Abstract
Background
In recent years, the development of new therapies for multiple myeloma has improved the survival of patients, but newer treatments may also affect healthcare costs. To date, no real-world study has examined the concurrent changes in survival and total healthcare costs over time in patients with multiple myeloma.
Objective
To examine the temporal changes in survival and healthcare costs among patients with multiple myeloma in the United States.
Method
This retrospective claims-based cohort study is based on death files in the Truven Health MarketScan Research Commercial and Medicare Supplemental databases. The study included adults who had at least 1 inpatient or 2 outpatient claims with a diagnosis of multiple myeloma between January 1, 2006, and December 31, 2014; continuous insurance enrollment for at least 12 months before and at least 30 days after the first diagnosis (ie, index date); and no previous malignancy. Patients were followed from the index date through the earliest among (1) the date of death recorded in the death files, (2) the end of enrollment in the MarketScan database, or (3) end of the study (September 30, 2015). The mortality rates and the total all-cause and multiple myeloma–specific healthcare costs per patient per month were compared between patients diagnosed in 2006–2010 and those diagnosed in 2011–2014.
Results
A total of 5199 patients were included in the study (2597 diagnosed between 2006 and 2010 and 2602 diagnosed between 2011 and 2014). We found a 35% decrease in the risk for death (hazard ratio, 0.65; 95% confidence interval, 0.57–0.74) among patients diagnosed in 2011–2014 compared with those diagnosed in 2006–2010. In addition, 18% and 26% increases were found in all-cause and multiple myeloma–specific healthcare costs, respectively, over the same time period (adjusted mean all-cause costs, $13,960 vs $16,449, respectively; adjusted mean multiple myeloma–specific costs, $7476 vs $9422, respectively).
Conclusion
The percent decrease in mortality in patients with multiple myeloma has been greater than the percent increase in healthcare costs in recent years, which may be attributable to improved treatments for multiple myeloma and changes in disease management. With the mortality rate having decreased more than the increase in healthcare costs over the same time period, the results of this study suggest that although healthcare spending has increased over time, there is a survival benefit.
Keywords: healthcare costs, mortality, multiple myeloma, survival
Approximately 30,000 new cases of multiple myeloma are diagnosed annually in the United States, and 12,650 deaths annually are attributed to multiple myeloma.1 In the past decade, the introduction of new drugs has markedly changed the treatment paradigm as well as survival outcomes for patients with multiple myeloma,2–6 although these novel regimens may also affect healthcare costs for patients.7–10
To date, studies that have assessed the impact of multiple myeloma treatments on survival relative to treatment costs have been largely based on data from clinical trials and have had conflicting results.8,11–13 We have found only a few studies involving real-world costs of treatments for patients with multiple myeloma, particularly in the United States, and those studies tend to be limited to assessments of only a few specific treatments.14–20
Researchers at the Moran Company conducted an analysis to estimate the volume-weighted Average Sales Price for cancer drugs administered through Medicare Part B in 2006–2014.21 The authors concluded that cancer drug costs have remained consistent with overall medical inflation. Because some multiple myeloma therapies were administered through Medicare Part B during that time, the study indicates that the costs of multiple myeloma treatments might have remained comparable with overall medical inflation.21
KEY POINTS
-
▸
New therapies for multiple myeloma have improved patient survival, but may have also affected the cost of care for patients.
-
▸
This study compared the changes in survival and healthcare costs among patients with multiple myeloma who were diagnosed in 2006–2010 versus those diagnosed in 2011–2014.
-
▸
Patients diagnosed in the more recent period had a 35% lower mortality risk than those diagnosed in the earlier period.
-
▸
The adjusted mean all-cause per-patient per-month (PPPM) healthcare costs were 18% higher in patients diagnosed in 2011–2014 than in 2006–2010.
-
▸
Similarly, the adjusted mean all-cause PPPM healthcare costs were 26% higher in those diagnosed in 2011–2014 than in 2006–2010.
-
▸
The percentage decrease in multiple myeloma mortality is greater than the percentage increase in healthcare costs in recent years, which may reflect improved therapies and treatment strategies.
-
▸
Tailoring treatment plans for patients with multiple myeloma based on specific risk factors may further optimize clinical outcomes.
In their real-world analysis of patients with multiple myeloma, Kumar and colleagues examined the changes in survival over a 10-year period (2001–2010).2 Kumar and colleagues concluded that the consistent improvement in survival they observed during that period was attributable to the use of newer agents in the initial treatment regimen.2 Similarly, Costa and colleagues showed significant improvements in the 5-year and 10-year relative survival rates of patients with multiple myeloma who were diagnosed in 2008–2012 compared with patients diagnosed in 1993–1997.17
To our knowledge, no study has explored the changes in survival and costs over time among patients with multiple myeloma. With the potential for real-world treatment costs to be consistent with medical inflation as suggested by the Moran Company analysis,21 and considering the increase in survival among patients with multiple myeloma suggested by Kumar and colleagues,2 our study hypothesized that the improvement in survival among patients with multiple myeloma has outpaced the increases in multiple myeloma treatment costs over time. Our study suggests that a dollar spent in more recent years is associated with better survival than a dollar spent in previous years.
To our knowledge, this is the first study to provide real-world evidence that simultaneously explores healthcare spending and mortality among patients with multiple myeloma.
Methods
This retrospective observational database study is based on administrative claims data in the Truven Health MarketScan Research Commercial Claims and Encounters (ie, Commercial) and Medicare Supplemental and Coordination of Benefits (ie, Medicare) databases from January 1, 2005, through September 30, 2015. This longitudinal study had a variable-length follow-up period (minimum of 30 days) that ran through the earliest of the date of a patient's death as recorded in the Social Security Administration (SSA) Death Master File; the end of a patient's enrollment in the MarketScan database; or the end of the study period (ie, September 30, 2015).
Data Sources
The MarketScan databases provide detailed cost, use, and outcomes data for healthcare services performed in inpatient and outpatient settings. For Medicare-eligible patients, the Medicare-covered portion of payment (which is represented as the Coordination of Benefits amount) and the employer-paid portion are included in the Medicare database. Together, the Commercial and Medicare databases comprise data for approximately 40 million individuals annually with employer-sponsored primary health insurance or Medicare supplemental health insurance. For this study, the MarketScan databases were further linked to the SSA Death Master File to obtain data on patient mortality from 2006 to 2015. The master file contains records for approximately 14.9 million individuals and is updated quarterly.
Our study was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996. We used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, and the study was, therefore, exempted from Institutional Review Board oversight.
Patient Selection
All patients included in the study were aged ≥18 years with at least 1 inpatient or 2 outpatient healthcare claims (≥30 days apart) with a diagnosis of multiple myeloma (International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 203.0x) between January 1, 2006, and December 31, 2014. In addition, all patients were required to be linkable to the SSA Death Master File to be included in the analysis. The date of the first multiple myeloma diagnosis claim was considered the index date.
To be eligible, patients were required to have at least 12 months of continuous enrollment in the MarketScan database, with medical and pharmacy benefits immediately before the index date (ie, the baseline period) and at least 30 days of postindex enrollment. Patients were excluded if they had a history of multiple myeloma or any other cancer before the index date.
Outcome Variables
One of the main outcome variables of this study was death and survival, which was identified by either having a death record in the SSA file or a claim for an inpatient admission with a discharge status of “death” anytime during the follow-up period.
The outcome variable of total healthcare costs was calculated as the sum of the total payment across all healthcare claims (medical plus pharmacy) during the follow-up period. Multiple myeloma–specific healthcare costs were calculated as the sum of payment across all inpatient claims with a primary diagnosis of multiple myeloma, all outpatient claims with a multiple myeloma diagnosis in any position, and all outpatient pharmacy claims for any multiple myeloma treatments during the follow-up period (Table).
Table.
Patient Demographic and Clinical Characteristics, by Period of Multiple Myeloma Diagnosis
| Patient characteristics | Multiple myeloma diagnosis, 2006–2010 (N = 2597) | Multiple myeloma diagnosis, 2011–2014 (N = 2602) |
| Age, mean (SD) | 65.9a (12.2) | 65.2 (12.3) |
| Age cohort, N (%) | ||
| <65 yrs | 1273b (49.0) | 1376 (52.9) |
| ≥65 yrs | 1324 (51.0) | 1226 (47.1) |
| Sex, N (%) | ||
| Male | 1514c (58.3) | 1440 (55.3) |
| Female | 1083 (41.7) | 1162 (44.7) |
| Payer, N (%) | ||
| Commercial | 1241c (47.8) | 1327 (51.0) |
| Medicare | 1356 (52.2) | 1275 (49.0) |
| Geographic region, N (%) | ||
| Northeast | 324b (12.5) | 547 (21.0) |
| North Central | 903 (34.8) | 693 (26.6) |
| South | 884 (34.0) | 972 (37.4) |
| West | 480 (18.5) | 385 (14.8) |
| Unknown | 6 (0.2) | 5 (0.2) |
| Total healthcare costs,d $, mean (SD) | 20,271a (39,697) | 23,572 (54,030) |
| Deyo-Charlson Comorbidity Index,d mean (SD) | 1.1b (1.5) | 1.2 (1.6) |
| Deyo-Charlson Comorbidity Index,d N (%) | ||
| 0 | 1288a (49.6) | 1240 (47.7) |
| 1 | 606 (23.3) | 572 (22.0) |
| 2 | 310 (11.9) | 315 (12.1) |
| 3+ | 393 (15.1) | 475 (18.3) |
| Number of ICD-9 diagnoses,d mean (SD) | 11.7b (7.8) | 15.0 (10.0) |
| Comorbidities,d N (%) | ||
| Cardiac conditions | 759 (29.2) | 724 (27.8) |
| Congestive heart failure | 223 (8.6) | 233 (9.0) |
| Cardiac dysrhythmias | 360 (13.9) | 408 (15.7) |
| Myocardial infarction | 61 (2.3) | 70 (2.7) |
| Other ischemic heart disease | 463a (17.8) | 408 (15.7) |
| Liver disease | 62a (2.4) | 91 (3.5) |
| Renal disease | 422b (16.2) | 535 (20.6) |
| Treatments during follow-up,e N (%) | ||
| Bone marrow/stem-cell transplant | 537b (20.7) | 648 (24.9) |
| Chemotherapy | 657b (25.3) | 507 (19.5) |
| Bendamustine | 22 (0.8) | 19 (0.7) |
| Cisplatin | 30 (1.2) | 18 (0.7) |
| Cyclophosphamide | 275b (10.6) | 418 (16.1) |
| Doxorubicin/liposomal doxorubicin | 195b (7.5) | 50 (1.9) |
| Etoposide | 1 (0.0) | 1 (0.0) |
| Melphalan | 306b (11.8) | 65 (2.5) |
| Vincristine | 69b (2.7) | 15 (0.6) |
| Hydroxamic acid | ||
| Panobinostat | 6 (0.2) | 4 (0.2) |
| Immunomodulatory drugs | 1220a (47.0) | 1137 (43.7) |
| Lenalidomide | 943b (36.3) | 1081 (41.5) |
| Thalidomide | 566b (21.8) | 127 (4.9) |
| Pomalidomide | 86 (3.3) | 102 (3.9) |
| Proteasome inhibitors | 858b (33.0) | 1123 (43.2) |
| Bortezomib | 851b (32.8) | 1116 (42.9) |
| Carfilzomib | 69b (2.7) | 110 (4.2) |
| Steroids | 1904 (73.3) | 1854 (71.3) |
| Dexamethasone | 1619 (62.3) | 1647 (63.3) |
| Prednisone | 947b (36.5) | 634 (24.4) |
P <.05,
P <.001,
P <.01 for the difference between 2006–2010 and 2011–2014 from t-tests or chi-square tests.
Measured during baseline period (12 months before multiple myeloma diagnosis).
Follow-up is ≥30 days after diagnosis, ending with the earliest of death, end of health plan enrollment, or end of the study period (September 30, 2015).
ICD-9 indicates International Classification of Diseases, Ninth Revision; SD, standard deviation.
The costs were based on paid amounts of adjudicated claims, including payer and patient out-of-pocket payments, and were adjusted for inflation using the Consumer Price Index and standardized to 2015 US dollars. The costs for services provided under capitated arrangements were estimated using payment proxies that were computed based on paid claims at the procedure level using the MarketScan Commercial and Medicare Supplemental databases.
The main independent variable in this study was the date of diagnosis, which was determined by the date of a patient's first healthcare claim with a multiple myeloma diagnosis during the patient selection period (January 1, 2006-December 31, 2014). A total of 2597 patients were categorized as being diagnosed between 2006 and 2010 (henceforth, 2006–2010) and 2602 diagnosed between 2011 and 2014 (henceforth, 2011–2014).
The covariates included patient demographics (eg, age, sex, geographic location [US Census region]) based on the index date and clinical characteristics (eg, Deyo- Charlson Comorbidity Index, specific comorbidities) assessed during the 12-month preindex period. The patients were also assessed for their use of various multiple myeloma treatments during the follow-up period, including bone marrow or stem-cell transplantation, chemotherapy, hydroxamic acid, immunomodulatory drugs, proteasome inhibitors, and steroids.
Statistical Analyses
Unadjusted bivariate analyses were completed for all outcomes and covariates, and were stratified by the date of diagnosis. The categorical variables are presented as the count and percentage of patients in each category, and the continuous variables are summarized by the means, standard deviations (SDs), and medians within each time period.
A Cox proportional hazards model was used to evaluate the association between the time of diagnosis (2006–2010 vs 2011–2014) and survival; generalized linear models with log-link and gamma error distribution were used to estimate the effect of the time of diagnosis on the total all-cause and multiple myeloma–specific healthcare costs, while controlling for covariates. The variance inflation factor was used to assess the multicollinearity of the models' independent variables, and the recycled prediction method was used to generate a predicted mean cost difference (ie, marginal and incremental costs) between the diagnosis periods. Estimation of the standard errors of the incremental costs was conducted using the delta method. All analyses were conducted using Statistical Analysis Software version 9.4 (SAS Institute Inc; Cary, NC), and P <.05 was considered statistically significant.
Results
During the patient selection period (ie, 2006–2014), a total of 55,945 adults were identified in the databases with a diagnosis of multiple myeloma. Of those patients, 23,207 (approximately 41%) met the continuous MarketScan database enrollment criteria, and 9020 (approximately 16% of the initial sample) of those patients had no history of having multiple myeloma or another cancer. Finally, 5199 patients (approximately 9.3% of the initial sample) were linked to the SSA death file and were included in this analysis. Approximately 50% (N = 2597) of the final sample was diagnosed in 2006–2010, and approximately 50% (N = 2602) of the sample was diagnosed in 2011–2014.
Baseline Demographic and Clinical Characteristics
The mean age of the patients was 65.2 years (SD, 12.3) among patients diagnosed in 2011–2014 and 65.9 years (SD, 12.2) among patients diagnosed in 2006–2010. A slightly smaller proportion of patients diagnosed in 2011–2014 were aged ≥65 years at diagnosis compared with those diagnosed in 2006–2010 (Table). More males than females were diagnosed in both periods, and the proportion of males was significantly greater among patients diagnosed in 2006–2010 than in 2011–2014 (P <.01).
The total healthcare costs during the 12 months before a multiple myeloma diagnosis were significantly higher among patients who were diagnosed in 2011–2014 compared with 2006–2010 (P <.05). The patients diagnosed in 2011–2014 had higher comorbidity indices (P <.001) and more total diagnoses in the baseline period (P <.001) compared with those diagnosed in 2006–2010. The incidence of cardiac comorbidities was similar in both time periods, although patients diagnosed in 2006–2010 had a significantly lower incidence of liver (P <.05) and renal (P <.001) diseases than those diagnosed in 2011–2014.
Patients diagnosed in 2006–2010 were more likely than those diagnosed in 2011–2014 to receive chemotherapy (25.3% vs 19.5%, respectively; P <.001) and immunomodulatory drugs (47.0% vs 43.7%, respectively; P <.05) during the postindex period. A greater proportion of patients diagnosed in 2011–2014 compared with 2006–2010 had a bone marrow or stem-cell transplantation (24.9% vs 20.7%, respectively; P <.001) and received proteasome inhibitors (43.2% vs 33.0%, respectively; P <.001; Table).
Survival Trends
Among the patients diagnosed in 2006–2010, 36.9% died during follow-up versus 15.3% in the patients diagnosed in 2011–2014. These proportions do not account for the variable length of follow-up; therefore, mortality rates were calculated based on person-time.
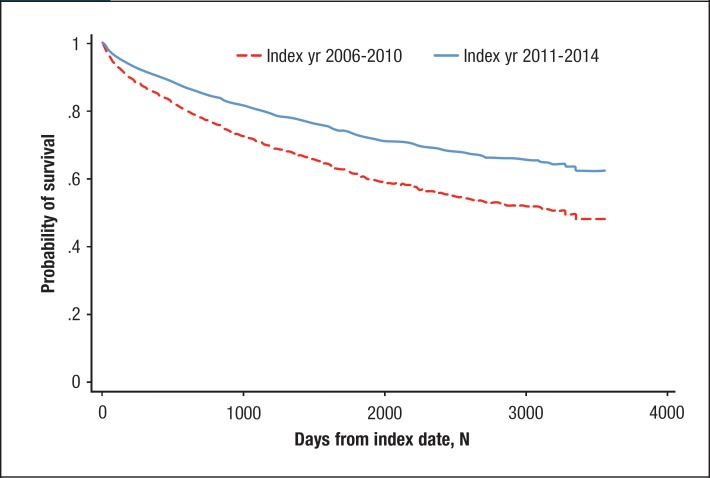
The unadjusted mortality rate was substantially lower for patients diagnosed in 2011–2014 (0.24 deaths per 1000 person-days) than in those diagnosed in 2006–2010 (0.32 deaths per 1000 person-days). Adjusting for patients' demographic and clinical characteristics, the patients diagnosed in 2011–2014 had a 35% lower risk for death than those diagnosed in 2006–2010 (hazard ratio, 0.65; 95% confidence interval [CI], 0.57–0.74). Figure 1 shows the adjusted survival probabilities during follow-up for both groups.
Figure 1.
Survival Probabilitiesa for Patients with Multiple Myeloma, by Period of Initial Diagnosis
aAdjusted for age, sex, insurance type, geographic region, baseline healthcare costs, baseline comorbidities, and postindex multiple myeloma treatments.
Healthcare Cost Trends
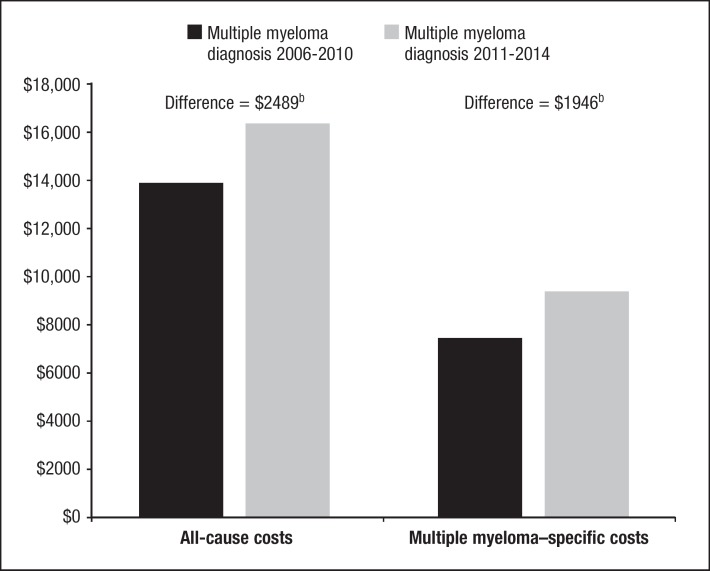
The mean unadjusted all-cause healthcare costs were 24% higher (median, 40% higher) among patients with multiple myeloma diagnosed in 2011–2014 (mean, $16,807; SD, $25,795; median, $10,534) than those diagnosed in 2006–2010 (mean, $13,534; SD, $23,316; median, $7510). After controlling for the patients' demographic and clinical characteristics, the total all-cause per-patient per-month (PPPM) costs were 18% (95% CI, 6–31) greater among those diagnosed in 2011–2014 (adjusted mean, $16,449; SD, $6142) than in those diagnosed in 2006–2010 (adjusted mean, $13,960; SD, $5213). The PPPM adjusted mean incremental cost was $2489 (SD, $929) comparing patients diagnosed in 2011–2014 with those diagnosed in 2006–2010 (Figure 2).
Figure 2.
Predicted Mean All-Cause and Multiple Myeloma–Specific PPPM Healthcare Costsa after Multiple Myeloma Diagnosis, by Period of Diagnosis
aAdjusted for age, sex, insurance type, geographic region, baseline healthcare costs, baseline comorbidities, and postindex multiple myeloma treatments.
bP <.01 for difference between time periods.
PPPM indicates per-patient per-month.
Similarly, the multiple myeloma–specific mean unadjusted healthcare costs were 40% higher (median, 59% higher) among patients diagnosed in 2011–2014 (mean, $9492; SD, $18,669; median, $4861) compared with patients diagnosed in 2006–2010 (mean, $6758; SD, $14,286; median, $3057). After controlling for covariates, the total multiple myeloma–specific costs PPPM were 26% (95% CI, 6–50) greater among those diagnosed in 2011–2014 (adjusted mean, $9422; SD, $6962) compared with patients diagnosed in 2006–2010 (adjusted mean, $7476; SD, $5524). The PPPM adjusted mean incremental cost was $1946 (SD, $1438), comparing patients diagnosed in 2011–2014 with those diagnosed in 2006–2010 (Figure 2).
Discussion
These results show a 35% decrease in the mortality rate among patients diagnosed with multiple myeloma in 2011–2014 compared with those diagnosed in 2006–2010. Furthermore, these findings show increases of 18% and 26% in the total all-cause and multiple myeloma–specific healthcare costs, respectively, over these periods.
The decrease in mortality rate was greater than the increase in healthcare costs over the same time period, suggesting that in recent years a dollar spent on healthcare may lead to improved patient outcomes compared with a dollar spent in previous years. Hence, the value of healthcare spending among patients with multiple myeloma has increased over time, and this increase may be attributed to advancements in drug therapies, coupled with advancements in disease management.22
Similar trends in survival and treatment costs have been observed when examined separately in the United States, as well as in other countries. A study of Japanese patients with multiple myeloma showed that younger patients (aged <65 years) who were diagnosed in 2001–2012 had improvements in overall survival compared with patients diagnosed and treated in 1999–2000.4 The authors attributed these improvements to the emergence of new drug classes for the treatment of multiple myeloma during that time.4
A US-based study during a similar time frame reported that significant improvements in overall survival from 2001–2005 to 2006–2010 were primarily seen among elderly patients (≥65 years) who received novel regimens.2 By contrast, Costa and colleagues observed significant improvements in the 5-year relative survival rates among patients with multiple myeloma of all ages in the United States who were diagnosed in 2008–2012 versus those diagnosed in 1993–1997, but the 10-year relative survival rates only improved among patients aged <65 years.17
The findings in our study show a significant decrease in mortality rates in 2006–2010 and in 2011–2014 among patients aged <65 years, as well as in those aged ≥65 years (unadjusted mortality rates for 2006–2010 vs 2011–2014, age <65 years: 0.19 vs 0.15 deaths, respectively, per 1000 person-days; P = .02; age >65 years: 0.47 vs 0.35 deaths, respectively, per 1000 person-days; P <.001).
Previous and current findings suggest that there have been changes in disease management in recent years that have contributed to improvements in multiple myeloma survival among patients of all ages.
The report by the Moran Company showed that changes in the volume-weighted costs of oncology drugs during the study period (2006–2014) did not exceed the rate of medical inflation, suggesting that the increased healthcare costs between patients diagnosed in 2006–2010 and in 2011–2014 are not a result of disproportionate increases in the cost of cancer treatments.21
However, that study did not specifically assess the cost of multiple myeloma treatments, which might have changed at a different rate from the general overall cost of cancer treatments. Some authors have previously concluded that increased multiple myeloma treatment costs over time are associated with the use of newer multiple myeloma treatments, suggesting a direct relationship between novel therapies and higher treatment costs.2,10,23
In a recent Swedish study, the gain in life expectancy among patients with multiple myeloma surpassed the high incremental costs associated with newer treatment regimens.24 Similarly, Lakdawalla and colleagues reported that the higher cost associated with the increased use of newer therapies over time was offset by concurrent health improvements and gains in quality-adjusted life-years among US patients with multiple myeloma.25
Our study adds to the available body of evidence that increases in healthcare costs among patients with multiple myeloma in recent years have been offset by improved outcomes.
The key strength of our study is the use of real-world data from a patient sample drawn from a large, heterogeneous database of patients with multiple years of clinical and cost data, which enabled a good depiction of temporal trends of multiple myeloma disease burden and costs.
Controlling for several patient characteristics allowed for a more unbiased estimate of healthcare costs compared with previous studies. Also, this analysis included similar numbers of patients diagnosed in each of the years within both time periods, thus providing a good representation of the survival and cost experience across each cohort.
Limitations
This study has limitations that should be considered when interpreting our findings. This study was limited to patients with commercial or Medicare supplemental health insurance coverage, so the results may not be generalizable to patients with other types of insurance or no insurance.
In addition, the healthcare claims data are subject to errors in coding and entry, which can result from the misclassification of some patients with regard to the study variables.
Furthermore, information on the patients' race and socioeconomic status, which may be associated with a patient's diagnosis, treatment, costs, and survival, were not available in the claims databases and could not be controlled for in multivariable analyses.
Conclusion
This study provides the first real-world evidence that patients diagnosed with multiple myeloma in recent years (ie, 2011–2014) incurred greater all-cause and disease-specific healthcare costs than patients diagnosed in earlier years (ie, 2006–2010), but that substantial improvements in multiple myeloma survival occurred during the same period. Among patients with multiple myeloma, survival has improved at a greater rate than the increase in healthcare costs, which may be a result of improved multiple myeloma therapies and changes in overall disease management. With the US Food and Drug Administration's approval of new treatment options for patients with multiple myeloma, tailoring treatment plans for the individual patient based on specific risk factors is even more feasible and may further optimize disease management and continue improvements in patient survival.
Source of Funding
This study was funded by Janssen Scientific Affairs.
Author Disclosure Statement
Dr Maiese is an employee of and owns stocks in Janssen; Dr Evans, Dr Chu, and Dr Irwin are employees of Truven Health Analytics, and received research funding from Janssen for this study. They have received research funding from many pharmaceutical and biotech companies for previous research.
Contributor Information
Eric M. Maiese, An employee of and owns stocks in Janssen.
Kristin A. Evans, Truven Health Analytics, and received research funding from Janssen for this study..
Bong-Chul Chu, Truven Health Analytics, and received research funding from Janssen for this study..
Debra E. Irwin, Truven Health Analytics, and received research funding from Janssen for this study..
References
- 1. American Cancer Society. Cancer Facts and Figures 2016. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf. Accessed September 25, 2016.
- 2. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozaki S, Handa H, Saitoh T, et al. Survival of multiple myeloma patients in the era of novel agents and autologous stem cell transplantation: a multicenter retrospective collaborative study of the Japanese Society of Myeloma. Blood. 2014;124:5684. [DOI] [PubMed] [Google Scholar]
- 5. Stewart AK, Rajkumar SV, Dimopoulos MA, et al; for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. [DOI] [PubMed] [Google Scholar]
- 6. Warren JL, Harlan LC, Stevens J, et al. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31:1984–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garrison LP Jr, Wang ST, Huang H, et al. The cost-effectiveness of initial treatment of multiple myeloma in the U.S. with bortezomib plus melphalan and prednisone versus thalidomide plus melphalan and prednisone or lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment. Oncologist. 2013;18:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oster G, Berger A, Bornheimer R, et al. Cost-effectiveness of lenalidomide and bortezomib in patients with previously untreated multiple myeloma (MM). Blood. 2013;122:5604. [Google Scholar]
- 9. Institute for Clinical and Economic Review. A look at treatments for multiple myeloma. May 2016. http://icer-review.org/wp-content/uploads/2016/06/icer_multiple_myeloma_v4.pdf. Accessed September 25, 2016.
- 10. Suzuki K, Yamamoto T, Ikeda M, et al. 10 years history of cost-effectiveness for 209 newly diagnosed multiple myeloma patients at a single-institute in Japan. Blood. 2016;128:5620. [Google Scholar]
- 11. Brown RE, Stern S, Dhanasiri S, Schey S. Lenalidomide for multiple myeloma: cost-effectiveness in patients with one prior therapy in England and Wales. Eur J Health Econ. 2013;14:507–514. [DOI] [PubMed] [Google Scholar]
- 12. Usmani SZ, Cavenagh JD, Belch AR, et al. Cost-effectiveness of lenalidomide plus dexamethasone vs bortezomib plus melphalan and prednisone in transplant-ineligible US patients with newly-diagnosed multiple myeloma. J Med Econ. 2016;19:243–258. [DOI] [PubMed] [Google Scholar]
- 13. Jakubowiak AJ, Campioni M, Benedict Á, et al. Cost-effectiveness of adding carfilzomib to lenalidomide and dexamethasone in relapsed multiple myeloma from a US perspective. J Med Econ. 2016;19:1061–1074. [DOI] [PubMed] [Google Scholar]
- 14. Song X, Cong Z, Wilson K. Real-world treatment patterns, comorbidities, and disease-related complications in patients with multiple myeloma in the United States. Curr Med Res Opin. 2016;32:95–103. [DOI] [PubMed] [Google Scholar]
- 15. Teitelbaum A, Ba-Mancini A, Huang H, Henk HJ. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data. Oncologist. 2013;18:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willenbacher E, Weger R, Rochau U, et al. Real-world use of 3rd line therapy for multiple myeloma in Austria: an Austrian Myeloma Registry (AMR) analysis of the therapeutic landscape and clinical outcomes prior to the use of next generation myeloma therapeutics. PLoS One. 2016;11:e0147381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaultney JG, Franken MG, Tan SS, et al. Real-world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38:41–47. [DOI] [PubMed] [Google Scholar]
- 19. Petrucci MT, Calabrese E, Levi A, et al. Cost of illness in patients with multiple myeloma in Italy: the CoMiM study. Tumori. 2013;99:e193–e202. [DOI] [PubMed] [Google Scholar]
- 20. Maiese EM, Dimova M, Baio G, Makin C. Cost per median overall month of survival in multiple myeloma patients with ≥3 lines of therapy or were double refractory. J Clin Oncol. 2016;34(15 suppl):8057. [Google Scholar]
- 21. The Moran Company. Trends in weighted average sales prices for prescription drugs in Medicare Part B, 2006–2014. September 2015. http://phrma-docs.phrma.org/sites/default/files/pdf/asp_report_2015.pdf. Accessed September 25, 2016.
- 22. Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360–376. Erratum in: Mayo Clin Proc. 2013;88:777. [DOI] [PubMed] [Google Scholar]
- 23. Zhou X, Xia J, Mao J, et al. Real-world outcome and healthcare costs of relapsed or refractory multiple myeloma: a retrospective analysis from the Chinese experience. Hematology. 2016;21:280–286. [DOI] [PubMed] [Google Scholar]
- 24. Borg S, Nahi H, Hansson M, et al. Cost effectiveness of pomalidomide in patients with relapsed and refractory multiple myeloma in Sweden. Acta Oncol. 2016;55:554–560. [DOI] [PubMed] [Google Scholar]
- 25. Lakdawalla D, Shafrin J, Lucarelli C, et al. Quality-adjusted cost of care: a meaningful way to measure growth in innovation cost versus the value of health gains. Health Aff (Millwood). 2015;34:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]