Abstract
Purpose
Distinct normal physiological patterns of fat conversion in vertebrae were described both for children and adults. Our aim was to evaluate the T1-weighted bone marrow pattern of the vertebral bodies in various sites along the scoliotic spine of children with adolescent idiopathic scoliosis (AIS).
Methods
We retrospectively evaluated spine MRI studies of children with AIS. Scoliosis radiographs were assessed for type of curvature according to the Lenke classification. A paediatric neuroradiologist assessed the T1-weighted signal of vertebral bodies in comparison with the adjacent disc and distinct patterns of fatty conversion within the apical and stable vertebral bodies. Statistical assessment was performed.
Results
MRI study of the spines of 75 children with AIS were assessed, 59 (79%) of whom were female, with an age range of nine to 19 years. The relative overall T1-weighted signal intensity of the vertebral body bone marrow relative to the intervertebral disc was hyperintense in 76% and isointense in 24%. Fatty conversion grade of the stable vertebra was higher than the apex vertebra (p = 0.0001). A significant tendency to have more advanced fat conversion patterns in the apex vertebra up to age 13.5 years old compared with adolescents above that (p = 0.015) was seen.
Conclusion
This preliminary study suggests a different pattern of bone marrow conversion in AIS from the normal physiologic pattern described in the literature. Whether these changes are secondary to the biomechanics of the curved spine or may suggest that bone marrow maturation rate and content have a role in the pathogenesis of AIS remains to be further researched.
Level of Evidence
Level III (Diagnostic Study)
Keywords: Paediatric, spine, scoliosis, bone marrow, MRI
Introduction
Adolescent idiopathic scoliosis (AIS) is the most common cause of scoliosis. According to White and Panjabi1 scoliosis may be defined biomechanically as an abnormal deformation between and within vertebrae; too much curvature of the spine in the frontal plane, and too much vertical axis rotation in the wrong direction. Such an occurrence can set off a chain of events which lead to asymmetrical loads on the epiphyseal plates, muscle and ligamentous imbalance and progression to scoliosis. With time, the unbalanced forces acting on the epiphyses lead to structural changes. These forces working on the segments of the growing spine can result in a progressive deformation of individual vertebrae because of Hueter-Volkmann’s low.1 Guo et al2,3 reported that scoliotic spines had, in the thoracic region, longer vertebral bodies and shorter pedicles with a larger interpedicular distance as compared with normal controls. These morphological changes were also shown by Parent et al4 who described a significant decrease in the height of the vertebral bodies on the concave side at the apex of the curve and pedicles which were smaller and shorter then on the convex side. Dayer et al5 reviewed the literature regarding suggested aetiology and pathogenesis, including the possibility that this is an inherited complex disease of childhood, and conclude that the primary aetiology of this common condition remains unclear. MR imaging research of patients with scoliosis has concentrated on morphometric measurements of skull and spine, assessment of T2-weighted changes in the intervertebral discs, evaluation of the spinal cord, cranio-cervical junction and brain morphometrics.6 Our search of the literature did not find any description of bone marrow conversion pattern in these children.
T1-weighted imaging of the bone marrow has been demonstrated to be sensitive to bone marrow maturation changes during life.7 An increase in T1-weighted signal of the vertebral marrow reflects conversion from haematopoietic rich marrow to fatty replacement. The most striking change in bone marrow content, and hence its appearance on T1-weighted imaging, takes place in infancy. Distinct normal physiological patterns of fat conversion in vertebrae were described both for children8 and adults.9
Our aim was to evaluate the T1-weighted bone marrow pattern of the vertebral bodies at various sites along the scoliotic curve in children with AIS.
Patients and methods
In our institution (Dana Children’s Hospital, Tel Aviv Sourasky Medical Center), children with AIS who are considered for surgery are evaluated with MRI of the entire spine as part of presurgical workup. After institutional review board approval, we retrospectively evaluated the records and imaging studies of 75 children with AIS that were evaluated with MRI study of the spine prior to surgical interventions. Scoliosis radiographs were assessed by a paediatric orthopaedic surgeon (LS) for type of curvature according to the Lenke classification 10, measurement of the Cobb angle, and depiction of the apex, end and stable vertebrae for each curve.
The sagittal T1-weighted sequence from each MRI study was evaluated by a paediatric neuroradiologist (SIS), and the following data was collected:
The T1-weighted signal of the vertebral body was compared with the T1-weighted signal of the adjacent disc and described as hypo-, iso-, or hyperintense.
Three distinct patterns of bone marrow fatty replacement as demonstrated by increased T1-weighted signal within the bone marrow of the vertebral body were noted: no distinct area of increased signal (type 1), linear increased signal along the basivertebral vein (type 2) and thicker areas of increased signal either along the basivertebral vein or wedge-like increased signal in the anterior aspect of the vertebral body (type 3), as shown in Figure 1.
Fig. 1.
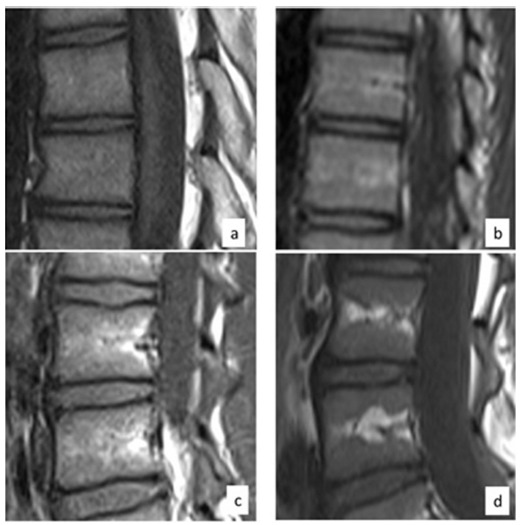
Type 1: overall T1-weighted signal increased compared with disc signal. No focal areas of increased T1-weighted signal, consistent with focal fatty change, are present (a). Type 2: linear areas of increase T1- weighted signal above and below the basivertebral vein, reflecting linear focal fatty change (b). Type 3: larger areas of increased T1-weighted signal reflecting increased areas of fatty change, either by thickening of the linear areas along the basivertebral vein (c) or wedge shape areas of increased T1-weighted signal along the peripheral central portion of the vertebral bodies (d).
The data was collected for the apical vertebra, the vertebra above and below the apex, the upper and lower end vertebra and the stable vertebra for both the primary and secondary curve, if present. A third of the studies were additionally evaluated by a second paediatric neuroradiologist (LB) and interobserver variation was assessed using the Kappa measure of agreement.
Statistical analysis
Statistical assessment for association between bone marrow pattern of the apex vertebra to age, gender, Lenke classification and degree of cob angle were performed using Pearson chi-squared tests. Association between bone marrow pattern of the apex vertebra to the bone marrow pattern of the stable vertebra were assessed with Pearson chi-squared test and the Wilcoxon signed-rank test. Relation of age by primary curve apex vertebra bone marrow pattern and age by stable vertebra bone marrow pattern were assessed with one-way analysis of variance (ANOVA).
Results
MRI studies of the spine of 75 children with AIS were assessed, 59 (79%) of whom were female and 16 (21%) of whom were male, with ages ranging from nine to 19 years. In all, 51% of children had scoliosis with primary thoracic curve, 42% had double curve pattern and 7% had primary (thoraco) lumbar curve pattern; Table 1 presents the prevalence of the different Lenke classification subtypes.
Table 1.
The prevalence of the different Lenke classification subtypes
| Primary thoracic curve pattern | |
|---|---|
| Lenke Ia | 7 (9%) |
| Lenke Ib | 19 (26%) |
| Lenke Ic | 8 (11%) |
| Lenke IIb | 4 (5%) |
| Primary (thoraco) lumbar curve pattern | |
| Lenke Vc | 5 (7%) |
| Double curve pattern | |
| Lenke IIIb | 3 (4%) |
| Lenke IIIc | 22 (29%) |
| Lenke VIc | 7 (9%) |
The relative overall T1-weighted signal intensity of the vertebral body bone marrow relative to the intervertebral disc was hyperintense in 76% and isointense in 24%, no patients demonstrated relative hypointense signal.
The vertebral body bone marrow patterns of focal fat distribution within the scoliotic spine are represented in Figure 2. There was a statistically significant difference (p = 0.0001) between the bone marrow pattern of the apex vertebra of the primary curve to the bone marrow pattern of the stable vertebra, with a tendency for the stable vertebra to show a higher grade of fat conversion compared with the apex vertebra. This was also demonstrated by the Wilcoxon signed-rank test (p = 0.00013). No statistically significant changes were found when correlating the bone marrow pattern of the apex vertebra to gender, Cobb angle or the Lenke classification. Assessing distribution of age by vertebral apex bone marrow pattern using one-way ANOVA demonstrated a significant tendency to have more advanced fat conversion patterns (57% type 3, 33% type 2) up to the age of 13.5 years compared with adolescents above that age (40% type 2, 40% type 1) (p = 0.015). No similar significant tendency was found for the stable vertebra bone marrow pattern. Interobserver agreement was substantial with mean kappa score 0.88 (0.68 to 1.00).
Fig. 2.
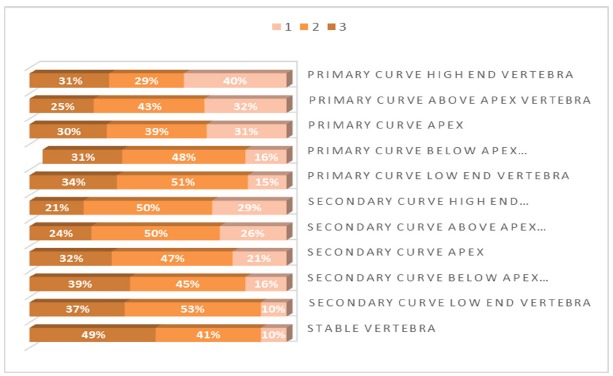
The vertebral body bone marrow patterns of focal fat distribution within the scoliotic spine.
Discussion
This is the first study that describes the patterns of bone marrow fat conversion in children with AIS and found it to be different than the normal age-related physiological pattern. We found a statistically significant difference in fat conversion pattern between the primary curve apical vertebra and the stable vertebra with more progressed fat conversion in the stable vertebra. We also found age-related difference regarding fat conversion pattern of the primary curve apical vertebra with younger children demonstrating more advanced fat conversion pattern.
On T1-weighted imaging, increased signal within the bone marrow reflects increased fat content in the tissue and hence conversion from red marrow to yellow marrow,7 with the most striking change from low signal intensity to high signal intensity taking place in the first year of life.11 In our study, we evaluated the overall T1 signal of the vertebral body bone marrow compared with the adjacent disc; no children demonstrated relative hypointense signal, which is consistent with the findings in normal lumbar spine of children as been described.8,12 In our study the overall bone marrow T1 signal relative to the adjacent disc was hyperintense in 76% of the children and isointense in 24% of the children. These findings are somewhat different from those described in normal children by Sebag et al;8 in their study 50 children were in the age group five to 15 years and within that group 90% demonstrated an overall increased T1 signal in the bone marrow compared with the disc, and 10% demonstrated isointense signal. Our study population age range included older children but with sufficient overlap to allow for comparison; this discrepancy suggests possible lag in bone marrow conversion in children with AIS compared with the normal population.
When assessing the more specific fatty marrow conversion patterns the most interesting finding is the relative heterogeneity of maturation pattern along different vertebrae of the curved spine. Studies describing bone maturation on MRI do not report significant difference between the cervical, thoracic and lumbar spine. In our study we found a statistically significant change between the apex vertebra and the stable vertebra with a tendency of the stable vertebra to have more advanced fatty replacement than that of the apex vertebra. The stable vertebra was a lumbar vertebra in all cases with 10% pattern 1, 41% pattern 2 and 49% pattern 3. In the normal population reported by Sebag et al8 36% pattern 1, 40% pattern 2 and 24% pattern 3 were noted in the similar age group. These results raise a question regarding the possible shift to more mature pattern of fatty conversion in the lumbar stable vertebra compared with the reported normal population.
An interesting observation is the tendency of younger patients to advanced patterns of fat replacement in the apex vertebra and similar patterns of fatty conversion in apex and stable vertebrae. As these MRI studies are preformed when surgery is planned, a younger age at the time of the study reflects severity of scoliosis leading to earlier intervention. Interestingly we did not find statistically significant correlation between the Cobb angles of the primary curve to the apex vertebra pattern.
Our results suggest that patients with AIS may have a different bone marrow conversion process then the expected physiologic changes. Studies have shown that AIS is regarded as a multifactorial disease and a variety of causes were postulated to be part of its aetiology.5,13 Unbalanced growth and loss of alignment can be affected by asymmetrical mechanical loading on the spine.14,15 The skeleton is characterized by its ability to adapt to external forces. This adapting/remodelling capacity raises an array of interesting questions regarding the mechanical control of the musculoskeletal system during growth and development as well as aging. Mechanical forces have an important role in embryonic development.16 Mechanical loads are transformed into biological signals affecting bone formation and resorption. It was suggested that osteocytes are involved in the continuous adaptation mechanism17-19 and there is a molecular basis for mechanotransduction that regulates the mechanical effects on the skeleton.20 The mechanical environment is important in regulating the structural adjustment of the skeleton.16 New bone is added in response to increase loading whereas bone is removed in response to unloading or disuse. The differential growth rate between the vertebral bodies and the posterior elements causes distortion and rotation.2,3 The structural curve is rigid and cannot be corrected by active muscle forces. This curve usually consists of deformation within vertebrae; there is wedging and distortion of the osseous structure, and the ligamentous components of the curve are stiff.13 Highly wedged discs were observed near the apex of the curve.21 It is well known that a balance exists between the adipocytes and the bone tissues within the marrow cavity.22 Both tissues are derived from the common pluripotent mesenchymal cell stem. There are cellular interrelationships between marrow adipocytes and osteoblasts. Specific stimuli such as growth factors may induce preferential differentiation of the stem cells to osteoblasts or adipocytes. This differentiation pathway has a reciprocal manner and the balance between the two cell populations may determine an increase or decrease in bone mass. Increased adipocytes within the marrow cavity come on the account of decreased hematopoiesis. It was shown23 that low-magnitude mechanical stimuli act at the level of the stem cells by enhancing osteoblastic activity and decreasing adipogenesis.24,25 Further research is needed to better understand the cause and effect relationship between the mechanical forces and the processes of osteoblastic activity and adipogenesis in the pathogenesis of AIS.
The main limitation of this study is related to its retrospective nature as the children were scanned in different MRI machines in different imaging centres, not allowing for consistency of technique. This major limitation requires caution in our interpretation of the bone marrow pattern compared with the normal pattern reported in the literature, although good interobserver correlation validates our results. An additional major limitation is lack of a normal control group, as we could not find a sufficient number of age-matched MRIs of the spine of teens without scoliosis and with no disease that interferes with the normal signal within the vertebral bodies; this is the reason we regard this work as preliminary. We believe this first attempt at assessing the bone marrow in scoliotic patients suggests changes in pattern of bone marrow conversion from the expected physiologic process, and further controlled prospective studies with more consistent and improved technique are warranted to elucidate if these changes reflect secondary changes to the biomechanics of the curved spine and/or may suggest that bone marrow maturation rate and content may have a role in the pathogenesis of AIS.
COMPLIANCE WITH ETHICAL STANDARDS
FUNDING STATEMENT
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
OA LICENCE TEXT
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
ETHICAL STATEMENT
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Informed consent: No consent was required. Only general non-identifiable data on a series of patients is included.
REFERENCES
- 1.White AA, Panjabi MM.. Clinical biomechanics of the spine. Lippincott Williams & Wilkins Philadelphia, 1990. [Google Scholar]
- 2.Guo X, Chau WW, Chan YL, et al. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis--result of disproportionate endochondral-membranous bone growth? Summary of an electronic focus group debate of the IBSE. Eur Spine J 2005;14:862-873. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Chau WW, Chan YL, Cheng JCY.. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral-membranous bone growth. J Bone Joint Surg [Br] 2003;85-B:1026-1031. [DOI] [PubMed] [Google Scholar]
- 4.Parent S, Labelle H, Skalli W, Latimer B, de Guise J.. Morphometric analysis of anatomic scoliotic specimens. Spine 2002;27:2305-2311. [DOI] [PubMed] [Google Scholar]
- 5.Dayer R, Haumont T, Belaieff W, Lascombes P.. Idiopathic scoliosis: etiological concepts and hypotheses. J Child Orthop 2013;7:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu WCW, Rasalkar DD, Cheng JCY.. Asynchronous neuro-osseous growth in adolescent idiopathic scoliosis--MRI-based research. Pediatr Radiol 2011;41:1100-1111. [DOI] [PubMed] [Google Scholar]
- 7.Vande Berg BC, Malghem J, Lecouvet FE, Maldague B.. Magnetic resonance imaging of the normal bone marrow. Skeletal Radiol 1998;27:471-483. [DOI] [PubMed] [Google Scholar]
- 8.Sebag GH, Dubois J, Tabet M, Bonato A, Lallemand D.. Pediatric spinal bone marrow: assessment of normal age-related changes in the MRI appearance. Pediatr Radiol 1993;23:515-518. [DOI] [PubMed] [Google Scholar]
- 9.Ricci C, Cova M, Kang YS, et al.. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 1990;177:83-88. [DOI] [PubMed] [Google Scholar]
- 10.Lenke LG, Betz RR, Harms J, et al.. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg [Am] 2001;83-A:1169-1181. [PubMed] [Google Scholar]
- 11.Sze G, Baierl P, Bravo S.. Evolution of the infant spinal column: evaluation with MR imaging. Radiology 1991;181:819-827. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor G, Abdulla S, MacIver D, Toms AP.. Paediatric vertebral marrow signal: defining a normal reference range for the marrow-to-disc signal ratio. Clin Radiol 2016;71:1069.e7-1069.e12. [DOI] [PubMed] [Google Scholar]
- 13.Hefti F. Pathogenesis and biomechanics of adolescent idiopathic scoliosis (AIS). J Child Orthop 2013;7:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little JP, Pearcy MJ, Izatt MT, et al.. Understanding how axial loads on the spine influence segmental biomechanics for idiopathic scoliosis patients: A magnetic resonance imaging study. Clin Biomech (Bristol, Avon) 2016;32:220-228. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuizen AG, Wever DJ, Webb PJ.. The aetiology of idiopathic scoliosis: biomechanical and neuromuscular factors. Eur Spine J 2000;9:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shwartz Y, Blitz E, Zelzer E.. One load to rule them all: mechanical control of the musculoskeletal system in development and aging. Differentiation 2013;86:104-111. [DOI] [PubMed] [Google Scholar]
- 17.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int 2014;94:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S.. Mechanosensation and transduction in osteocytes. Bone 2013;54:182-190. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler MB, Cheung WY, Majeska R, Kennedy O.. Osteocytes: master orchestrators of bone. Calcif Tissue Int 2014;94:5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yavropoulou MP, Yovos JG.. The molecular basis of bone mechanotransduction. J Musculoskelet Neuronal Interact 2016;16:221-236. [PMC free article] [PubMed] [Google Scholar]
- 21.Porter RW. Idiopathic scoliosis: the relation between the vertebral canal and the vertebral bodies. Spine 2000;25:1360-1366. [DOI] [PubMed] [Google Scholar]
- 22.Nuttall ME, Shah F, Singh V, et al. Adipocytes and the regulation of bone remodeling: a balancing act. Calcif Tissue Int 2014;94:78-87. [DOI] [PubMed] [Google Scholar]
- 23.Rubin CT, Capilla E, Luu YK, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA 2007;104:17879-17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 2009;24:50-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luu YK, Pessin JE, Judex S, Rubin J, Rubin CT.. Mechanical signals as a non-invasive means to influence mesenchymal stem cell fate, promoting bone and suppressing the fat phenotype. Bonekey Osteovision 2009;6:132-149. [DOI] [PMC free article] [PubMed] [Google Scholar]


