Abstract
In early years, Dr. Donald S. Coffey fostered the development of a new model for the organization of DNA in the sperm nucleus that had many unexpected similarities to that of somatic cells. This was surprising because it was well known that mammalian sperm DNA is more compact than the DNA in mitotic chromosomes, and the fact that some of the major structural components of somatic cell DNA organization were retained in the sperm cell was not predicted. The highly compact sperm DNA is organized into loop domains that are attached at their bases to a proteinaceous nuclear matrix, at specific matrix attachment regions (MARs). This organization is required for DNA synthesis of the paternal genome hours after fertilization, and also participates in an apoptotic-like degradation of the DNA under certain conditions. The tight packaging of the sperm chromatin is due entirely to the replacement of histones by protamines, but the sperm DNA loop domain organization is not changed by this transition, and is probably inherited by the paternal pronucleus in the newly formed embryo.
Keywords: Spern DNA, nuclear matrix, pronucleus, embryo
Tribute to Don S. Coffey
In this special issue commemorating the scientific accomplishments of Donald S. Coffey, one of his many unique contributions to one of many fields is highlighted. He fostered the development of a new model for the organization of DNA in the sperm nucleus that had many unexpected similarities to that of somatic cells.
Decondensing sperm DNA
This article is part of a special issue, dedicated to Donald S. Coffey, Ph.D.. In 1985, Coffey’s lab was focused on cancer, but since he encouraged young scientists to explore interesting avenues of research that were even mildly related to the overall questions at hand, a young postdoctoral fellow who had recently joined him (the author of this review) was allowed to explore the higher order structure of sperm DNA packaging. The argument was that since sperm DNA was essentially functionally silent - there was no ongoing DNA replication or transcription of RNA - it was the perfect model for understanding the basics of all chromatin structure.
Previous attempts to study general aspects of sperm chromatin were frustrated by the tendency of the sperm nucleus to completely decondense, releasing all of its DNA when protamines were extracted with high salt and disulfide reducing reagents [1,2]. Researchers therefore had to capture the decondensation process in stages and these studies revealed that sperm chromatin was partitioned in lamellae, but they did not illuminate a model for the packaging that was remotely similar to that of somatic cells. We performed similar decondensation studies and identified a small ring-like structure we termed the sperm nuclear annulus that anchored all the decondensing DNA to the end of the tail (Figure 1E and 1F) [3]. But this was not a comprehensive model that bore any relationship to somatic cell DNA structure. Was it possible that sperm DNA was so completely different from somatic cell DNA that nothing could be learned about the latter from the former?
Figure 1.
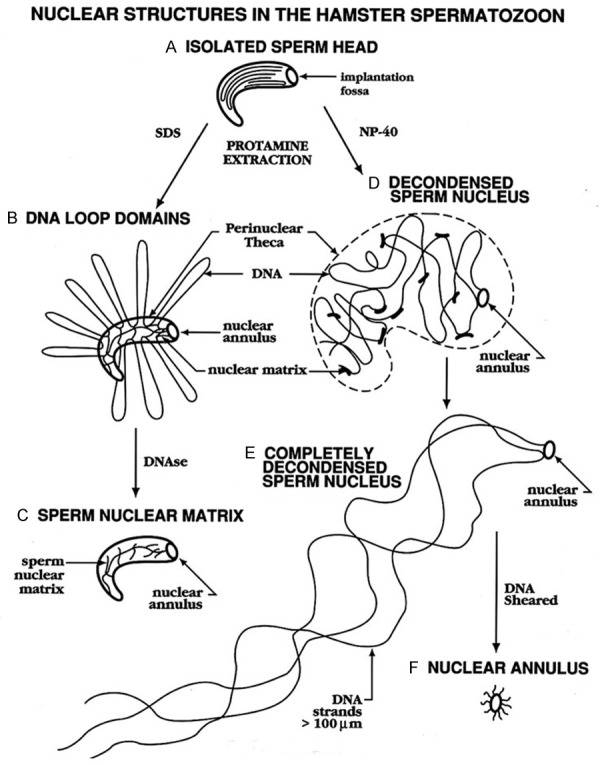
Fates of Mammalian Sperm Nuclei when the Protamines are Extracted. When mammalian sperm nuclei are extracted with high salt and dithiothreitol (DTT) to extract the protamines, they decondense completely, leaving only the nuclear annulus (D-F). When sperm are washed with SDS before salt extraction, the nuclear matrix remains intact (A-C) and DNA loop domains are visible. This figure was originally published in [25].
Sperm loops take shape
During one of the many discussions about sperm DNA, Coffey mentioned that he believed that DNA must be organized into loop domains. This was such a universal feature of chromatin structure, being present in interphase cells and essential for DNA replication [4,5], as well as the major structural component of mitotic chromosomes [6,7], that Coffey was convinced they must be present in sperm chromatin. The problem was that nothing supported the existence of loop domains in the sperm at that time. The traditional method to expose loop domains was to extract cell nuclei with high salt to remove the protamines, and then stain them with ethidium bromide to visualize the DNA. This revealed a halo of DNA surrounding the extracted loop domains that was composed of naked DNA loops attached at their bases to a sperm nuclear matrix (Figure 2E). All of our attempts to extract the protamines, the DNA binding proteins of sperm, resulted in the complete decondensation of the nucleus. If there was a sperm nuclear matrix there, it was being destroyed by the extraction procedure. The existence of loops domains in sperm also went against everything we knew at the time about protamines binding to DNA. The current models suggested that protamine-bound DNA was essentially linear, not folded into anything resembling a loop [8]. But Coffey’s absolute certainty that somewhere in the sperm cell we would find remnants of loops was unshakeable. Anyone who worked with Coffey soon learned that his hunches had a habit of paying off. His lifelong scientific mantra “If this is true, what does it imply?” had lead him to think long and hard about DNA loops over the previous fifteen years, and he had convinced me to believe in their likely universal importance in organizing the DNA. When one thinks about this for even a few minutes, one sees the logic in this - how could three feet of DNA be folded into a 10 µm long, 0.5 µm wide sperm nucleus without some kind of folding that involved loops? With Coffey’s strong intuition that loops were, in fact, present in the sperm cell, it was back to the drawing board.
Figure 2.
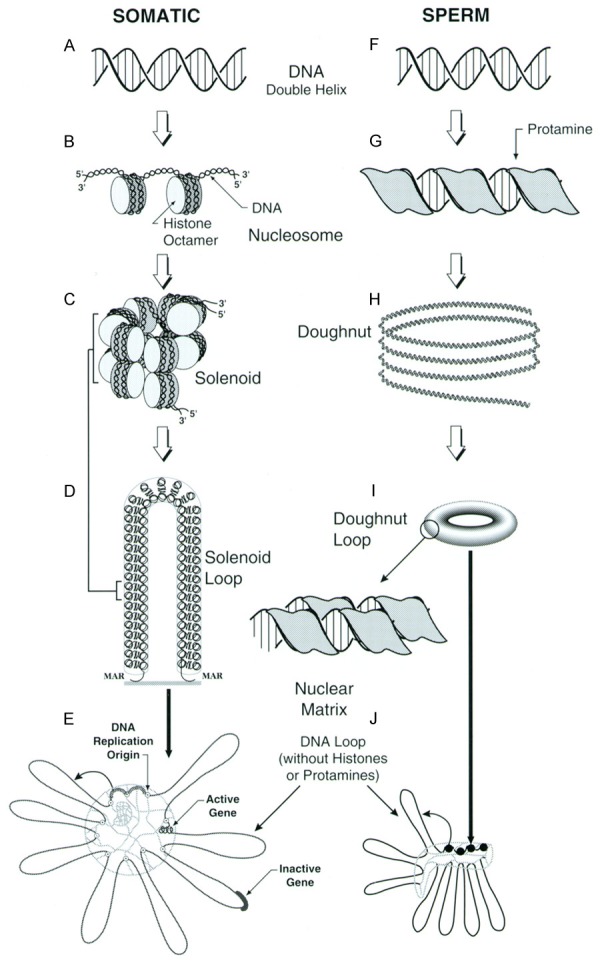
Comparison of DNA Packaging in Somatic and Sperm Cells. Somatic cell DNA (A) is coiled around histone octamers (B), then into a 30 nm filament that is often depicted as a solenoid (C). These 30 nm chromatin fibers are then attached at their bases to the sperm nuclear matrix to form loop domains (D). Loop domains can be examined experimentally by isolating nuclei and extracting the histones with 2 M NaCl (E). In sperm chromatin, the histones have been replaced by protamines that bind the DNA (F) in the major groove, completely neutralizing the negative charges of the DNA (G). This chromatin coils in to toroids (H and I). When sperm are extracted with salt after washing with SDS, the DNA loop domains are revealed (J). We have proposed that each protamine toroid represents one DNA loop domain. This figure was originally published in Biology of Reproduction [25] and a modified version published in Genetics of Human Male Fertility [33].
When one thinks about the function of the sperm cell, it seems logical that the nucleus would be spring loaded to decondense to release its compact DNA into the oocyte upon fertilization so that the embryo could functionally incorporate the paternal genome into the new being. On the other hand, this decondensation during normal fertilization is very controlled. Shortly after fertilization, the sperm chromatin divests itself of the very tightly binding protamines and replaces them with the much more functional histones (compare Figure 2C, 2D with 2H, 2I). During this time, the compact sperm nucleus expands to the size of a very large nucleus, then condenses again to a smaller pronucleus before DNA replication begins [9,10]. This is a process that could involve a nuclear matrix that could expand and contract. It was possible, therefore, that the complete decondensation of sperm nuclei that we and others had experienced when extracting sperm with high salt was an aberration of normal process. It was known that the sperm cell contained proteases that could digest the protamines [11], but these authors also showed that the proteinases were not necessary for fertilization. Perhaps these proteinases digested other sperm proteins that allowed the nuclei to decondense after fertilization? Other researchers had shown that mammalian sperm could survive a wash in the ionic detergent SDS [12]. This was the result of the extreme stability of sperm chromatin conferred by the protamines. Protamines are insoluble in SDS, and they are crosslinked by disulfide bonds. The condensed, insoluble chromatin appears to protect other nuclear proteins that are tightly integrated with the chromatin. We found that if we washed sperm with SDS, then washed out the SDS and extracted with high salt and dithiothreitol, hamster sperm nuclei formed “halos”, the classic structure that defined the existence of loop domains (Figures 1B and 2J) [13]. These halos fit all the criteria for DNA loop domain organization: they were topologically constrained, and had definitive sizes. A clue to the model for chromatin structure that would emerge ten years later, these DNA loop domains were not highly supercoiled. This corroborated an earlier report in Xenopus sperm, which also contain protamines, that protamine bound DNA was not supercoiled [14]. Without Coffey’s certainty that DNA was organized into loop domains in the sperm cell, we might not have pushed those experiments so hard to find them.
This work was compiled in a review that Coffey and I co-authored that was one of the most highly cited reviews on sperm chromatin ever published [15]. Another example of Coffey’s selflessness when it came to his trainees (and everyone else, for that matter) was the publication of this review. It was published shortly after I left Hopkins, and Coffey strongly admonished me not to include his name. He predicted this would have a huge impact on the field and he wanted me to have all the credit (the review has been cited 441 times). I am glad I did not take that one piece of advice. The years since have shown the importance of this level of chromatin organization in sperm. We, and other labs demonstrated that sperm loops are attached at their bases at specific sequences termed MARs [16-19]. Lawrence and colleagues showed the world the first images of individual loop domains identified by fluorescent in situ hybridization (FISH) in somatic cells [20], and this inspired us to identify them in sperm cells [21]. Both papers showed something else that Coffey had long before predicted - that loop domain organization would be different in different cell types. Others had provided evidence for cell-specific matrix attachment sites [22], but Lawrence and colleagues were the first to visualize it. This work, and others not cited, firmly demonstrated that the evolutionary pressure to condense the sperm chromatin which sacrificed much of the canonical structural elements in somatic cell chromatin did not eliminate the organization of sperm DNA into loop domains. This suggested to us a model for the transmission of sperm loop domain organization from the sperm nucleus to the embryo that has yet to be experimentally verified.
A model for sperm chromatin structure
The foundation of the sperm work that we did between 1985 and 1990 lead to an entire career in the field. For the next ten years, when I was located at Robert Wood Johnson Medical School in New Brunswick, New Jersey, I maintained frequent contact with my mentor Don Coffey. The models and work progressed with the help of his insights and encouragement. The work that Coffey and I did together lead to the next question that had been plaguing us all during that time, how do we reconcile the idea that sperm DNA was folded into loops and that it was condensed into an almost crystalline like structure by the protamines? Protamines are small proteins that are essentially poly-arginines. The positive charges of the arginine residues neutralize the negative charges of the phosphodiester backbone of the DNA [8,23]. Each individual loop had to condensed or folded in some way, or the DNA must be tangled. If our previous data were correct, what did this imply for sperm packaging? Then, Balhorn and colleagues solved one important piece of this puzzle. They demonstrated that protamine bound DNA was coiled into toroids that contained roughly 20 to 50 kb of DNA [24]. This fit with our observation that sperm DNA was not supercoiled. We calculated that 50 kb of DNA coiled into a toroid with the dimensions Balhorn’s group had shown would have some, but very little supercoiling, not enough to have been detected by our ethidium bromide assay [25]. This brought an obvious model to mind in which each loop domain condensed into one protamine toroid. To test that model, we took advantage of a second property of protamine bound DNA, its resistance to digestion by nucleases. If it was true that each loop domain was one protamine toroid, what would that imply? It would mean that the DNA that linked each toroid would also be the sites of the matrix attachment regions (MARs). This also implied that these sections of the chromatin would not be bound to protamines, but to the 1% to 10% of histones that remain in mammalian sperm. These toroid linker regions would be susceptible to degradation by nucleases. We tested this model by treating fully condensed hamster sperm nuclei with varying doses of DNAse I. The model predicted that the chromatin would be degraded into loop-sized fragments of 20 to 50 kb, and would not be further digested. The experiments supported this model for chromatin organization [26], which was recently more fully described in a review [27].
The active sperm nuclear matrix
“Growing up” in Coffey’s vibrant laboratory at Johns Hopkins meant that one was a part of an exciting group of like-minded scientists who loved to think about chromatin structure and its function. Topoisomerase 2 (TOP2) was key player in these discussions since it was discovered that it was one of the scaffolding proteins of the mitotic chromosomes - the proteins that held the loops together [28]. TOP2 is a homodimeric enzyme that cleaves both strands of DNA, passes another double strand through the break, then reseals the breaks [29]. The laboratory of Leroy Liu, another of Coffey’s many collaborators at Hopkins, showed that during apoptosis, TOP2 cleaved the DNA into loop sized fragments in somatic cells [30]. This first step of DNA degradation during apoptosis could go one of two ways; if apoptosis was fully activated, nucleases would irreversibly digest the chromatin further, but if the “threat” to the cell was removed in time, TOP2 would repair the initial double stranded breaks. In our original foray into the doughnut-loop model for sperm chromatin, in addition to showing that DNAse I could digest the toroid linkers, we also showed that sperm had the ability to cleave its own DNA [26]. Later, we demonstrated that this activity was reversible, just as in somatic cell apoptosis, in epididymal sperm, but in vas deferens sperm the degradation proceeded further to an irreversible stage [31]. This mirrored the TOP2 mediated degradation in somatic cell apoptosis. More interestingly, it showed that the highly compact sperm chromatin was capable of responding to its environment.
Don Coffey and DNA structure
The science in the story described above has already been detailed in several different reviews [15,27,32]. But the real story behind it lies with the man who made it all possible. Don Coffey was a rare scientist who always put his concerns for the progress of the scientists whom he took under his wing above any of his own. He cared very deeply for all the people with whom he worked. One of the most memorable examples of this I witnessed was when I was a postdoctoral fellow in his laboratory in 1986. Coffey was invited to give a plenary lecture at the annual meeting of the American Fertility Society in Toronto. Because of the unpublished work we had done on the sperm DNA loop domain organization, he was a good choice for this meeting. But Coffey always tried to have the student or postdoc present the work the first time it appeared in public. So, in his own indomitable way, Coffey made a decision without informing the program committee. He would give the first 30 min of the talk, then invite me up to the stage to present the last 10 min of the talk. He coached me on this for weeks, and though I was a little afraid of the repercussions, I trusted his instincts. The talk went well, and even 20 years later, I was invited to give a talk at a meeting because the organizer had been in that 1986 audience. I have described Don Coffey many times as a truly great scientist who made it by always helping others. I think this is the legacy of which he was most proud, his humanity.
Acknowledgements
This work was supported by NIH Grants P20GM10345 and 1 R01 GM123048 to W.S.W.
References
- 1.Livolant F. Cholesteric organization of DNA in the stallion sperm head. Tissue Cell. 1984;16:535–555. doi: 10.1016/0040-8166(84)90029-6. [DOI] [PubMed] [Google Scholar]
- 2.Tsanev R, Avramova Z. Nonprotamine nucleoprotein ultrastructures in mature ram sperm nuclei. Eur J Cell Biol. 1981;24:139–145. [PubMed] [Google Scholar]
- 3.Ward WS, Coffey DS. Identification of a sperm nuclear annulus: a sperm DNA anchor. Biol Reprod. 1989;41:361–370. doi: 10.1095/biolreprod41.2.361. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM, Vogelstein B, Coffey DS. A fixed site of DNA replication in eucaryotic cells. Cell. 1980;19:527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Pardoll DM, Coffey DS. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980;22:79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- 6.Paulson JR, Laemmli UK. The structure of histone-depleted metaphase chromosomes. Cell. 1977;12:817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- 7.Mirkovitch J, Mirault ME, Laemmli UK. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984;39:223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- 8.Balhorn R. A model for the structure of chromatin in mammalian sperm. J Cell Biol. 1982;93:298–305. doi: 10.1083/jcb.93.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adenot PG, Szollosi MS, Geze M, Renard JP, Debey P. Dynamics of paternal chromatin changes in live one-cell mouse embryo after natural fertilization. Mol Reprod Dev. 1991;28:23–34. doi: 10.1002/mrd.1080280105. [DOI] [PubMed] [Google Scholar]
- 10.Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod. 2006;75:442–51. doi: 10.1095/biolreprod.106.053223. [DOI] [PubMed] [Google Scholar]
- 11.Perreault SD, Zirkin BR. Sperm nuclear decondensation in mammals: role of sperm-associated proteinase in vivo. J Exp Zool. 1982;224:253–257. doi: 10.1002/jez.1402240215. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien DA, Bellve AR. The mammalian spermatozoon: structure and temporal assembly. In: Hartmann JF, editor. Mechanism and control of animal fertilization. New York: Academic Press; 1983. pp. 55–137. [Google Scholar]
- 13.Ward WS, Partin AW, Coffey DS. DNA loop domains in mammalian spermatozoa. Chromosoma. 1989;98:153–159. doi: 10.1007/BF00329678. [DOI] [PubMed] [Google Scholar]
- 14.Risley MS, Einheber S, Bumcrot DA. Changes in DNA topology during spermatogenesis. Chromosoma. 1986;94:217–227. doi: 10.1007/BF00288496. [DOI] [PubMed] [Google Scholar]
- 15.Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–574. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- 16.Ostermeier GC, Liu Z, Martins RP, Bharadwaj RR, Ellis J, Draghici S, Krawetz SA. Nuclear matrix association of the human beta-globin locus utilizing a novel approach to quantitative real-time PCR. Nucleic Acids Res. 2003;31:3257–3266. doi: 10.1093/nar/gkg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid C, Heng HH, Rubin C, Ye CJ, Krawetz SA. Sperm nuclear matrix association of the PRM1-->PRM2-->TNP2 domain is independent of Alu methylation. Mol Hum Reprod. 2001;7:903–911. doi: 10.1093/molehr/7.10.903. [DOI] [PubMed] [Google Scholar]
- 18.Kalandadze AG, Bushara SA, Vassetzky YS Jr, Razin SV. Characterization of DNA pattern in the site of permanent attachment to the nuclear matrix located in the vicinity of replication origin. Biochem Biophys Res Commun. 1990;168:9–15. doi: 10.1016/0006-291x(90)91667-h. [DOI] [PubMed] [Google Scholar]
- 19.Ward WS, Coffey DS. Specific organization of genes in relation to the sperm nuclear matrix. Biochem Biophys Res Commun. 1990;173:20–25. doi: 10.1016/s0006-291x(05)81015-0. [DOI] [PubMed] [Google Scholar]
- 20.Gerdes MG, Carter KC, Moen PT Jr, Lawrence JB. Dynamic changes in the higher-level chromatin organization of specific sequences revealed by in situ hybridization to nuclear halos. J Cell Biol. 1994;126:289–304. doi: 10.1083/jcb.126.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadel B, de Lara J, Finkernagel SW, Ward WS. Cell-specific organization of the 5S ribosomal RNA gene cluster DNA loop domains in spermatozoa and somatic cells. Biol Reprod. 1995;53:1222–1228. doi: 10.1095/biolreprod53.5.1222. [DOI] [PubMed] [Google Scholar]
- 22.Cockerill PN, Garrard WT. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986;44:273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- 23.Prieto MC, Maki AH, Balhorn R. Analysis of DNA-protamine interactions by optical detection of magnetic resonance. Biochemistry. 1997;36:11944–11951. doi: 10.1021/bi971061l. [DOI] [PubMed] [Google Scholar]
- 24.Hud NV, Allen MJ, Downing KH, Lee J, Balhorn R. Identification of the elemental packing unit of DNA in mammalian sperm cells by atomic force microscopy. Biochem Biophys Res Commun. 1993;193:1347–1354. doi: 10.1006/bbrc.1993.1773. [DOI] [PubMed] [Google Scholar]
- 25.Ward WS. Deoxyribonucleic acid loop domain tertiary structure in mammalian spermatozoa. Biol Reprod. 1993;48:1193–1201. doi: 10.1095/biolreprod48.6.1193. [DOI] [PubMed] [Google Scholar]
- 26.Sotolongo B, Lino E, Ward WS. Ability of hamster spermatozoa to digest their own DNA. Biol Reprod. 2003;69:2029–2035. doi: 10.1095/biolreprod.103.020594. [DOI] [PubMed] [Google Scholar]
- 27.Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16:30–36. doi: 10.1093/molehr/gap080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earnshaw WC, Halligan B, Cooke CA, Heck MM, Liu LF. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985;100:1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 30.Li TK, Chen AY, Yu C, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamauchi Y, Shaman JA, Ward WS. Topoisomerase II mediated breaks in spermatozoa cause the specific degradation of paternal DNA in fertilized oocytes. Biol Reprod. 2007;76:666–672. doi: 10.1095/biolreprod.106.057067. [DOI] [PubMed] [Google Scholar]
- 32.Ward WS, Zalensky AO. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr. 1996;6:139–147. doi: 10.1615/critreveukargeneexpr.v6.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 33.Ward WS. Chromosome organization in mammalian sperm nuclei. In: Barratt CL, de Jong JH, Mortimer D, Parinaud J, editors. Genetics of human male fertility. Paris: Editions E.D.K.; 1997. pp. 147–163. [Google Scholar]


