Abstract
Background
The bitter taste receptor T2R38, expressed in the tongue and nasal epithelium, has been shown to trigger sinonasal innate immunity contributing to the prevention of gram-negative upper airway bacterial infections. Common polymorphisms of the T2R38 gene, correlating with bitter taste sensitivity to phenylthiocarbamide (PTC), have been linked to differences in sinonasal innate immune response, with specific genotypes significantly more common in medically recalcitrant chronic rhinosinusitis patients. The purpose of this study was to examine this association between T2R38 function and sinonasal infection or symptoms in a healthy population.
Methods
A survey of the frequency of sinus infections, as well as other nasal symptoms such as colds, allergies, and overall nasal quality of life (nQOL), was administered to healthy adult participants. nQOL was measured using a 0 to 3 scale of worsening symptoms. A PTC compound taste strip was administered with T2R38 taste sensitivity classified as extremely, somewhat, or not sensitive.
Results
Among 217 participants (55% female, 70% Caucasian, 42% age 21 to 25 years), 30% did not detect bitterness (nontasters), 34% were moderate tasters, and 36% were “supertasters,” experiencing a strong, unpalatable bitterness. Supertasters were associated with less frequent sinus infections (p = 0.04), and PTC sensitivity was predictive of nasal symptoms: Supertasters had the best nQOL scores, followed by moderate tasters and non-tasters (means: 0.65, 0.81, 1.00, respectively; p = 0.014 for trend). There were no significant associations with other variables.
Conclusion
This study provides evidence that T2R38 functionality in the tongue correlates with nasal symptoms in healthy individuals.
Keywords: rhinitis, quality of life, sinusitis, bitter taste receptor, allergy, T2R38, T2R gene
Sinonasal disease is a frequent cause of morbidity in the United States. Twelve to thirty percent of adults may experience rhinitis from allergies,1 acute sinusitis has accounted for over 12 million outpatient visits annually,2 and chronic rhinosinusitis (CRS) is experienced by 14% to 16% of the population,3 with costs exceeding $8 billion.4
Recently, expression of taste receptors in respiratory epithelial cells have been demonstrated to contribute to innate immunity defenses.5–7 One of these receptors, T2R38, has been shown to be activated by acyl-hormone lactones (AHLs), molecules that are secreted by Gram-negative bacteria such as Pseudomonas aeruginosa, and used to regulate microbial processes such as biofilm formation and virulence.8, 9 In sinonasal epithelial cells, activation of T2R38 results in nitric oxide (NO) production leading to two biologic consequences: (1) diffusion of the NO into the airways where it kills bacteria, and (2) increased ciliary beat frequency (clearing bacteria from the epithelium).10 Thus the T2R38 receptor detects Gram-negative bacteria and triggers their destruction and clearance. Furthermore, genetic variability in this bitter taste receptor alters this response, leading to different bacterial defense mechanisms in the general population. There are two common haplotypes expressed in T2R38 depending on the amino acid residues at positions 49, 262, and 296; the functional allele of the receptor contains a sequence of proline, alanine, and valine (PAV), whereas the nonfunctional allele of the receptor contains alanine, valine and isoleucine (AVI) at these respective positions. These two common haplotypes generate three common genotypes (PAV/PAV, PAV/AVI, and AVI/AVI). Pseudomonas activation of PAV/PAV receptors will generate substantial NO release, whereas PAV/AVI will generate a slight release, and AVI/AVI will generate even less.10 These genotypes follow near-classic Mendelian genetics with a respective 20%, 50%, and 30% population distribution.11
The expression of a functional T2R38 receptor can be detected phenotypically by the taste of the bitter compound phenylthiocarbamide (PTC), which is an agonist for T2R38.12 This correlation between genotype and phenotype has been well studied; individuals with a strong bitter taste response (PAV/PAV) have been classified as “supertasters,” individuals with some taste (PAV/AVI) have been called “tasters,” and individuals with no taste to PTC (AVI/AVI) have been labeled “nontasters.”11
The effect of this variability has been studied in vivo by two recent studies of patients undergoing surgery for CRS. The prevalence of a functional T2R38 receptor in patients receiving the surgery was significantly lower than the prevalence in the general population, suggesting that those with functional T2R38 may require less sinus surgery and may have a lower incidence of CRS.13, 14
Nonetheless, the impact of the T2R38 receptor remains unknown in healthy patients. It is possible that supertasters with fully functional T2R38 receptors (approximately 20% of the U.S. population) may experience less frequent low-grade sinonasal disease, especially due to Gram-negative bacteria, or require less therapy for sinonasal symptoms. Since the T2R38 functionality can be determined by a simple taste test, this question may be answerable without invasive genotyping or tissue biopsy.
We thus conducted a survey examining common sinonasal symptoms and the T2R38 phenotype in healthy adults. We hypothesized that supertasters might have fewer sinonasal symptoms, as well as less frequent nasal disease, due to better immune clearance. Participants were asked to rate nasal quality of life (nQOL), as well as their perceived frequency of “sinus infections,” a way to conceptualize recurrent nasal disease. Since this was a pilot study on a relatively unexamined receptor, questions on other sinonasal comorbidities, such as asthma, allergies, and otologic disease, were also included.
Subjects and methods
Study design and setting
T2R38 receptor sensitivity and sinonasal symptoms were surveyed in individuals at the University of Pennsylvania. The Institutional Committee on Research Involving Human Subjects approved the study protocol.
Selection of participants
A convenience sample of adults associated with the University of Pennsylvania was used. Participants were recruited from graduate and undergraduate classes and lectures. A recruiter explained that a study was being conducted about taste sensitivity and sinonasal disease, and asked for volunteers to participate. All adults (age >18 years) willing to take both the survey and taste test were enrolled.
Data collection
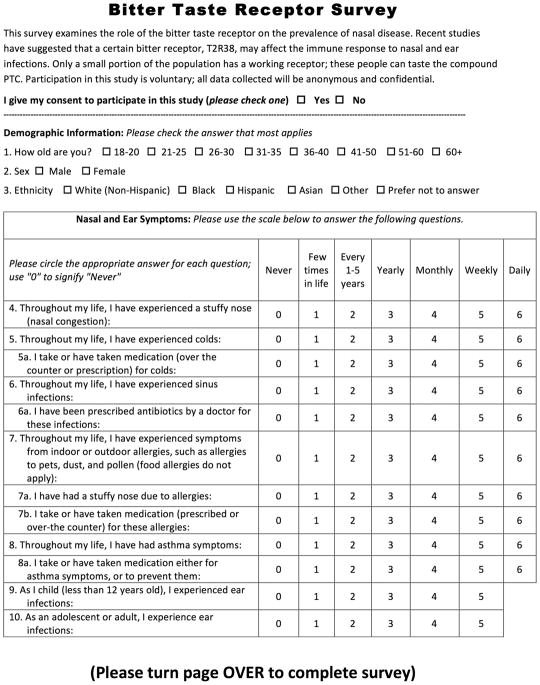
A survey assessing demographic factors, nasal symptoms, nasal comorbidities, nasal disease treatment, and nQOL was given to all participants. The demographic section of the survey included questions on age, gender, and ethnicity. For sinonasal symptoms and diseases, the frequency was assessed on a 7-point Likert scale; options were “never,” “few times in life,” “every 1 to 5 years,” “yearly,” “monthly,” and “daily.” The effect of sinonasal symptoms on QOL was assessed on a 4-point scale, with options “not at all,” “a little,” “moderately,” and “a lot” (Fig. 1). This question was validated against the 22-item Sino-Nasal Outcome Test (SNOT-22) score in additional participants.
FIGURE 1.
Bitter taste receptor survey used in this study.
A taste test was administered to all participants by one of the authors after the survey was completed. Each participant placed a strip of paper soaked in PTC solution on his or her tongue for 5 seconds. A participant then classified the taste of the strip as “like paper,” “somewhat bitter,” or “strongly/unpalatably bitter.” Initial participants requested a blank strip of paper to taste as a control; all participants were subsequently given a sterile strip of normal paper for optional tasting. Methods were adapted from previous studies examining PTC sensitivity and dental carries.15
Outcomes
The primary outcome measure was self-reported nQOL. Secondary outcomes were the frequency of self-reported sinonasal infections, as well as other nasal symptoms and comorbidities. The independent variable was PTC sensitivity, indicating T2R38 receptor function. Participants were assumed to be masked to their PTC sensitivity until the taste test, which was performed after the questionnaire.
Data analysis
Standard summary statistics were used to describe the populations of nontasters, tasters, and supertasters. Since we hypothesized a difference between those with a PAV/PAV genotype and all others, Fisher’s exact test was used to compare demographics, nasal disease, and comorbidities between supertasters and all other participants. The Mantel-Haenszel extension of the Wilcoxon rank sum test was used to test for trend between nQOL and T2R38 sensitivity, as well as allergy symptoms and T2R38 sensitivity. A multivariate logistic regression model was created using nQOL of nasal symptoms bothering an individual “a lot” or “all of the time” as the outcome variable, and T2R38 status, age, and gender as independent values. All data analyses were performed using STATA statistical software (version 13; StataCorp, College Station, TX). A probability threshold of <0.05 was used to determine statistical significance.
Results
Demographics of participants
A total of 217 volunteers participated in the taste test and survey. Recruiters estimated participation at approximately 85%. Fifty-five percent of those enrolled (n = 119) were female. Seventy percent (n = 152) were Caucasian, and 42% were aged 21 to 25 years. Thirty percent of participants (n = 64) did not detect bitterness (nontasters), 34% (n = 74) were moderate tasters, and 36% (n = 79) were supertasters, experiencing a strong, unpalatable bitterness (Table 1).
TABLE 1.
Demographics of population*
| No or slight bitter taste (n = 138) | Strong bitter taste (n = 79) | All patients (n = 217) | p | |
|---|---|---|---|---|
| Age (years) | ||||
| 18–25 | 54 (68) | 90 (65) | 144 (66) | 0.120a |
| 26–35 | 16 (20) | 29 (21) | 45 (21) | |
| 36–45 | 5 (6) | 3 (2) | 8 (4) | |
| 46–56 | 2 (3) | 12 (9) | 14 (6) | |
| 56+ | 1 (1) | 3 (2) | 4 (2) | |
| Gender | ||||
| Female | 52 (66) | 67 (49) | 119 (56) | 0.022 |
| Male | 26 (33) | 68 (50) | 94 (44) | |
| Ethnicity | ||||
| Caucasian | 50 (63) | 102 (74) | 152 (70) | 0.316b |
| Asian | 10 (13) | 18 (13) | 28 (13) | |
| Hispanic | 8 (10) | 7 (5) | 15 (10) | |
| Black | 4 (5) | 6 (4) | 10 (5) | |
Some percentages do not add up to 100 because some participants declined to respond. Values are n (%) except where indicated. Bold p values are significant.
Value of p is for >36 vs <36 years.
Value of p is for Caucasian vs non-Caucasian.
Low PTC sensitivity associated with poor nQOL
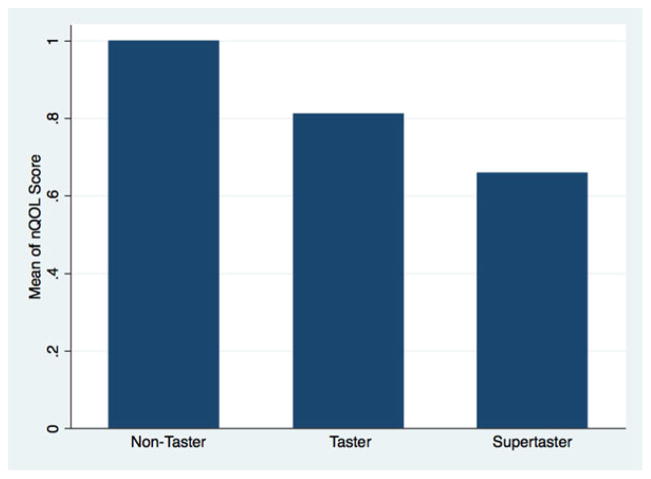
The question “[How much do] nasal symptoms affect my quality of life?” used a Likert scale ranging from 0 (“not at all”) to 3 (“a lot”). Mean responses ranged from 1.00 for nontasters, to 0.81 for tasters and 0.65 for supertasters (Fig. 2). This trend toward worse nQOL with decreasing PTC sensitivity reached significance (p = 0.014). A logistical regression model was also constructed using a 2 or a 3 on the Likert scale as an outcome; decreasing PTC sensitivity was associated with increased odds of reporting nasal symptoms affecting QOL (odds ratio [OR] = 1.9, p = 0.010).
FIGURE 2.
Averaged responses to the Likert scale question “[How much do] nasal symptoms affect my quality of life?” p = 0.014 for the trend in the means, using the Mantel-Haenszel extension of the Wilcoxon rank sum test (a nonparametric test for trend). Possible responses to the question ranged from “not at all” (0) to “a lot” (4).
Female gender and age (categorized as 18 to 30 years, 31 to 50 years, and 50+ years), both associated with supertasters, were added to the model to control for potential confounding effects. Nonetheless the multivariate model found the same odds of reporting a poor nQOL as PTC sensitivity decreased (OR = 1.9, p = 0.013) (Table 2).
TABLE 2.
Multivariate logistic regression with poor nQOL response as outcome
| Odds ratio | P | 95% confidence interval | ||
|---|---|---|---|---|
| Low | High | |||
| PTC sensitivity | 1.89 | 0.013 | 1.14 | 3.13 |
| Age | 1.16 | 0.620 | 0.65 | 2.09 |
| Female gender | 3.44 | 0.003 | 1.53 | 7.72 |
nQOL = nasal quality of life; PTC = phenylthiocarbamide.
Validation of nQOL question
A separate survey with the nQOL question and the SNOT-22 measurement was subsequently given to 116 participants recruited by the same means as the study cohort. There was a significant correlation between the total SNOT-22 score and the nQOL score (p = 0.0001, R2 = 0.24)
Nasal infections and female gender associated with high PTC sensitivity
Female participants were significantly more likely to be supertasters (p = 0.02). Supertaster status was significantly associated with less frequent sinonasal infections (by retrospective report), with 10% of supertasters reporting yearly to monthly sinus infections vs 22% of other individuals (p = 0.04) (Table 3). Notably, there were no significant associations between supertasters and the frequency of any other variables examined, including allergy symptoms, nasal congestion secondary to allergy symptoms, asthma symptoms, otologic symptoms, and sinus surgery (Table 3).
TABLE 3.
Nasal and ear diseases and comorbidities*
| No or slight bitter taste (n = 138) | Strong bitter taste (n = 79) | All patients (n = 217) | p | |
|---|---|---|---|---|
| Sinus infections | ||||
| Never to a few times | 71 (90) | 108 (78) | 179 (82) | 0.04 |
| Yearly to monthly | 8 (10) | 30 (22) | 38 (18) | |
| Allergy symptoms | ||||
| Never to a few times | 42 (53) | 64 (46) | 106 (49) | 0.5 |
| Yearly to monthly | 30 (38) | 54 (39) | 84 (39) | |
| Weekly or greater | 7 (9) | 19 (14) | 26 (12) | |
| Stuffy nose from allergies | ||||
| Never to a few times | 44 (56) | 65 (47) | 109 (50) | 0.29 |
| Yearly to monthly | 30 (38) | 59 (43) | 89 (41) | |
| Weekly or greater | 4 (5) | 14 (10) | 18 (8) | |
| Asthma symptoms | ||||
| Never to a few times | 70 (89) | 122 (89) | 192 (89) | 0.25 |
| Yearly to monthly | 6 (8) | 14 (10) | 20 (9) | |
| Weekly or greater | 3 (4) | 1 (1) | 4 (2) | |
| Ear infections as child | ||||
| Never to a few times | 60 (76) | 99 (72) | 159 (73) | 0.62 |
| Yearly to monthly | 19 (24) | 37 (27) | 56 (26) | |
| Weekly or greater | 0 (0) | 2 (1) | 2 (1) | |
| Ear infections as adult | ||||
| Never to a few times | 73 (92) | 132 (96) | 205 (95) | 0.22 |
| Yearly to monthly | 6 (8) | 5 (4) | 11 (5) | |
| Sinus surgery | ||||
| No | 75 (95) | 131 (96) | 206 (95) | 1 |
| Yes | 4 (5) | 6 (4) | 10 (5) | |
Some percentages do not add up to 100 because some participants declined to respond. Values are n (%) except where indicated. Bold p values are significant.
Individuals with previous sinus surgery did not affect results
There were 10 individuals (5% of the sample) who had previous sinus surgery. All associations continued to be significant when they were excluded from the sample.
Discussion
To date, research has focused on the association between bitter taste receptor genotype and disease severity in patients with clinically diagnosed chronic rhinosinusitis, studying only individuals with documented and prolonged sinus disease. However, this study demonstrates that the T2R38 bitter taste receptor may play a role in the sinonasal symptoms of otherwise healthy individuals as well. Specifically, this study supports a link between phenotype and sinonasal symptoms in the healthy population, despite limitations in sample size and retrospective reporting. Finally, it introduces the utility of a simple clinical taste test that may predict nQOL by determining T2R38 receptor expression (phenotype).
It is possible that the lack of a functional T2R38 receptor partially compromises sinonasal innate immunity resulting in increased sinonasal symptoms in otherwise healthy individuals. These may range from severe disease requiring surgery to subclinical infections. This disposition could explain the association with poor nQOL and the self-reported increased frequency of sinus infections in individuals with a nonfunctional T2R38 receptor. While many cases of sinusitis have a viral cause, participants may conceptualize common, recurrent, or chronic nasal irritation and infection, which may be caused by continual bacterial disease, as frequent “sinus infections.” Likewise, these individuals may have an increased propensity to develop CRS ultimately requiring surgical intervention, which would explain our prior findings.13, 14
It is important to note the limitations of this pilot study. The survey was designed to capture a broad range of nasal comorbidities and yet be answered quickly by adults who received no compensation. Thus it was partially validated against the SNOT-22 survey instead of using the full instrument, which would have led to more clinically valid results. In addition, the term “sinus infections” is ambiguous, and has multiple etiologies, including viral infections that are unlikely to be affected by T2R38-mediated immunity. Nonetheless, this study paves the way for future research that could use more clinically validated instruments, and would also warrant more specific queries on topics such as facial pain and the color of nasal drainage, in order to more precisely determine the symptoms experienced and their etiology. Future studies could finally include chart reviews with outcomes monitoring, to alleviate the bias of retrospective self-reporting.
Another limitation of this study is that PTC sensitivity was reported subjectively by participants. Nonetheless, the taste of a paper strip containing PTC has been closely associated with genetic expression of the T2R38 receptor.16 In this study, the proportion of nontasters (30%), tasters (34%), and supertasters (36%) was close to that expected based on gene distribution in the general population (of 30%, 50%, and 20%, respectively). With variable expression among participants who carry one or more copies of the supertaster allele, and subjectivity in the distinction between taster and supertaster, it is likely that there was some overlap in the reported supertaster and moderate taster groups, which could explain the difference in the genotypic and phenotypic distributions. This study was further limited by a small sample size (n = 217), convenience sampling, and retrospective reporting of symptoms. Nonetheless, these distinctions do not affect the conclusion, that those who reported greater T2R38 sensitivity also reported fewer nasal symptoms.
Some participants were a part of the medical community, and may have previously heard of the hypothesized link between the T2R38 receptor. However, prior knowledge should not affect any results, as all participants were assumed blind to their T2R38 functionality until they had taken a taste test, which was given after the survey.
While a wide range of ages were included in the study population, participant recruitment in the university setting skewed the data toward a younger age, with a mean in the second decade of life. As the hypothesized relationship between nasal symptoms and reported bitter taste is based largely on gene expression, age should not introduce significant confounding variables to the study.
The association between female gender and supertasters has been found in other studies, and helps to validate the taste test.12 The multivariate analysis indicates that the association between supertasters and female gender is independent of the association between supertasters and poor nQOL. The question on nQOL was found to correlate well with the SNOT-22 score; however, as previously mentioned, a limitation of this study is that it has not been rigorously validated against multiple disease-specific instruments.
In the future, this simple “taste-test” protocol could prove useful to predict individual susceptibility to sinus disease. Ultimately it may aid clinicians identify the extent of treatment required, potentially treating nontasters more aggressively medically than supertasters. As a pilot investigation, this study demonstrates promising sinonasal outcomes in correlation with T2R38 genetics, but future investigation is necessary to elucidate the link between PTC sensitivity and nasal disease before true clinical implementation is feasible.
Conclusion
PTC sensitivity serves as an accurate predictor of T2R38 bitter taste receptor expression. Our study shows that supertaster individuals for the PTC strips are likely to experience less sinonasal symptoms that lead to a poor nQOL. It would be beneficial to further study this effect using validated QOL measures to better characterize severity and frequency of sinonasal symptoms in an otherwise healthy population.
Footnotes
Potential conflict of interest: None provided.
References
- 1.Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29:600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 2.Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1996. Vital Health Stat 13. 1998;134:1–37. [PubMed] [Google Scholar]
- 3.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009;242:1–157. [PubMed] [Google Scholar]
- 4.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 5.Braun T, Mack B, Kramer MF. Solitary chemosensory cells in the respiratory and vomeronasal epithelium of the human nose: a pilot study. Rhinology. 2011;49:507–512. doi: 10.4193/Rhino.11.121. [DOI] [PubMed] [Google Scholar]
- 6.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M, Kofonow J, Nayak JV, et al. Biofilms in chronic rhinosinusitis: a review. Am J Rhinol Allergy. 2009;23:255–260. doi: 10.2500/ajra.2009.23.3319. [DOI] [PubMed] [Google Scholar]
- 9.Suh JD, Cohen NA, Palmer JN. Biofilms in chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:27–31. doi: 10.1097/MOO.0b013e328334f670. [DOI] [PubMed] [Google Scholar]
- 10.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed DR, Knaapila A. Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci. 2010;94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiology Behavior. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 13.Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184–187. doi: 10.1002/alr.21140. [DOI] [PubMed] [Google Scholar]
- 14.Adappa ND, Adappa ND, Zhang Z, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma P, Shetty V, Hegde AM. Propylthiouracil (PROP)—a tool to determine taster status in relation to caries experience, Streptococcus mutans levels and dietary preferences in children. J Clin Pediatr Dent. 2006;31:113–117. doi: 10.17796/jcpd.31.2.34302r2857511268. [DOI] [PubMed] [Google Scholar]
- 16.Bufe B, Breslin PAS, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]