Abstract
Clinical risk assessment involves absolute risk measures, but information on modifying risk and preventing cancer is often communicated in relative terms. To illustrate the potential impact of risk factor modification in model-based risk assessment, we evaluated the performance of the IBIS Breast Cancer Risk Evaluation Tool, with and without current body mass index (BMI), for predicting future breast cancer occurrence in a prospective cohort of 665 postmenopausal women. Overall, IBIS’s accuracy (overall agreement between observed and assigned risks) and discrimination (AUC concordance between assigned risks and outcomes) were similar with and without the BMI information. However, in women with BMI > 25 kg/m2, adding BMI information improved discrimination (AUC = 63.9 % and 61.4 % with and without BMI, P < 0.001). The model-assigned 10-year risk difference for a woman with high (27 kg/m2) versus low (21 kg/m2) BMI was only 0.3 % for a woman with neither affected first-degree relatives nor BRCA1 mutation, compared to 4.5 % for a mutation carrier with three such relatives. This contrast illustrates the value of using information on modifiable risk factors in risk assessment and in sharing information with patients of their absolute risks with and without modifiable risk factors.
Keywords: Breast cancer, IBIS, BMI, Prediction model, Risk evaluation
Introduction
Accurate assessment of a woman’s breast cancer risk is needed for the clinical management of decisions about preventive interventions (e.g., mammographic screening, risk-reducing surgery, and chemoprevention). Clinical guidelines use absolute risk, either as remaining lifetime risk (RLR) or as within a fixed time horizon (e.g., 5 or 10 years), to guide these decisions. For example, consideration of annual mammograms and MRI starting at age 30 years is recommended for women with RLR of 20 % or higher (as determined by risk models that are largely dependent on family history) [1] and consideration of risk-reducing strategies is recommended for women aged 35 years or more whose 5-year invasive breast cancer risk, as determined by the Breast Cancer Risk Assessment Tool (BCRAT) [2–4], is 1.7 % or higher.
Since breast cancer is caused by a complex interplay of many genetic and non-genetic risk factors, risk assessment is complex. Various statistical models (reviewed in [5]) have been developed for assigning absolute breast cancer risks. As the models differ with respect to their risk factors and their assumptions about the role of the competing risk of death, they can yield substantially different risk estimates. Therefore, the choice of a particular prediction model is an important aspect of clinical counseling. A further challenge is how best to communicate personal risk to individuals and physicians. Currently, counseling is based on stratification of women as “high risk,” (e.g., RLR ≥ 20 %) versus average risk < 20 % RLR [1]. Further, many modifiable risk factors, such as physical activity and alcohol intake, are not included in current risk assessment tools. Thus, integration of risk reduction strategies based on modifiable factors is limited to the modifiable factors present in a given risk model and to the ability to integrate relative risk concepts into communication of absolute risk estimates.
We have found that the IBIS model performed well on prospective data from 1857 pre- and postmenopausal women from the New York site of the breast cancer family registry (NYBCFR) [6]. We also have noted limitations of clinical guidelines based on the concept of RLR and instead recommended risk stratification based on shorter time periods [7]. Body mass index (BMI) is one of the few modifiable risk factors included in the IBIS model. Here, we use a cohort of high-risk women to investigate two effects of IBIS’ inclusion of BMI: (i) the extent of IBIS’ overall performance improvement and (ii) variation of BMI’s impact on IBIS-assigned risk across subgroups of women with different inherited risks.
Methods
Study sample
We studied women enrolled in the NYBCFR, using inclusion criteria described elsewhere [6–8]. We restricted eligibility to postmenopausal women with at least one subsequent update on cancer and vital status, and who at cohort enrollment were aged 20–70 years and had no prior history of bilateral prophylactic mastectomy. We excluded women with a personal history of breast cancer and women without information on weight and height. At enrollment, we collected data on demographics, lifestyle and environmental factors, and family history of cancer. All cohort participants provided written informed consent, and the study was approved by the relevant local ethics committees.
Risk model
We used the software packages IBIS v7 [9] (http://www.ems-trials.org/riskevaluator/) to predict a woman’s probability of developing breast cancer during a subsequent period after enrollment defined as her 10-year risk. The breast cancer hazard rate assumed by the IBIS model depends on a woman’s BRCA1 and BRCA2 mutation status, on her (unobserved) carrier status of a single hypothetical dominantly acting gene (to accommodate residual familial clustering), and on several non-genetic factors. These include age at menarche, parity, age at first live birth, age at menopause, prior use of hormone replacement therapy, history of hyperplasia/atypical hyperplasia, history of lobular carcinoma in situ, height, and BMI [11]. The BMI-associated hazard ratios relative to the referent group (BMI < 21 kg/m2) are 1.14 (21–23 kg/m2), 1.15 (23–25 kg/m2), 1.26 (25–27 kg/m2) 1.32 (>27 kg/m2) [9].
Statistical analysis
We evaluated the calibration of the IBIS model by comparing its 10-year assigned risks to breast cancer incidence within 10-year post-enrollment. We compared calibration and discrimination (as described elsewhere [6, 7]) for women (i) with actual reported information on BMI to (ii) a hypothetical scenario where all women had given no information on BMI (default setting of weights). We calculated the mean RLR for the entire cohort and compared for women (i) with actual reported information on BMI to (ii) a hypothetical scenario where all women had given no information on BMI (default setting of weight).
We also examined BMI-related differences in assigned 10-year risk in women with different levels of hereditary risk.
Results
BMI’s effect on IBIS performance
Calibration
During the 10-year post-enrollment risk period, 38 of the 665 women developed breast cancer, 42 died from other causes, 179 were last observed breast cancer-free during the period, and 406 were alive and free of breast cancer after 10-year follow-up. 342 (51 %) women reported a BMI exceeding 25 kg/m2. The overall mean observed 10-year breast cancer probability was 6.61 % (95 % CI 4.84–8.97 %), which was similar to the mean IBIS-assigned risk of 7.06 % (including BMI) and 7.00 % (without BMI). The IBIS-assigned risks also were well calibrated within subgroups of women determined by assigned risk level (data not shown).
Discrimination
IBIS’ receiver operating characteristic (ROC) plots also were similar with and without BMI information, with a common area under the curve (AUC) of 61.93 % (95 % CI 51.44–72.42 %) with BMI and of 61.87 % (95 % CI 51.44–72.3 %) without BMI. However, in the subgroup of women with a BMI greater than ≥ 25 kg/m2, the AUC was 63.9 % with BMI and 61.4 % without it (P value < 0.001).
Effect of BMI on IBIS-assigned risk
Since IBIS was well calibrated to this cohort, we use the risks assigned by this model to investigate BMI’s effect on a woman’s 10-year breast cancer risk. Figure 1 shows a scatterplot of 10-year risks as assigned by IBIS with and without inclusion of reported BMI information. Overall, the differences in absolute risk are modest, in agreement with the above performance results. However, they are higher for women having high risk due to other factors than for women at lower risk. Using the 10-year risk threshold of 3.4 % to define women at high risk (horizontal and vertical lines in the figure), we find that inclusion of BMI information reclassifies 22 (3.3 %) of the 665 women: 11 women are deemed high risk with BMI information but not without it, and conversely, another 11 women are deemed at lower risk with BMI information but not without it.
Fig. 1.
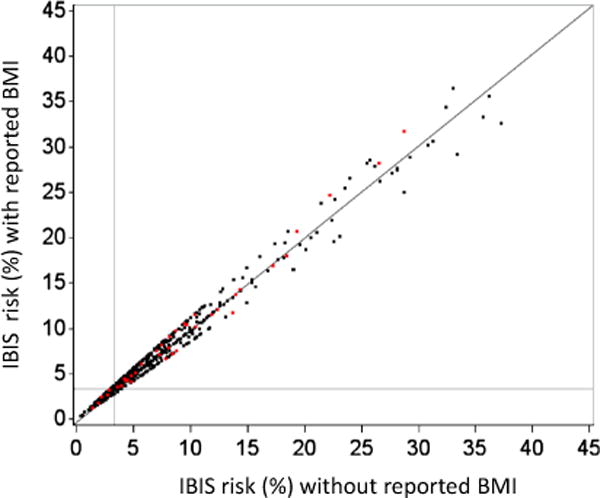
Scatterplot of 10-year risks with and without BMI in model. Points denote 10-year risks for 665 postmenopausal women as assigned by IBIS model with and without self-reported BMI. Women who developed breast cancer (N = 38) are highlighted in red. Horizontal and vertical lines indicate the commonly used 3.4 % 10-year threshold for high-risk status. The addition of BMI information reclassified 11 women from low to high risk, and 11 women from high to low risk
We also analyzed BMI’s influence on IBIS’ 10-year risks for five hypothetical women aged 50 years with varying levels of hereditary risk, keeping all other risk factors constant. For each woman, we examined the difference between her IBIS risk with BMI of 27 versus 21 kg/m2 in terms of number of affected first-degree relatives and BRCA1 mutation carrier status. Table 1 shows that this difference increases with a woman’s hereditary risk, ranging from 0.3 % for women without affected relatives or BRCA1 mutation to 4.5 % for those with three affected relatives and a BRCA1 mutation. This contrast shows that a woman at high hereditary risk can move across the 10-year threshold of 3.4 % used to increase screening strategies by increasing her BMI.
Table 1.
Effect of including BMI in IBIS model according to inherited risk factors
| Inherited risk factors No. affected first-degree relatives |
10-year risk (%)
|
||
|---|---|---|---|
| BMI = 27 kg/m2 | BMI = 21 kg/m2 | Risk difference | |
| 0 | 1.8 | 1.5 | 0.3 |
| 1 | 3.8 | 3.0 | 0.8 |
| 2 | 5.0 | 4.0 | 1.0 |
| 3 | 9.7 | 7.8 | 1.9 |
| 3 + BRCA1 mutation carrier | 24.6 | 20.1 | 4.5 |
Discussion
We have applied the IBIS breast cancer risk model to our prospective cohort of postmenopausal women at risk for breast cancer. We found that, although adding current BMI information does not improve the calibration or discrimination of the model, it does improve discrimination for the subgroup of postmenopausal women with a BMI greater ≥ 25 kg/m2. We also found that the BMI-induced change in IBIS-assigned 10-year risk was greater for women with high hereditary risks than for those with low hereditary risks. Moreover, obese women classified as high risk (i.e., as having ≥ 3.4 % 10-year risk) could be reclassified as low risk by changing BMI alone, although this would require a large weight reduction.
We focused on the role of BMI in postmenopausal women because of its positive association with breast cancer risk among postmenopausal, but not premenopausal women [10, 11]. The reasons for the specificity of this association are thought to involve circulating estrogen concentrations due to estrogen production in adipose tissues [12, 13]. In premenopausal women, estrogen levels (produced predominantly in the ovaries) are homeostatically regulated by a negative feedback system involving gonadotropins [12, 13].
The IBIS model, and others that ignore potential interactions between hereditary and nonhereditary factors (5, 9), assumes that the relative risk of a nonhereditary risk factor remains constant across the entire risk continuum. This assumption may be valid for BMI, as epidemiologic studies that have shown that (i) BMI does not alter risk ratios associated with family history of breast cancer [14] and (ii) the BMI-associated breast cancer risk ratio among postmenopausal women is similar for BRCA1/2 mutation carriers women in the general population [15].
Nevertheless, even if the assumption of constancy of relative risks across the risk spectrum holds, risk factor modification will, on the absolute risk scale, have higher impact on women with high hereditary risks than on those will lower hereditary risks. Thus communicating changes in absolute risk from modifiable risk factors may have a large impact at the individual level, particularly for women with high absolute risks.
Absolute risk thresholds for developing a cancer within a fixed time horizon (e.g., 5 or 10 years) are regularly quoted in clinical guidelines as key bases for decisions regarding prevention strategies these shorter term fixed time horizon may be more useful to guide clinical practice than overall lifetime risk [7]. For example, women whose 10-year breast cancer risks are 3.4 % or higher may be classified as high risk, and thus deemed appropriate for aggressive preventive strategies. Here we have illustrated how an obese woman can change her high-risk status by substantially reducing her weight. More generally, this argument indicates that even when risk factors have limited impact on a population level, they can have a large impact on how particular women are classified into categories affecting their screening and chemoprevention counseling. We recommend that women be informed about both absolute and relative risk reductions when counseled for breast cancer prevention.
Acknowledgments
This work was supported by Grant UM1 CA164920 from the USA National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the USA Government or the BCFR.
Funding: The authors declare that they do not have a financial relationship with the organization that sponsored the research.
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
References
- 1.Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE, Heerdt AS, Helvie M, Huff JG, Khakpour N, Khan SA, Krontiras H, Lyman G, Rafferty E, Shaw S, Smith ML, Tsangaris TN, Williams C, Yankeelov T, National Comprehensive Cancer N NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Cancer Netw. 2009;7(10):1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 2.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 3.Gail MH, Costantino JP, Pee D, Bondy M, Newman L, Selvan M, Anderson GL, Malone KE, Marchbanks PA, McCaskill-Stevens W, Norman SA, Simon MS, Spirtas R, Ursin G, Bernstein L. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 4.Matsuno RK, Costantino JP, Ziegler RG, Anderson GL, Li H, Pee D, Gail MH. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103(12):951–961. doi: 10.1093/jnci/djr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meads C, Ahmed I, Riley RD. A systematic review of breast cancer incidence risk prediction models with meta-analysis of their performance. Breast Cancer Res Treat. 2012;132(2):365–377. doi: 10.1007/s10549-011-1818-2. [DOI] [PubMed] [Google Scholar]
- 6.Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB. Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 2012;14(6):R144. doi: 10.1186/bcr3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quante AS, Whittemore AS, Shriver T, Hopper JL, Strauch K, Terry MB. Practical problems with clinical guidelines for breast cancer prevention based on remaining lifetime risk. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd N, Buys SS, Daly MB, O’Malley FP, Santella RM, Southey MC, Venne VL, Venter DJ, West DW, Whittemore AS, Seminara D. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–R389. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 10.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 11.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One. 2012;7(12):e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Key TJ, Allen NE, Verkasalo PK, Banks E. Energy balance and cancer: the role of sex hormones. Proc Nutr Soc. 2001;60(1):81–89. doi: 10.1079/pns200068. [DOI] [PubMed] [Google Scholar]
- 13.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 15.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, van Asperen CJ, Meijers-Heijboer H, Ausems MG, van Os TA, Gomez-Garcia EB, Brohet RM, van Leeuwen FE, Rookus MA. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2011;126(1):193–202. doi: 10.1007/s10549-010-1120-8. [DOI] [PubMed] [Google Scholar]


