccine. J Infect Dis 201: 580–589. [DOI] [PubMed] [Google Scholar]
- van den Berg RA, Coccia M, Ballou WR, Kester KE, Ockenhouse CF, Vekemans J, Jongert E, Didierlaurent AM, van der Most RG. 2017. Predicting RTS,S vaccine-mediated protection from transcriptomes in a malaria-challenge clinical trial. Front Immunol 8: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Kappe SH. 2012. Malaria vaccine development: Persistent challenges. Curr Opin Immunol 24: 324–331. [DOI] [PubMed] [Google Scholar]
- Woods JA, Vieira VJ, Keylock KT. 2006. Exercise, inflammation, and innate immunity. Neurol Clin 24: 585–599. [DOI] [PubMed] [Google Scholar]
- Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, Krishnamurty AT, Chang JT, Adams DJ, Hensley TR, et al. 2012. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci 109: E3503–E3512. [DOI] [PMC free article] [PubMed] [Google Scholar]