Abstract
Objective
To perform a systematic review and meta-analysis on the prevalence of TDP-43 proteinopathy in cognitively normal older adults.
Methods
We systematically reviewed and performed a meta-analysis on the prevalence of TDP-43 proteinopathy in older adults with normal cognition, evaluated by the Mini-Mental State Examination or the Clinical Dementia Rating. We estimated the overall prevalence of TDP-43 using random-effect models, and stratified by age, sex, sample size, study quality, antibody used to assess TDP-43 aggregates, analyzed brain regions, Braak stage, CERAD score, hippocampal sclerosis, and geographic location.
Results
A total of 505 articles were identified in the systematic review, and seven were included in the meta-analysis with 1,196 cognitively normal older adults. We found an overall prevalence of TDP-43 proteinopathy of 24%. Prevalence of TDP-43 proteinopathy varied widely across geographic location (North America: 37%, Asia: 29%, Europe: 14%, and Latin America: 11%). Estimated prevalence of TDP-43 proteinopathy also varied according to study quality (quality score >7: 22% vs. quality score <7: 42%), antibody used to assess TDP-43 proteinopathy (native: 18% vs. hyperphosphorylated: 24%), and presence of hippocampal sclerosis (without 24% vs. with hippocampal sclerosis: 48%). Other stratified analyses by age, sex, analyzed brain regions, sample size, and severity of AD neuropathology showed similar pooled TDP-43 prevalence.
Conclusions
Different methodology to access TDP-43, and also differences in lifestyle and genetic factors across different populations could explain our results. Standardization of TDP-43 measurement, and future studies about the impact of genetic and lifestyle characteristics on the development of neurodegenerative diseases are needed.
Keywords: TDP-43 proteinopathy, brain aging, elderly, dementia, postmortem
Introduction
Abnormal accumulation of transactive response DNA-binding protein 43 (TDP-43) is found in several age-related diseases, including frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease (AD) [1, 2]. Indeed, expression of clinical symptoms during disease progression follows regional and cell-specific patterns of pathological TDP-43 accumulation [3]. For example in FTD behavioral variant, TDP-43 inclusions are present in brain regions that play a central role in emotional control, such as in the orbitofrontal cortex and the amygdala, while in ALS the encephalic regions responsible for motor control are primarily affected [4, 5]. In the semantic variant of FTD, left lateral temporal cortex atrophy is associated with frontotemporal lobar degeneration with accumulation of TDP-43 (FTLD-TDP). TDP-43 pathology has been also associated with aggravated memory loss and medial temporal atrophy in AD, which are major features of the disease [6]. Furthermore, TDP-43 proteinopathy have been independently associated with more rapid cognitive decline and impairment of episodic memory in older adults [7].
Pathological changes in the brain may be present decades before the onset of clinical symptoms [8, 9]. Early detection of neurodegenerative diseases is crucial to better understand their pathophysiology and improve the diagnosis and treatment of these diseases. Tools to assess pathological accumulation of proteins related to other neurodegenerative diseases in patients, such as tau and β-amyloid [10–12], are now possible to be used in patients. However, approaches to detect TDP-43 accumulation in vivo in humans are not yet available [13]. A way to better understand the early changes in TDP-43 protein is studying its prevalence through post-mortem neuropathological evaluation in cognitively normal older adults. These studies have shown a wide variation in TDP-43 frequency, ranging from 8 to 52% [14–17]. In this study, we performed a systematic review of the literature on the prevalence of TDP-43 proteinopathy in cognitively normal older adults.
Methods
The systematic review and meta-analysis were performed based on the guidelines of both the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [18–20] and the Meta-analyses of Observational Studies in Epidemiology (MOOSE) [21].
Selection of studies
We conducted a systematic review of the literature from January 2006 to November 2015 on the prevalence of TDP-43 proteinopathy, using the search terms: "TDP-43 AND cognitively normal elderly" OR "TDP-43 AND health aging” OR “TDP-43 AND normal aging” OR “TDP-43 AND controls”. All search terms were used across three different databases: Pubmed (which comprises MEDLINE, Mesh, and NLM), EMBASE, and Web of Science. Three investigators conducted the search independently of each other (ATA, CB, and CN).
We included studies that had data on the prevalence of TDP-43 proteinopathy in cognitively normal subjects without FTLD-TDP. Normal cognition was evaluated by the Clinical Dementia Rating (CDR=0) [22] or by the Mini-Mental State Examination [23]. To be included in this meta-analysis, articles had to state ethical approval, and the assessment of TDP-43 inclusions had to be performed using immunohistochemistry in post-mortem brain tissue, which is the gold-standard method to diagnose TDP-43 proteinopathy [24]. Because TDP-43 proteinopathy can be found widespread in the brain [1, 4, 17, 25], we considered studies that analyzed these inclusions in any encephalic region. Since TDP-43 proteinopathy in neurodegenerative diseases was first described in 2006, we included studies published from 2006 to 2015. Only articles published in English were included.
Duplicate articles among the databases were excluded and the remaining articles were organized in numeric codes. Two blinded investigators (ATA and CN) screened the titles and the abstracts of all articles and selected those that were considered eligible to this study. We also checked the references of these articles for additional eligible studies. Subsequently, ATA and CN read the entire content of the eligible articles and reached a consensus on those that met the inclusion criteria. For data extraction, authors consulted all available information in the included articles, such as figures and tables, as well as the main text. Besides the sample size and the number of subjects with TDP-43 proteinopathy, we also included information about other variables that could explain heterogeneity among studies. These variables were age, sex, where the study was conducted, type of antibody used to assess TDP-43 inclusions, and the brain regions in which TDP-43 proteinopathy was analyzed. If any variable of interest was missing, CN contacted the corresponding authors of the articles to request the missing data by e-mail.
Quality control
Quality control of the included studies was performed based on a guideline for the evaluation of prevalent studies [26]. We used nine of the ten original guideline questions to assess the quality of the included studies. One question was excluded because it was regarding recruitment of the patients and our study focused on post-mortem neuropathological data. A score from 0 to 9 was given independently by each one of the investigators (ATA and CN), according to sample size, reliability of the study, and quality of the statistical analysis. Higher scores indicate better study quality. The final score was achieved by consensus between the two investigators when necessary.
The minimum required sample size to be included in this meta-analysis was calculated, according to the formula [26]: , where n= sample size, Z= Z-statistic for the level of confidence, P= expected prevalence or proportion, and d=precision.
We considered an overall prevalence of 40%, since this was the average prevalence found in studies with larger sample sizes and good quality control scores [15, 17]. Therefore, the minimum sample size required to be included in this study was 47.
Data analysis
Meta-analytic methods enable the quantitative integration of data across multiple observational studies in order to derive pooled prevalence estimates stratified across key variables, such as different diagnostic criteria for TDP-43 diagnosis or the geographical region of the population sampling [18–21]. Data analysis was carried out using Stata software (version 12, StataCorp, College Station, TX, USA). We used the following user-defined procedures: metaprop, metafunnel, and metabias [27, 28].
The level of statistical heterogeneity was reported using the I2 statistic [(Q statistic—degree of freedom) /Q statistic) × 100%], which is a measure of the percentage of variability in the pooled prevalence estimate due to inter-study heterogeneity (tau-squared: τ2) [29]. Since significant heterogeneity was present in all analyses, a random-effects model was used. In random-effects models, the inverse variance is corrected by a measure of between-study variation (τ2), thus reducing the effects of sample size. Because prevalence is a proportion, study estimates were combined using a log transformation to help normalize the data. Confidence intervals were calculated using the exact method [30], which is the gold-standard method for binomial data [28].
We performed sensitivity analyses to explore possible explanations for variations in TDP-43 prevalence: (1) sample size (<300 and ≥300); (2) mean age (<80 and ≥80 years old); (3) sex; (4) antibody used to asses TDP-43 aggregates (antibody that recognizes total TDP-43 protein, or antibody that recognizes its hyperphosphorylated form, with a specific site of phosphorylation at serine 409/410, mentioned throughout the text as pser409/410); (5) analyzed brain regions (limbic and non-limbic); and (6) geographic location (North America, Europe, Asia, and Latin America), (7) geographic location, but considering only studies that analyzed limbic brain regions; and (8) study quality (quality score ≥7 versus quality score <7).
In addition, we requested additional data from all included studies to investigate TDP-43 prevalence according to Braak & Braak stage and CERAD scores [31, 32]. Braak & Braak classification was categorized in Braak stage ≤II and ≥III, and CERAD score in ≤A and ≥B [33]. Similarly, we investigated TDP-43 prevalence among participants with and without hippocampal sclerosis (HS).
Funnel plots of the prevalence logit plotted against the standard error were assessed for asymmetry to look for evidence of publication bias. Begg’s rank correlation test and Egger’s asymmetry test were also conducted to provide a formal assessment of publication bias across studies [34, 35].
Results
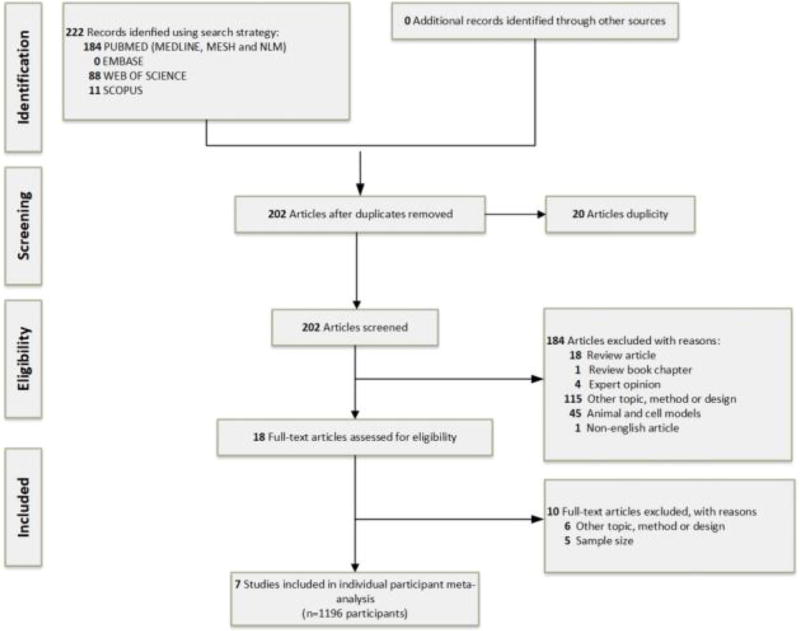
We identified a total of 505 abstracts regarding the prevalence of TDP-43 accumulation in cognitively normal subjects throughout all databases. From these, 202 abstracts were suitable according to the inclusion criteria, and 18 articles were selected for full-text assessment. After this step, we contacted authors from these studies to retrieve missing data. We finally included seven studies in the systematic review and meta-analysis that included data from 1,196 participants (Figure 1).
Figure 1.
Flow diagram of the literature search and study selection through the different phases of the systematic review.
Characteristics of the subjects from these seven studies are shown in Table 1. The mean age at death was 80.2±8.5 years. Two studies were from North America, two from Europe, two from Asia, and one from Latina America. The mean quality score of the studies was 6.5±2.0 (with minimum score of 2.5 and maximum of 8.0).
Table 1.
Studies selected for meta-analysis according to the inclusion criteria (n = 7)
| Author, year |
sample size (n) |
TDP-43 proteinopathy (n) |
Mean (SD) age at death |
Male (n) |
Geographic location |
Bruin regions analyzed |
Quality control score |
|---|---|---|---|---|---|---|---|
| Yu et al., 2015 | 363 | 136 | 86.6 (6.9) | 41 | North America | Limbic and neocortex | 5 |
| Keage et al., 2014 | 101 | 18 | 90.6 (4.6) | 40 | Europe | Only Limbic | 8 |
| Kovacs et al., 2013 | 51 | 4 | 81.8 (2.8) | 26 | Europe | Limbic and neocortex | 8 |
| Mizuno et al., 2012 | 112 | 18 | 74 (n/a) | 72 | Asia | Brainstem | 2.5 |
| Nascimento et al 2015 | 323 | 34 | 69.9 (11.7) | 155 | Latin America | Limbic, neocortex and other regions (subcortical nuclei and brainstem) | 7 |
| Arnold et al., 2013 | 110 | 40 | 86 (6) | 54 | North America | Limbic and neocortex | 7 |
| Uohino et al 2015 | 136 | 55 | 78.5 (9.7) | 95 | Asia | Limbic, neocortex and other regions (subcortical nuclei and brainstem) | 8 |
SD=standard deviation; INA = Information not available
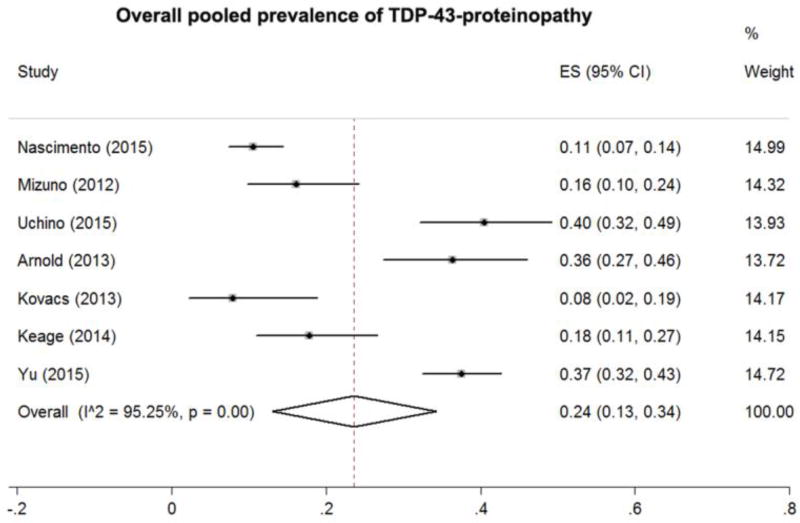
Our meta-analysis of pooled prevalence of TDP-43 in cognitively normal subjects showed an overall prevalence of 24% (effect size [ES] = 0.24, 95% confidence interval [CI] = 0.13–0.34, Figure 2), with a high heterogeneity across studies (ES varying from 0.08 to 0.40, I2 = 126%, p = 0.001).
Figure 2.
Estimated prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy of the included articles and overall meta-analysis pooled prevalence
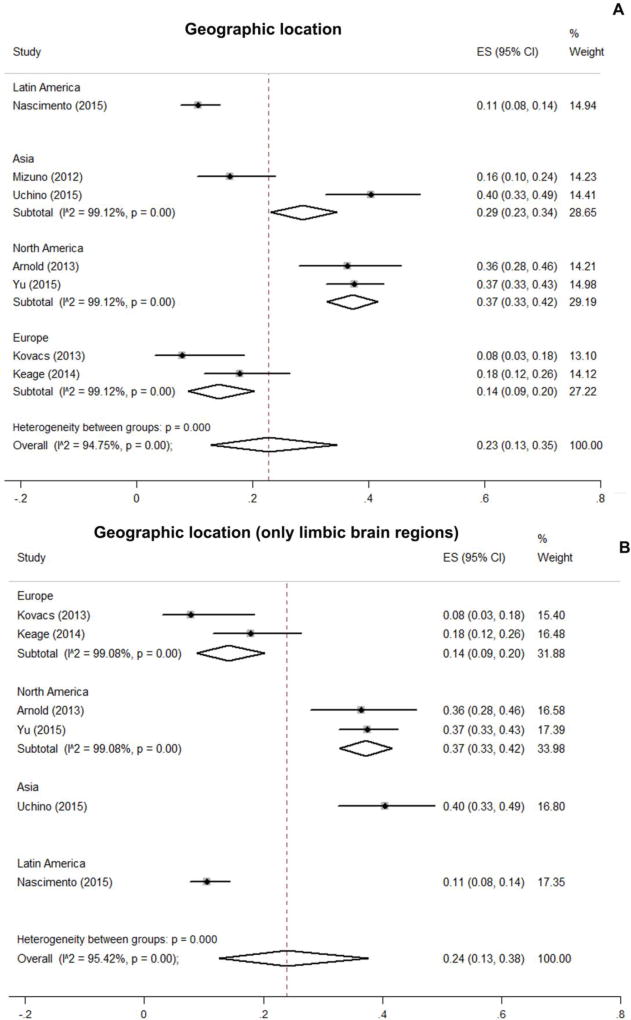
When pooled prevalence was stratified across geographical region, the highest prevalence found was in North America at 37%, followed by Asia at 29%. Europe and Latin America showed the lower prevalence, 14% and 11% respectively (Figure 3A). Interestingly, effect sizes were similar within studies from the same geographic location, except for Asia, where prevalence varied from 16% to 40%. In the case of North America, TDP-43 prevalence was 36% [15] and 37% [36], while TDP-43 prevalence in Europe was lower and varied from 8% [14] to 18% [37]. Because previous studies [16, 17] showed that limbic brain regions (including hippocampus and amygdala) are preferentially affected in cognitively normal subjects, we performed a sensitivity analysis of the pooled prevalence of TDP-4-proteinopahty, considering neuropathological findings only in hippocampus and amygdala (Figure 3B). While a slight difference was found in the pooled prevalence, the variation across geographic location remained similar.
Figure 3.
Estimated prevalence values of transactive response DNA-binding protein 43 proteinopathy of the included articles grouped by geographic location (all brain regions, A vs. only limbic brain regions, B).
Pooled TDP-43 prevalence among cognitively normal subjects were similar regarding mean age at death (<80 years old: ES = 0.21, 95% CI = 0.06–0.41; and 80 years old: ES = 0.24, CI = 0.13–0.39; Supplementary Figure 1), sex (Male: ES = 0.24, 95% CI = 0.11 – 0.38; and Female: ES = 0.24, 95% CI = 0.13 – 0.36, Supplementary Figure 2A and B, respectively) and brain regions analyzed (limbic ES = 0.25, 95% CI = 0.12–0.41; and non-limbic: ES = 0.17, 95%CI=0.05 – 0.50, Supplementary Figure 3A).
Regarding the type of antibody used to identify the TDP-43 protein, the antibody against the pser409/410 showed pooled prevalence of 24% (95%CI = 0.12 – 0.37), while the one that recognizes the native form of the protein 18% (95%CI = 0.12 – 0.26, Supplementary Figure 2B). Higher quality studies (quality control ≥7) showed a TDP-43 prevalence of 21%, while lower quality studies had a prevalence of 32% (quality control <7, Supplementary Figure 2C). We find similar TDP-43 prevalence when we pooled studies with sample size ≥300 (ES = 0.23, 95%CI = 0.20 – 0.27), and <300 participants (ES = 0.23, 95%CI = 0.12 – 0.35; Supplementary Figure 2D).
Five out of seven studies provided additional data about TDP-43 prevalence in participants with lower AD neuropathology and higher AD neuropathology (Supplementary Figures, 4 and 5). The prevalence of TDP-43 among participants with Braak ≤II was 20% (ES=0.20, 95%CI=0.06–0.34, Supplementary Figure 4A) and 23% in Braak ≥III (ES=0.23, 95%CI=0.08–0.38, Supplementary Figure 4B). Similarly, TDP-43 prevalence was 23% in participants with CERAD ≤A (ES=0.23, 95%CI=0.08–0.37, Supplementary Figure 5A) and CERAD ≥B (ES=0.23, 95% CI=0.08–0.37, Supplementary Figure 5B). Four out of seven studies had information on TDP-43 and HS. Among these four studies, only 2 studies had participants with HS pathology in the brain. The prevalence of TDP-43 was 24% in participants without HS (ES=0.24, 95%CI=0.07–0.40, Supplementary Figure 6A) and 48% in participants with HS (ES=0.48, 0.28–0.68, Supplementary Figure 6B); however this last estimate varied widely between the two available studies (27% in Nascimento et al. to 75% in Yu et al.).
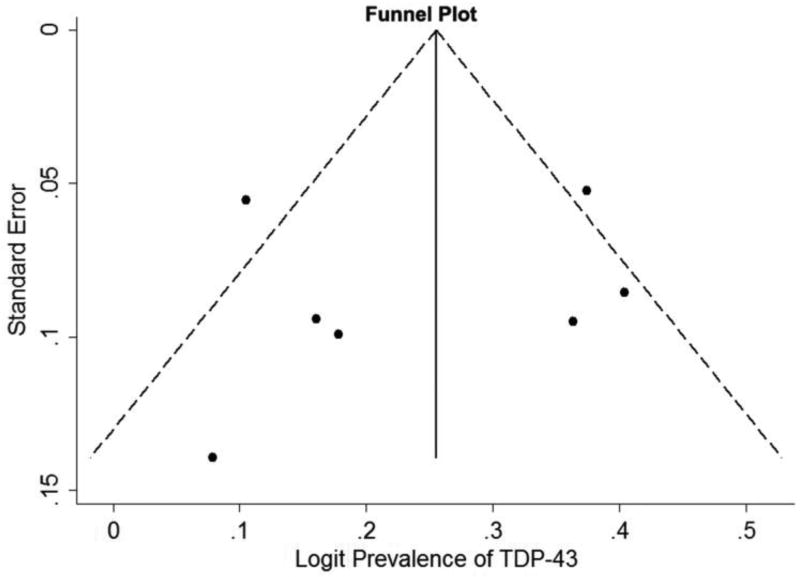
We looked for evidence of publication bias using the funnel plot, and the Egger’s and Begg’s tests. Figure 4 shows the funnel plot of the logit prevalence of TDP-43, plotted against the logit standard error. Egger’s test for small-study effects was not significant (t = −0.35, p = 0.74). The Begg’s adjusted rank correlation test for funnel plot asymmetry also did not show evidence of publication bias (z=0.90, p=0.37).
Figure 4.
Publication bias assessed by the funnel plot of the logit prevalence of transactive response DNA-binding protein 43 (TDP- 43) proteinopathy plotted against the standard error
Discussion
In a meta-analysis of seven studies with 1,196 cognitively normal older adults, we found an overall prevalence of TDP-43 proteinopathy of 24%. We observed high heterogeneity with a wide range of TDP-43 prevalence across studies. We also found large differences in the pooled prevalence estimation of TDP-43 when we stratified the meta-analysis by geographic location, with higher prevalence in North America and lower prevalence in Europe and Latin America. Higher TDP-43 prevalence values were found in subjects with HS. We found similar prevalence estimations of TDP-43 among cognitively normal subjects when we stratified the meta-analysis by age, sex, analyzed brain regions, and Braak and CERAD scores.
Unfortunately, there is a lack of systematic reviews and meta-analysis studies to estimate overall prevalence of misfolded proteins in cognitively normal older adults with neurodegenerative diseases. The available data are from post-mortem neuropathological studies, and only a few originally focused on cognitively normal subjects. A previous neuropathological study on these subjects showed a prevalence of 23% of α-synuclein, which is another misfolded protein present in the brain of patients with Parkinson’s disease [38]. This result is similar to what we observed for TDP-43 proteinopathy overall prevalence after meta-analysis (24%). On the other hand, the reported prevalence of other misfolded proteins, such as tau and β-amyloid found in cognitively normal elderly adults is twice as high compared to what was found for TDP-43 or α-synuclein. Up to 45% of non-demented subjects meet neuropathological criteria for AD based on the National Institute on Aging (NIA)-Reagan, which means they have considerable neuropathological burden of two misfolded proteins (tau and β-amyloid) without manifestation of clinical symptoms [39–42]. The relationship between the presence of protein aggregates in the brain and the clinical manifestation in neurodegenerative diseases is not completely understood. Since in cognitively normal subjects TDP-43 and α-synuclein were less frequent than tau and β-amyloid, we may speculate that TDP-43 and α-synuclein protein aggregates seem to be more toxic than tau and β-amyloid, since less burden of pathology is found in cognitively normal subjects who present these types of aggregates in the brain. Tau protein aggregates may be influenced by environmental/ lifestyle factors [43]. In the case of TDP-43 pathology, several underlying genetic factors have been identified [44], but little is known about the role of environmental/ lifestyle factors. These factors should receive more attention, since TDP-43 functions are particularly important under cellular stress conditions [45–48].
Other important factor to be considered is the co-occurrence of different types of aggregates [14]. Because misfolded protein clearance systems may not be able to cope with the amount of malformed proteins that are produced during the aging process, a combination of these protein aggregates in the aged brain has been verified [14, 15, 49]. However, when we performed sensitivity analyses by AD neuropathology severity (considering Braak ≤II or Braak ≥III, and CERAD ≤A or CERAD ≥B), we did not find differences in the pooled prevalence of TDP-43 proteinopathy. Likewise, Arnold et al. also showed lack of association between AD neuropathology and TDP-43 proteinopathy in cognitively normal subjects. On the other hand, association between TDP-43 proteinopathy and AD neuropathology is consistently found in cognitively impaired subjects [14, 49]. Interestingly, TDP-43 stage showed a dose-response relationship with clinical Alzheimer’s disease [49]. We found higher TDP-43 prevalence values in individuals with HS (48%) than in those without HS (27%). Association between HS and TDP-43 has been consistently found in the literature [49–51]. However, we could only investigate this association in four studies.
Previous post-mortem neuropathological studies focusing on TDP-43 proteinopathy in cognitively normal elderly adults had found wide range prevalence [14–17]. For this reason, we sought to conduct a systematic review of the literature and present results obtained by a meta-analysis to estimate an overall pooled prevalence of TDP-43 proteinopathy and understand factors that could explain the high heterogeneity across studies. Primarily, we would assume that differences in study design and methodology would explain these variations. Indeed, our results showed variations in the pooled prevalence estimations according to the quality of the studies and the antibody used to assess TDP-43 proteinopathy. In fact, one study that assessed concordance of 25 independent evaluators on the diagnosis of AD-related hyperphosphorylated-tau pathology showed poor agreement (50%) for mild burden of lesions [52]. Standardized methods across different brain banks regarding the use of the same antibodies [53] and guidelines to assess lesions (including the number of evaluators) could make results more comparable. We found higher pooled prevalence values in studies that used antibody against the phosphorylated TDP-43 (24%), when compared to non-phosphorylated TDP-43 (18%). In agreement with our findings, the study that classified pathological FTLD-TDP subtypes using antibodies against phosphorylated (pTDP43) and non-phosphorylated TDP-43 (iTDP-43) showed that pTDP43 identified a greater severity of pathological inclusions in comparison to iTDP43, as well as a higher inter-observer agreement of subtype classification [54]. Indeed, previous published data using immunoblot analysis showed phosphorylation as the major component of TDP-43 inclusions in FTLD an ALS, but not in AD subjects [55]. In our meta-analysis, we included older adults that had normal cognition at the time of death. For this reason, it is not clear whether these subjects would evolve to FTLD-TDP or AD with TDP-43 inclusions [1]. The use of antibodies against the phosphorylated TDP-43 may contribute to the uniformity of TDP-43 proteinopathy classification across brain banks. Additionally, immunoblot analysis using antibodies that can recognize different epitopes of the protein, may contribute to our understanding of biochemical modification along the formation of TDP-43 aggregates in aging and neurodegenerative disease. When we examined other variables related to the study design (e.g. sample size and analyzed brain regions), we did not observe differences in the prevalence estimations.
Although we did not find changes in the pooled prevalence of TDP-43 proteinopathy when individuals were grouped by age or sex, similar prevalence estimations were found within the same geographic locations, except for Asia. The reason why the two studies from Asia showed variations in prevalence estimations may be because the distribution of TDP-43 varies across brain regions [56, 57]. While one study from Asia [17] analyzed TDP-43 changes in the limbic system, Mizuno et al. only analyzed a specific brainstem nucleus, the oculomotor nucleus. We performed a sensitivity analysis to certify that difference in geographic location were not due to differences in studied brain region. After this analysis, the different prevalence estimations across geographic location still remained. Our hypotheses is, that differences in population characteristics due to environmental and genetic factors could influence the neural microenvironment, and modify the presence of TDP-43 aggregates in the brain. Epidemiological studies have shown that changes in lifestyle can modify the chance of developing dementia in older ages [58]. These lifestyle changes include enrollment in social and physical activities, changes in dietary habits, as well as control of vascular risk factors [59–62]. Indeed, this is particularly important for TDP-43 since cerebral vascular changes may play a role [63]. Ethnicity also impacts the prevalence of age-related diseases, such as hypertension, cardiovascular disease [64, 65], and dementia [66]. Interestingly, our recently published data showed that cognitively normal Asians were more prone to exhibit TDP-43 proteinopathy, when compared to Caucasians, even after we controlled for age, sex, and education [16].
The high heterogeneity across studies included in our meta-analysis is a clear limitation, as reported previously in meta-analysis studies of prevalence [29, 67]. However, we conducted a funnel plot analysis accompanied by Egger’s and Begg’s tests that did not show evidence of publication bias. We also did not have individual participant data, which could have allowed for more comprehensive analyses. Additionally, the investigation of the coexistence of other neurodegenerative diseases and TDP-43 proteinopathy was limited to five out of seven studies that agreed to share the data for this meta-analysis.
In conclusion, we found a wide range of estimates when we examined the prevalence of TDP-43 proteinopathy in cognitively normal older adults from different geographic locations. Different methodology used across brain banks to access TDP-43 may explain these results. Another possible explanation for these results is the difference in lifestyle and genetic factors in different populations. Investigating the ethnical, genetic, and lifestyle factors that could affect the presence of TDP-43 related neuropathological findings, as well as standardization of the methods to measure TDP-43 protein, are of extreme importance to move forward our understanding of TDP-43 pathophysiology.
Supplementary Material
Acknowledgments
We thank the brain banks (Cambridge City over-75s Cohort Study [CC75C] and Rush Alzheimer’s Disease Center [RADC]) for providing data regarding AD pathology and hippocampal sclerosis. Neuropathological data from the study Yu et al was obtained with the grant numbers: P30AG10161, R01AG15819, R01AG17917, and R01AG42210.
CN was supported by FAPESP, PhD and post-doctorate scholarships (FAPESP: 2011/19833-7 and 2015/17365-7, respectively); ATDLA was supported by Albert Einstein post doctorate fellowship (2012/1620); CBGA, REPL, RN, WJ, CAP, SRKH, SH, HK, GGK, LTG and CKS report no disclosures.
List of abbreviations
- TDP-43
transactive response DNA-binding protein 43
- ALS
amyotrophic lateral sclerosis
- AD
Alzheimer’s disease
- FTLD-TDP
Frontotemporal lobar degeneration with transactive response DNA-binding protein 43
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- MOOSE
Meta-analyses of Observational Studies in Epidemiology
- CDR
Clinical Dementia Rating
- AD
Alzheimer’s disease
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- ES
effect size
- CI
confidence interval
Footnotes
Ethical Approval
Only ethically approved articles were included in our systematic review and meta-analysis.
Author Contributions
Camila Nascimento, study concept and design, acquisition and interpretation of data
Ana Tereza Di Lorenzo Alho, acquisition of data
Caroline Bazan Conceição Amaral, acquisition of data
Renata Elane Paraizo Leite, critical revision of the manuscript for important intellectual content and study supervision
Ricardo Nitrini, critical revision of the manuscript for important intellectual content
Wilson Jacob-Filho, study supervision
Carlos Augusto Pasqualucci, study supervision
Suvi Rosa Kastehelmi Hokkanen, acquisition of data and critical revision of the manuscript for important intellectual content and study supervision
Sally Hunter, acquisition of data and critical revision of the manuscript for important intellectual content and study supervision
Hannah Keage, acquisition of data and critical revision of the manuscript for important intellectual content and study supervision
Gabor Kovacs, acquisition of data and critical revision of the manuscript for important intellectual content and study supervision
Lea Tenenholz Grinberg, critical revision of the manuscript for important intellectual content
Claudia Kimie Suemoto, analysis and interpretation of data, critical revision of the manuscript for important intellectual content and study supervision
Units of measurement
Symbols and abbreviations were used in conformity with the guidelines laid down in Baron D.N. (1988) Units, Symbols and Abbreviations, 4th ed. published and supplied by the Royal Society of Medicine, 1 Wimpole Street, London, W1M 8AE.
References
- 1.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR, Kiernan MC, Halliday GM. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–3122. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- 4.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of neurology. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Lee EB, Fang L, Van Deerlin VM, Ludolph AC, Lee VM, Braak H, Trojanowski JQ. Erratum to: Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta neuropathol. 2015;129:929. doi: 10.1007/s00401-015-1428-x. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR, Jr, Parisi JE, Petersen RC, Dickson DW. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70:1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, Morris JC, Holtzman DM Dominantly Inherited Alzheimer N. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Sci Transl Med. 2014;6:226ra230. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 12.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 13.Geser F, Prvulovic D, O'Dwyer L, Hardiman O, Bede P, Bokde AL, Trojanowski JQ, Hampel H. On the development of markers for pathological TDP-43 in amyotrophic lateral sclerosis with and without dementia. Prog Neurobiol. 2011;95:649–662. doi: 10.1016/j.pneurobio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126:365–384. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 15.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126:51–57. doi: 10.1007/s00401-013-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, Pasqualucci CA, Jacob-Filho W, Grinberg LT. Higher Prevalence of TDP-43 Proteinopathy in Cognitively Normal Asians: A Clinicopathological Study on a Multiethnic Sample. Brain Pathol. 2016;26:177–185. doi: 10.1111/bpa.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, Saito Y, Arai T, Nishiyama K, Murayama S. Incidence and extent of TDP-43 accumulation in aging human brain. Acta neuropathol commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22:128. doi: 10.1097/EDE.0b013e3181fe7825. author reply 128. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM Consortium for Frontotemporal Lobar D. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edition JBIRM. Joanna Briggs Institute Reviewers’ Manual: 2014 edition/supplement. Australia: Joanna Briggs Institute: University of Adelaide; 2014. [Google Scholar]
- 27.Sterne JAC, Bradburn MJ, Egger M. Meta—Analysis in Stata™. Systematic Reviews in Health Care: BMJ Publishing Group. 2008:347–369. [Google Scholar]
- 28.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Clopper CJP, ES The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 31.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 33.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015;84:927–934. doi: 10.1212/WNL.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keage HA, Hunter S, Matthews FE, Ince PG, Hodges J, Hokkanen SR, Highley JR, Dening T, Brayne C. TDP-43 pathology in the population: prevalence and associations with dementia and age. J Alzheimers Dis. 2014;42:641–650. doi: 10.3233/JAD-132351. [DOI] [PubMed] [Google Scholar]
- 38.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elobeid A, Rantakomi S, Soininen H, Alafuzoff I. Alzheimer's disease-related plaques in nondemented subjects. Alzheimers Dement. 2014;10:522–529. doi: 10.1016/j.jalz.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 42.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 43.Farfel JM, Nitrini R, Suemoto CK, Grinberg LT, Ferretti RE, Leite RE, Tampellini E, Lima L, Farias DS, Neves RC, Rodriguez RD, Menezes PR, Fregni F, Bennett DA, Pasqualucci CA, Jacob Filho W Brazilian Aging Brain Study G. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology. 2013;81:650–657. doi: 10.1212/WNL.0b013e3182a08f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scotter EL, Chen HJ, Shaw CE. TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets. Neurotherapeutics. 2015;12:352–363. doi: 10.1007/s13311-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SS, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, Clare AJ, Badders NM, Bilican B, Chaum E, Chandran S, Shaw CE, Eggan KC, Maniatis T, Taylor JP. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu-Yesucevitz L, Lin AY, Ebata A, Boon JY, Reid W, Xu YF, Kobrin K, Murphy GJ, Petrucelli L, Wolozin B. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. J Neurosci. 2014;34:4167–4174. doi: 10.1523/JNEUROSCI.2350-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 48.Diaper DC, Adachi Y, Sutcliffe B, Humphrey DM, Elliott CJ, Stepto A, Ludlow ZN, Vanden Broeck L, Callaerts P, Dermaut B, Al-Chalabi A, Shaw CE, Robinson IM, Hirth F. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum Mol Genet. 2013;22:1539–1557. doi: 10.1093/hmg/ddt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016 doi: 10.1093/brain/aww224. this is missing page numbers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cykowski MD, Takei H, Van Eldik LJ, Schmitt FA, Jicha GA, Powell SZ, Nelson PT. Hippocampal Sclerosis but Not Normal Aging or Alzheimer Disease Is Associated With TDP-43 Pathology in the Basal Forebrain of Aged Persons. J Neuropathol Exp Neurol. 2016;75:397–407. doi: 10.1093/jnen/nlw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Annals of neurology. 2015;77:942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H. Staging of neurofibrillary pathology in Alzheimer's disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goossens J, Vanmechelen E, Trojanowski JQ, Lee VM, Van Broeckhoven C, van der Zee J, Engelborghs S. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies. Acta neuropathol commun. 2015;3:15. doi: 10.1186/s40478-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan RH, Shepherd CE, Kril JJ, McCann H, McGeachie A, McGinley C, Affleck A, Halliday GM. Classification of FTLD-TDP cases into pathological subtypes using antibodies against phosphorylated and non-phosphorylated TDP43. Acta neuropathol commun. 2013;1:33. doi: 10.1186/2051-5960-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Annals of neurology. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Lee EB, Fang L, Van Deerlin VM, Ludolph AC, Lee VM, Braak H, Trojanowski JQ. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josephs KA, Dickson DW. TDP-43 in the olfactory bulb in Alzheimer's disease. Neuropathol Appl Neurobiol. 2016;42:390–393. doi: 10.1111/nan.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paganini-Hill A, Kawas CH, Corrada MM. Lifestyle Factors and Dementia in the Oldest-old: The 90+ Study. Alzheimer Dis Assoc Disord. 2016;30:21–26. doi: 10.1097/WAD.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71:1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 61.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 62.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang WX, Kryscio RJ, Neltner JH, Kukull WA, Cykowski MD, Van Eldik LJ, Ighodaro ET. "New Old Pathologies": AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS) J Neuropathol Exp Neurol. 2016;75:482–498. doi: 10.1093/jnen/nlw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao B, Jose PO, Pu J, Chung S, Ancheta IB, Fortmann SP, Palaniappan LP. Racial/ethnic differences in hypertension prevalence, treatment, and control for outpatients in northern California 2010–2012. Am J Hypertens. 2015;28:631–639. doi: 10.1093/ajh/hpu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, Cullen MR, Palaniappan LP. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64:2486–2494. doi: 10.1016/j.jacc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37:1009–1015. doi: 10.2337/dc13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol. 2014;43:476–493. doi: 10.1093/ije/dyu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.